INTRODUCTION
Dabrafenib chemically designated as N- {3- [5- (2-amino pyrimidin-4-yl) -2-tert- butyl-1, 3-thiazol -4-yl]-2-fluorophenyl} -2, 6-difluoro benzene-1-sulfonamide. Its chemical formula and molecular mass are C23H20F3N5O2S2 and 519.56 g/mol, respectively (Fig. 1). It (Tafinlar is a trade name) is concomitant with a mutated type version of a BRAF gene and useful in the management of cancer (Gibney et al., 2013; Huang et al., 2013; Lawrence et al., 2014). This drug is related to the B-RAF enzyme, which will exhibit important activity in cellular growth regulation, and inhibits to produce anticancer action. During the phase I and II trials, this drug has shown safety management profiles in all the patients associated with mutated metastatic melanoma B-RAF (V600) (Maverakis et al., 2015; Suttle et al., 2015).
First, this drug is utilized singly for the management of metastatic melanoma or unresectable with B-RAF (V600E) mutations as an inhibitor of kinases. This was identified by US-FDA confirmed tests (Ascierto et al., 2012; FDA, 2013,2018). Utilization of combined formulation was created a demonstrational strong response rate. Development in disease-related symptoms or complete existence has not at confirmed for this drug when combined with trametinib (Dhillon et al., 2007; Hauschild et al., 2017; Long et al., 2017).
Literature review on dabrafenib reveals that only two analytical approaches on LC-tandem mass spectroscopy (Merienne et al., 2017; Svante et al., 2017) were described for the assessment of dabrafenib in sample plasma. The reported works were in combination with the other components. Therefore, current work was aimed to develop an accurate, specific, and robust liquid chromatography–tandem mass spectrometry (LC–MS/MS) technique for the assessment of dabrafenib in human’s plasma, as a single drug.
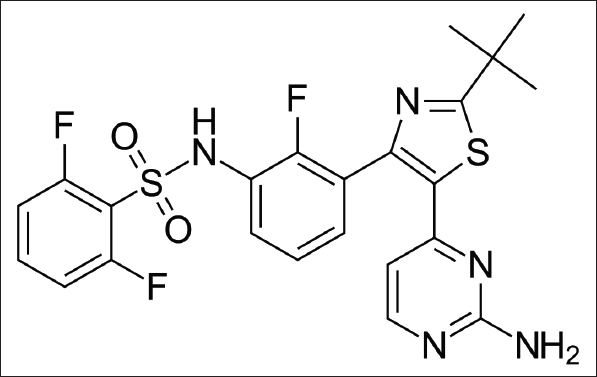 | Figure 1. Dabrafenib chemical structure. [Click here to view] |
MATERIALS AND METHODS
Chemical reagents
The dabrafenib (purity: 99.68%) standard and sorafenib (purity: 99.87%) utilized as internal standard (IS) were obtained from the MSN Labs, Hyderabad, India. Acetonitrile (ACN) and methyl alcohol of LC purity were acquired from Merck, Mumbai, India. Deionization of water has been processed from Milli-Q waters system (Millipore, USA).
LC–MS/MS system and its settings
The LC–MS/MS system comprising an Agilent 1200 liquid chromatographic instrument with a dual pumps-SL and an Agilents/6164 triple quadrupole mass spectroscopic system with electro spraying ionizations source (CA). Chromatographic data was processed by a Mass Hunters version B.010.004 software. Through Phenominex (50 × 4.6mm id, 5 μm), C18 analytical column, an isocratic solvent system comprised ACN and HCOOH (0.1%), (85:15, %v/v) was passed. The assessment of analytes was executed in a triple quadrupoles mass system retaining electro spraying ionizations method, functioning in multiple reactions monitoring, and transitions were m/z 520.10–176.98, m/z 465.09–244.10 for dabrafenib, sorafenib, respectively, in the positive ionizations mode (Raviraj et al., 2016; Vijaykumar and Raviraj, 2019). The MS/MS settings were fixed as: nebulize gas pressures, 45.0 psi, source temperature, 500ºC; capillaries voltage, 6.00 kV, and dryer gas (N2) ?ow, 10 l/minute. The injection volume and auto-sampler temperatures were set to 10 μl and 5.0ºC, respectively. The ?owing rates of 0.80 ml.minute−1 and collisional energies of 20 eV were utilized in the chromatographic elution.
Protocol quality control samples
1.0 mg/ml individual dabrafenib stock solution and IS were executed in ACN (diluent) separately. The stock solution of dabrafenib was then subjected for serial dilution with diluent to attain the operational standards. An IS operational solution at 250 ng.ml-1 executed by dilution of IS primary solutions with diluent. Processed standard samples were retained at −20ºC before the utilization.
Calibration standards of dabrafenib (74, 180, 450, 850, 1,300, 1,800, 2,350, and 2,956 ng/ml,) were processed by spiking method applied to working standards to blank plasmas. Quality controls at lower, medium, and higher concentration (207, 1,478, and 2,217 ng.ml−1), were executed separately in a similar mode.
Protocol for samples preparation
A 200 μl aliquots of plasmas samples were located in 10 ml plastic tubes, follows by adding 100 μl of IS operational solution was added in all the sample solutions excluding the blank sample. The resulting mixture was extracted with 5.0 ml of ethyl acetate after the sonication for 20 minutes. Next, the sample was subjected for centrifugation for 15.0 minutes at 5.0ºC and 5,000 rpm. The supernant organic solvent phase was relocated into fresh glass tube and nitrogen steam was applied to evaporate the same. Resultant dried residue was reconstructed with 100 μl of movable solvent and 10μl aliquots were infused to and LC–MS/MS equipment for examination.
Analytical method validation
Developed analytical technique was validated as per the regulatory guidelines of US-FDA for different validation constraints to meet the respective guidelines (EMA, 2011; FDA, 2001).
RESULT AND DISCUSSIONS
Mass spectrometric instrument
In the development stage, fresh dabrafenib solution was injected for the optimization of the product and parent ion. Precursor ion at 520.10 m/z value was detected in the positive ionizations approach. Upon fragmentation of the precursor ion, fragments of m/z 176.98 and 94.04 were noticed. The daughter ion of dabrafenib at 176.98 m/z was identified with a maximum intensity value. Sorafenib is having similar physicochemical properties with the dabrafenib to select as an IS for this bioanalytical method development and for good recovery during the sample preparation and validation process. Multiple reactions monitoring (MRM) scan was executed for the identification of the product and parent ions for both drugs and transitions finalized as m/z 520.10 –176.98 for dabrafenib and m/z 465.09–244.10 for sorafenib. (Bhamare et al., 2019; Kulkarni et al., 2016a, 2016b; Patel et al., 2017)
Speci?city
Plasma blank and spiked plasma at lower limit of quantifiacation (LLOQ) level (74 ng/ml) of dabrafenib and IS were ran in the LC-MS/MS system and the outcomes were represented in Figure. 2. No intervention peaks were noticed for dabrafenib and sorafenib from sample plasmas. The drug and the IS were isolated from the system within 4 minutes and the retaining times of dabrafenib and sorafenib were 2.40 and 3.0 minutes, correspondingly (ICH, 2005; Vikingsson et al., 2017).
Sensitivity and linearity
The LLOQQC of drug component was fixed at 74 ng.ml−1, because at this concentration level the signal/noises findings were >10.0, and the accurateness and precision findings were <2.82 %relative standard deviation (RSD). Rectilinear plots were processed for every batch between the concentration levels of 74.0–2,596.0 ng.ml−1 for dabrafenib in plasmas (Table 1). The equation of regression plot was calculated to form the average values of six replica calibration standards and was found as: y = 0.00034 x + 0.00018 for dabrafenib, where “x” represents the plasmas concentration (Nirav et al., 2017; Jaivik et al., 2017) and “y” indicates the peaks ratio, i.e., analytes/IS.
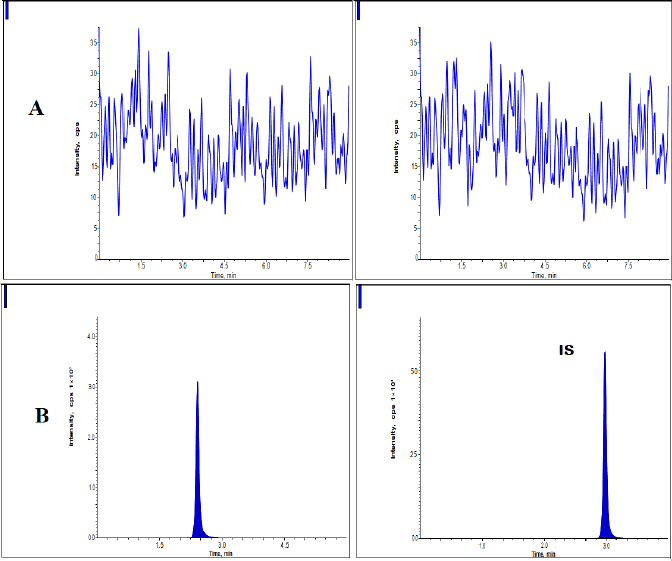 | Figure 2. Dabrafenib processed chromatograms at A) Blank and B) LLOQ samples. [Click here to view] |
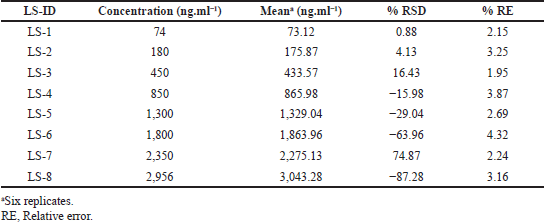 | Table 1. Linearity standard levels for dabrafenib. [Click here to view] |
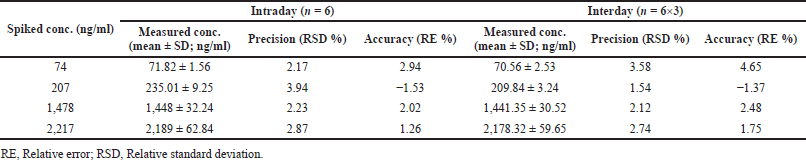 | Table 2. Intra, and inter-day accurateness and precision of dabrafenib in plasma. [Click here to view] |
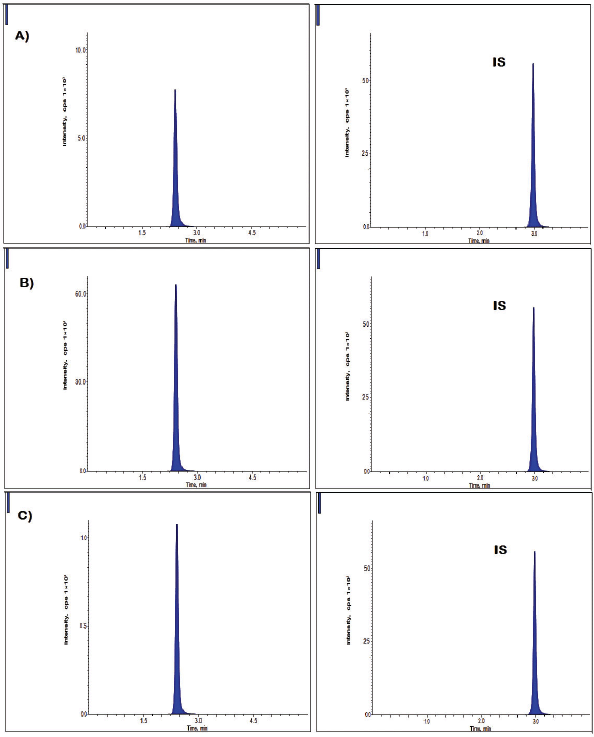 | Figure 3. Representative dabrafenib chromatograms at A) LQC B) MQC, and C) HQC levels. [Click here to view] |
Recovery, accuracy, and precision
Inter-day, and intra-day accurateness, and precision findings were given in Table 2 and Figure. 3. Within a day precision finding existed in between 2.17% and 3.94% (RSD) for dabrafenib, whereas the accurateness findings were within -1.53%–2.94% of relative error. Likewise, for between the days experimental, precision was changed between 1.54% and 3.58% (RSD) for dabrafenib, whereas the accurateness was between −1.37% and 4.65% of relative error (Patel et al., 2011).
The dabrafenib average recovery findings were in the range of 95.35%–101.37% at 3-QC concentrations (Table 3). The executed LLE for the sampling process proved that dabrafenib and sorafenib (97.49%) were recovered with higher percentage values from plasma (Titier et al., 1997). These resulted findings were proven the accuracy in terms of recovery and precision in terms of %RSD.
Matrix effect
The findings of matrix effect values were represented in Table 4. The respective peaks area ratio of the drug/IS solubilized in the plasma blank extract to those solubilized with movable solvent existed in between 95.62% and 102.34% for dabrafenib at lower quality control (LQC) level and 95.62%–102.76% at high quality control (HQC) level. These results proposed that interfering matrix component effect of the analytes were insignificant when exposed to the developed LC–MS/MS circumstances (Jaivik et al., 2017; Titier et al., 1997).
Stability tests
The dabrafenib stability of was established subsequently subjecting control sample solutions to dissimilar storage environments (FDA, 2001; Nirav et al., 2017). The subjected environments comprise longer time stabilities after the storing at -20.0°C for 30.0 days, shorter time stabilities at the room conditions for a period of 8.0 hours, processed samples (extracts) stabilities after 24.0 hours at 4.0°C, and three completely freeze-thawed cycle (frozen at −20.0°C for 12.0 hour). The finding for stabilities for control sample solutions in the plasmas was represented in a Table 5. The assessed accurateness values for dabrafenib drug were existed in between 94.98% and 103.27%, which were acceptable as per the regulatory guidelines.
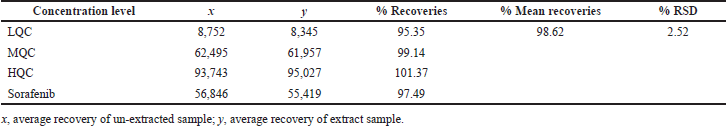 | Table 3. Recovery rates of dabrafenib and sorafenib. [Click here to view] |
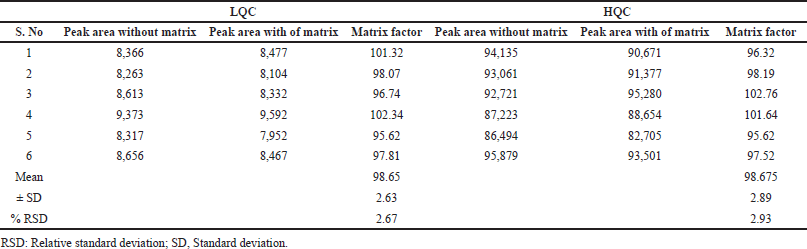 | Table 4. Matrix effect for dabrafenib at LQC and HQC levels. [Click here to view] |
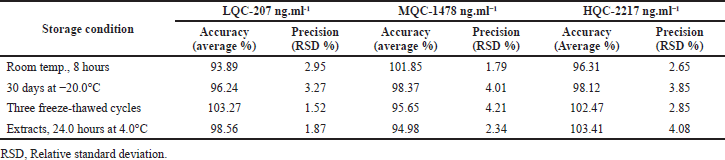 | Table 5. The stabilities of dabrafenib in humans plasma under dissimilar storage environments (n = 3). [Click here to view] |
CONCLUSION
A specific, accurate, and new validated LC–MS/MS technique was produced for the quantitation of US-FDA approved dabrafenib anticancer drug in plasmas of humans. Within a day precision finding existed in between 2.17% and 3.94% (RSD) for dabrafenib, whereas the accurateness findings were within −1.53%–2.94% of relative error. Likewise, for between the days experimental, precision was changed in between 1.54% and 3.58% (RSD) for dabrafenib, whereas the accurateness was in between −1.37% and 4.65% of relative error. The findings of matrix effect values were existed in between 95.62% and 102.34% for dabrafenib at LQC level and 95.62%–102.76% at HQC level. The LLOQQC of drug component was fixed at 74 ng.ml−1, because at this concentration level the Signal/Noise findings were >10.0. Equation of regression plot was y = 0.00034 x + 0.00018 for dabrafenib. Finally, the produced method was within the limits of bioanalytical method validation guidelines and can be successfully applicable to quantitate the dabrafenib in variable bio-samples.
The intra-day and inter-day accuracies were within −1.37% to 4.65% of relative error and the RSD of precision were less than 3.94%. The drug was sufficiently stable under different analytical conditions. liquid liquid extraction method was optimized for dabrafenib extracting from plasma with mean percent recoveries of 98.62% by utilizing the sorafenib as an IS. The sensitivity, good validation criteria, and high percent recoveries from plasma of the proposed method rendered it applicable for the bioequivalence and pharmacokinetic studies.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM, Mozzillo N. The role of BRAF V600 mutation in melanoma. J Transl Med, 2012; 10:85. CrossRef
Bhamare VS, Kulkarni RM. Palladium (II)-catalyzed oxidation kinetics of azidothymidine by heptavalent manganese during water treatment: kinetics, mechanism, and degradation. Desalin Water Treat, 2019;144:211–23. CrossRef
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene, 2007; 26(22):3279–90. CrossRef
European Medicines Agency. Guideline on bioanalytical method validation, 2011.
FDA approves adjuvant combo for BRAF+ Melanoma. Available via http://www.medscape.com. WebMD LLC (Accessed 2 May 2018).
FDA. Clinical pharmacology and biopharmaceuticals review(s) application number 202806Orig1s000. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, 2013. Available via http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202806Orig1s000ClinPharmR.pdf (Accessed 11 February 2017).
FDA Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), 2001.
Gibney GT, Zager JS. Clinical development of dabrafenib in BRAF mutant melanoma and other malignancies. Expert Opin Drug Metab Toxicol, 2013; 9(7):893–9. CrossRef
Huang T, Karsy M, Zhuge J, Zhong M, Li D. B-Raf and the inhibitors: from bench to bedside. J Hematol Oncol, 2013; 6:30. CrossRef
Hauschild A, Dummer R, Schadendorf D. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600–mutant stage III melanoma. J Clin Oncol, 2018; 36:3441–9. CrossRef
ICH guidelines for validation of analytical procedures: text and methodology. Q2(R1) ICH, Geneva, Switzerland,pp 1–14, 2005.
Jaivik VS, Shaha Priyanka A, Shaha Priya V, Shahb Mallika, Sanyalc Pranav S, Shrivastav. Fast and sensitive LC–MS/MS method for the simultaneous determination of lisinopril and hydrochlorothiazide in human plasma. J Pharm Anal, 2017; 7:163–9. CrossRef
Kulkarni PM, Bhamare VS, Santhakumari B. Oxidative transformation of antiretroviral drug zidovudine during water treatment with permanganate: reaction kinetics and pathways. Desalin Water Treat, 2016a; 57(52):24999–5010. CrossRef
Kulkarni RM, Bhamare VS, Santhakumari B. Mechanistic and spectroscopic investigations of Ru3+-catalyzed oxidative degradation of azidothymidine by heptavalent manganese at environmentally relevant pH. Desalin Water Treat, 2016b; 57(58):28349–62. CrossRef
Lawrence SK, Nguyen D, Bowen C, Richards-Peterson L, Skordos KW. The metabolic drug-drug interaction profile of dabrafenib: in vitro investigations and quantitative extrapolation of the P450–mediated DDI risk. Drug Metab Dispos, 2014; 42(7):1180–90. CrossRef
Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Chiarion-Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JBAG, Hansson J, Utikal J, Ferraresi V, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, Davies MA, Lane SR, Legos JJ, Mookerjee B, Grob JJ. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol, 2017; 28:1631–9. CrossRef
Merienne C, Rousset M, Ducint D, Castaing N, Titier K, Molimard M, Bouchet S. High throughput routine determination of 17 tyrosine kinase inhibitors by LC-MS/MS. J Pharm Biomed Anal, 2017; 150:112–20. CrossRef
Maverakis E, Cornelius LA, Bowen GM, Phan T, Patel FB, Fitzmaurice S, He Y, Burrall B, Duong C, Kloxin AM, Sultani H, Wilken R, Martinez SR, Patel F. Metastatic melanoma – a review of current and future treatment options. Acta Derm Venereol, 2015; 95(5):516–24.
Patel NP, Sanyal M, Sharma M, Patel DS, Shrivastav PS, Patel BN. Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study. J Pharm Anal, 2017; 6:145–50.
Patel DS, Sharma N, Patel MC, Patel BN, Shrivastav PS, Sanyal M. Development and validation of a selective and sensitive LC–MS/MS method for determination of cycloserine in human plasma: application to bioequivalence study. J Chromatogr B, 2011; 879:2265–73. CrossRef
Suttle AB, Grossmann KF, Ouellet D, Richards-Peterson LE, Aktan G, Gordon MS, LoRusso PM, Infante JR, Sharma S, Kendra K, Patel M, Pant S, Arkenau HT, Middleton MR, Blackman SC, Botbyl J, Carson SW. Assessment of the drug interaction potential and single- and repeat-dose pharmacokinetics of the BRAF inhibitor dabrafenib. J Clin Pharmacol, 2015;55(4):392–400. CrossRef
Titier K, Castaing N, Le-Deodic M, Le-Bars D, Moore N, Molimard M. Quantification of tricyclic antidepressants and monoamine oxidase inhibitors by high-performance liquid chromatography-tandem mass spectrometry in whole blood. J Anal Toxicol, 1997;21:200–7. CrossRef
Vikingsson S, Dahlberg JO, Hansson J, Höiom V, Gréen H. Simple and cost-effective liquid chromatography-mass spectrometry method to measure dabrafenib quantitatively and six metabolites semi-quantitatively in human plasma. Anal Bioanal Chem, 2017; 409(15):3749–56. CrossRef