INTRODUCTION
Lung cancer (LC) is the second most prevalent cancer but is the leading cause of cancer death worldwide [1]. It is mostly diagnosed in an advanced stage and metastatic [2,3]. Adenocarcinoma (ADC) is the most prevalent histology type of LC, with about 85% being caused by nonsmall cell lung cancer (NSCLC). Around 50%–80% of NSCLCs have epidermal growth factor receptor (EGFR) overexpression [4–7]. A higher proportion of Asians are positive for EGFR mutations (EGFRm+), around 40%–60%, compared with 10%–20% of Caucasians [8,9]. In general, the activity of EGFR tyrosine kinase inhibitors (TKIs) does not differ significantly between Asian and non-Asian populations [10]. However, there are likely to be differences in the mutation frequencies of NSCLC between Asian sub-groups, particularly South and Southeast Asian sub-groups [11].
Based on the improved overall survival (OS) for the human epidermal growth factor receptor 2 (HER2/ERBB2) mutations and mesenchymal-epithelial transition (MET) amplification with targeted therapies compared to conventional chemotherapy, the National Comprehensive Cancer Network (NCCN panel) recommends molecular testing to target driver mutations in LC diagnosis [12]. A pertinent achievement in NSCLC research, EGFR gene mutation identification is considered to be the most robust predictive biomarker of response to EGFR TKIs [13]. In clinical practice, they increased the OS approach by 22–34 months [14]. First- and second-generation EGFR TKIs are the first-line treatments for patients with metastatic NSCLC who have EGFR mutation in e19del or L858R, while third-generation is a second-line setting [10,15]. The distribution rates of common (e19del and L858R) and rare common (exon 18 G179X, exon 20 S768I, and exon 21 L861Q) activating EGFR mutations vary across the region [16,17]. In general, e19del represents 45% and L858R 40% of patients and confers a prognostic advantage, significantly improving progression-free survival (PFS) in advanced EGFR + NSCLC compared to L858R mutation [18]. For EGFR single mutations, L858R was found more frequently than e19del in South and North Asia [17], while e19del is higher than L858R in Southeast Asia [19,20]. Gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib are the five EGFR TKIs available globally, and icotinib is exclusive to China. The type of EGFR mutation is one of many considerations when deciding on first-line treatment for an EGFRm+ patient. With numerous drugs available for EGFR-mutated NSCLC, a long-term treatment plan is needed to maximize survival [21]. Comparative effectiveness research (CER) seeks effectiveness by comparing outcomes through a direct comparison of interventions or studies in everyday clinical care which will lead to a health care improvement, better health outcomes, and lower costs [22]. Recently, published network meta-analysis (NMA) on the efficacy of first-line EGFR TKIs are limited by single osimertinib randomised control trial (RCT) investigation [23,24]. In Asian patients, Farris et al. concluded that dacomitinib showed a numerical improvement in OS compared to all other EGFR TKIs, while osimertinib showed no significant improvement in OS compared to the first generation [10]. In general, Qi et al. updated that osimertinib and second-generation EGFR TKIs were more effective than first-generation monotherapy in improving OS and PFS.
Therefore, data concerning the effectiveness of the choices of LC treatment based on EGFR mutation are presently of paramount importance in the Asian population. It is important to note that CER on EGFR TKIs is an alternative strategy to support decisive decision-making toward increasing the effectiveness of treatment while reducing health expenditures. Based on these findings, we focus on comparing effectiveness between Asian populations.
This study aimed to compare the clinical effectiveness of EGFR TKIs as a first-line therapy based on identified EGFR gene mutations in Asian populations. A systematic synthesis was conducted by accessing quantitative and qualitative data and a meta-analysis was done as a deeper investigation.
METHODS
Search strategy
Six comprehensive electronic databases were systematically searched: ScienceDirect, ProQuest, EBSCOhost, Scopus, Cochrane Library, and PubMed. PICO (Population Intervention Comparison Outcome) table and search terms were provided in Appendix A. All direct EGFR TKIs comparison articles before August 2022 which conformed with eligibility criteria were included, i.e., (i) patients with NSCLC harboring EGFR aberrant, (ii) treated with EGFR TKIs in the first-line setting, (iii) has primary either secondary efficacy outcomes, i.e., PFS or OS or time to treatment failure (TTF), and (iv) conducted in Asian population. Studies were excluded when it is a review, post hoc analysis of trial data, and without available full text. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline was used to report this systematic review [25].
Study selection and screening
After the removal of any duplication, selected articles were skimmed by title and abstract. Subsequently, full-text articles were retained for further appraisal. One author (MW) performed the study search and reference tracking was conducted to obtain comparative effectiveness results. Two authors (MW and EK) independently reviewed data extraction. An agreement was sought through discussion in any case of differences between reviewers.
Quality assessment and data extraction
All 30 selected articles were quantitatively assessed using the Good Research for Comparative Effectiveness (GRACE) checklist. The GRACE checklist has been tested for validity. It was designed as a tool to access the quality of observational CER [26]. We used the GRACE checklist tailored to lung oncology, and a final score was based on a range of 0 to 11 [22]. In addition, JADAD’s scale is used to estimate the robustness of clinical trial comparative effectiveness studies [27]. The data extraction approach with thorough management of overlapping information and data at the synthesis stage addressed the potential scenario of sample overlap across multiple studies used in meta-analysis [28].
We extracted the following information: name of the first author, year of publication, the number of patients studied, ethnicity, treatments compared, study design, characteristic aberrant, statistical method, results of effectiveness, and conclusions.
Statistical analysis
The GRACE scores were analyzed using a t-test to compare the mean values of single-center to multicenter studies. This review investigated the association between EGFR aberrant and effectiveness through pooled hazard ratio (HR) of clinical outcome and 95% confidence interval (CI) in all. The significance effect of pooled HR was determined using the Z test (p < 0.05 considered statistically significant). The heterogenicity test was measured using I2 and showed significant heterogeneous results (p < 0.05 or I2 > 50%). We used a fixed-effect model if I2 < 50%, whereas I2 50%–90% used a random-effects model. This meta-analysis was conducted using Review Manager Software version 5.3 (RevMan v5.3, The Cochrane Collaboration, Oxford, UK).
RESULTS
Literature search
There were 1,373 finding articles and 799 articles were excluded by title. A total of 541 abstracts were screened and 106 full-text articles were assessed. In addition, 30 eligible articles were critically appraised using the GRACE instrument. The literature search process is described in Figure 1.
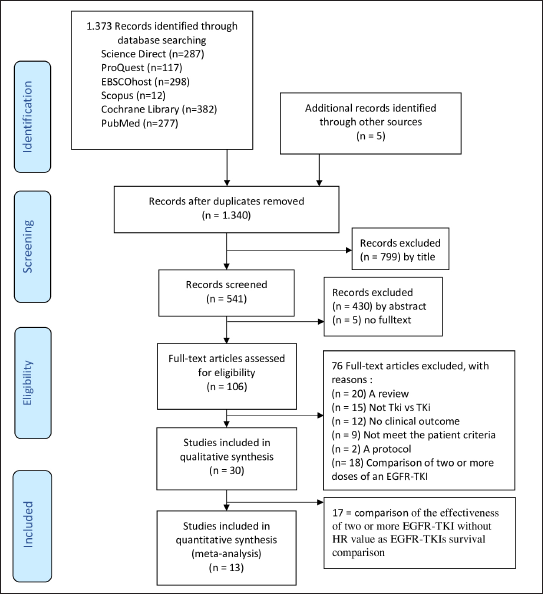 | Figure 1. Flowchart of the literature search process. [Click here to view] |
Characteristics of included studies
Fifteen of 30 eligible articles were conducted by multicenter and eight of 30 eligible articles were from five trials which were conducted as prospective randomized phase II and phase III clinical trials, i.e., FLAURA [29], ARCHER 1009 [30], ARCHER 1050 [31–33], LUX lung 7 [34,35], and ICOGEN [36]. Moreover, most of these articles had a cohort retrospective study design.
Furthermore, these findings were tabulated in two categories: first, there were 17 articles (Table 1) without HR of EGFR TKIs as survival effectiveness comparison and the second was 13 articles (Table 2) with the effectiveness comparison. There were seven ethicists revealed based on geographical locations, i.e., Chinese, Japanese, Indian, Indonesian, Korean, Singaporean, and Taiwanese. We found the most frequently used comparator therapy was gefitinib.
From these eligible articles, we compared the efficacy of the first generation of EGFR-TKIs (gefitinib or erlotinib or icotinib) with the second (afatinib or dacomitinib) and third generation (osimertinib). Meta-analyses were performed on 13 articles (Table 3), 8 clinical trial comparative effectiveness articles [29–36], and 5 retrospective cohorts [37–41]. We found potential patient overlap when comparing the efficacy outcome of dacomitinib and gefitinib/erlotinib, i.e., PFS [31,32] and OS [32,33] from ARCHER 1050 studies [31–33]. Based on geographical location, we found data on the effectiveness comparison of Chinese [29,36], Japanese [37], Korean [40], and Taiwanese [38,39,42]. In addition, there were two Taiwanese [39,42] and one Korean [40] articles comparing PFS and OS of afatinib and first-generation TKIs. A meta-analysis of efficacy in these two ethnicities is, therefore, possible.
Quality assessment
Articles tabulated in Table 1 and statistically analyzed in Appendix B (a). The average GRACE score was 6.15 with minimum–maximum (min–max) 3.5–9.5 and a standard deviation (SD) of 1.59. It did not differ significantly (p = 0.14) between studies from multicenter and single center. Similarly, the assessment results of Table 2 revealed a scoring average of 9.35 (min–max: 6.0–11.5; SD 3.68) and were not statistically significant (p = 0.25) under the statistical analysis shown in Appendix B (b). In addition, assessing the JADAD score for RCT articles according to the Cochrane Assessment Criteria resulted in a mean score of 3.5 (min–max: 3–4; SD 0.55).
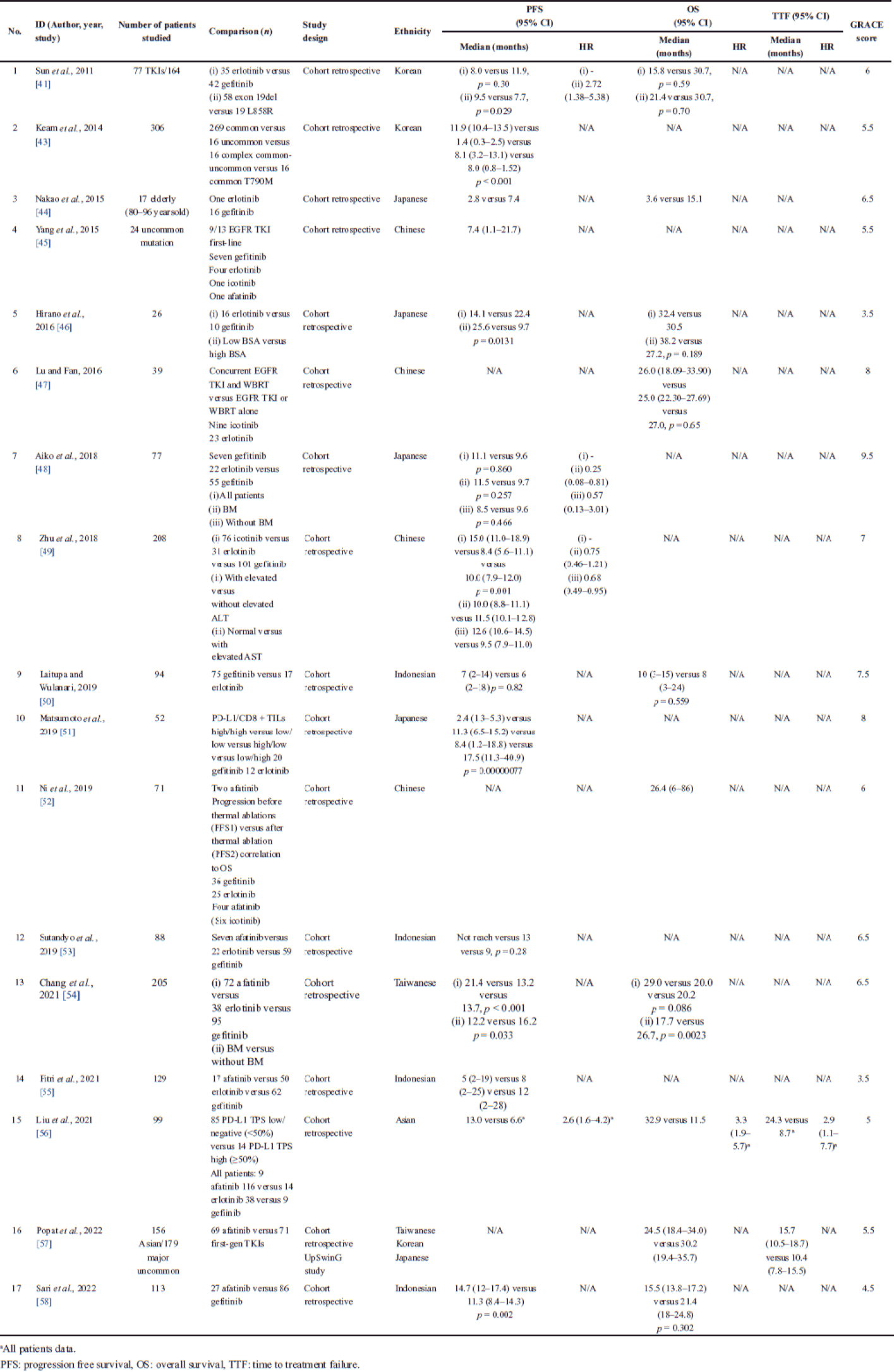 | Table 1. The comparative effectiveness result of EGFR TKI without HR as survival effectiveness comparison. [Click here to view] |
First-generation EGFR TKIs
Gefitinib and erlotinib have shown acceptable efficacy in NSCLC patients aged ≥ 80 years, but a dose reduction was required due to adverse events [44]. A study in Japan revealed that low-dose EGFR TKIs may provide sufficient disease control without side effects in lung cancer patients with a body surface area (BSA) <1.45 m2. PFS was significantly prolonged in the low BSA group, but no significant correlation was found between OS and BSA in those who received a reduced dose of gefitinib or erlotinib [46].
Gefitinib was associated with more cases of alanine aminotransferase (ALT) and aspartate transaminase (AST) elevation compared to erlotinib and icotinib. The increased ALT was more pronounced than the increased AST. Those with normal ALT levels had a significantly longer PFS than those with increased ALT levels [46]. A Korean study found that older people with EGFR-mutant NSCLCs had significantly shorter survival compared with younger people treated with gefitinib/erlotinib. In addition, the patients receiving erlotinib were more likely to have moderate to severe rash than those receiving gefitinib [41].
A multivariate analysis showed that erlotinib was associated with longer PFS than gefitinib (p = 0.025). Although with a 0.33 times higher rate, the uncommon EGFR aberrations had no significant association (p = 0.309) [37]. Icotinib was noninferior to gefitinib for PFS or OS with HR 0.84 95% CI: 0.67–1.05 and HR 1.02 95% CI: 0.82–1.27, respectively [36].
Microenvironment tumor
The tumor microenvironment based on programmed death-ligand 1 (PD-L1) tumor expression and CD8+ tumor-infiltrating lymphocytes (TIL) had a potential impact on the efficacy of EGFR TKI. There was no difference in outcome in high PD-L1 (≥ 50%) or low tumors stratified by type of TKI received. Tumors with high expression of PD-L1 were more likely to have de novo resistance and numerically less likely to receive subsequent treatment after progression [56]. Patients with high PD-L1 had a significantly shorter median PFS than those with low PD-L1, with 5.9 months and 13.2 months (p = 0.0059), respectively. A PD-L1 high/CD8+ TIL high phenotype may be the subset that would not benefit or poorly progress from EGFR TKI treatment [51].
Beyond progression
Continued TKI treatment beyond disease progression has been used to delay the need for chemotherapy and to avoid the risk of relapse. Thermal ablation of isolated oligoprogressive lesions in combination with continuous EGFR TKI may extend the use of TKI therapy in acquired TKI resistance [52]. Osimertinib was the clear treatment of choice for T790M [57].
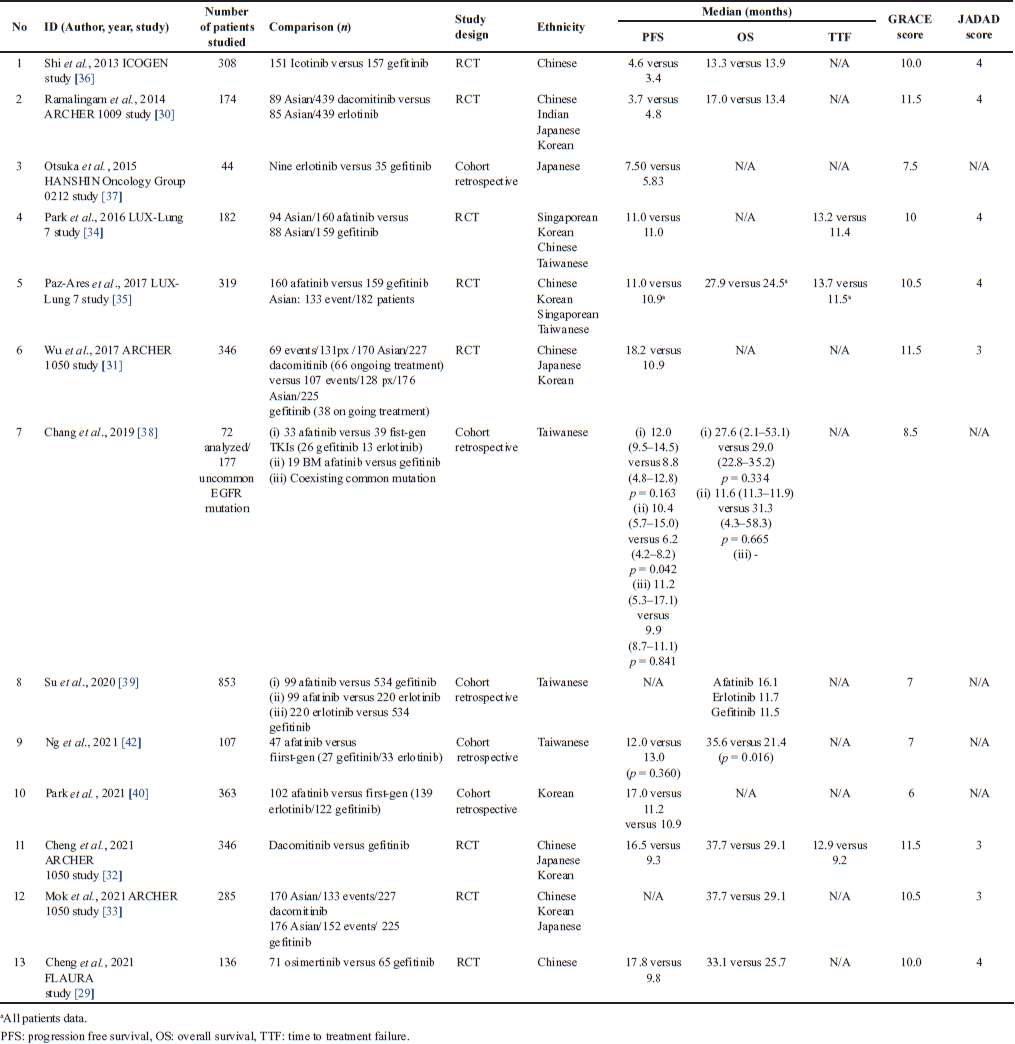 | Table 2. The comparative effectiveness result of EGFR TKI with HR as survival effectiveness comparison. [Click here to view] |
Uncommon EGFR mutation
There were 10.3% uncommon mutation frequencies among EGFR mutation-positive patients [43]. These uncommon mutations are exon 18 G179X, exon 20 S768I, and exon 21 L861Q. The use of erlotinib for L858R and gefitinib for uncommon EGFR mutations should be approached with caution [40]. Compared with first-generation EGFR TKIs, afatinib may be more appropriate [45]. A multicenter at three hospitals in Taiwan showed that afatinib may provide a better treatment response but no survival benefit compared with its alternatives. Afatinib had a longer median PFS and median OS than first-generation EGFR TKIs, but it was not statistically significant, p = 0.163 and p = 0.334, respectively. In contrast, uncommon patients with brain metastases (BMs) treated with afatinib had significantly longer median PFS and not significantly shorter OS [38]. An update revealed that both afatinib and first-generation EGFR TKIs have robust activity, with an overall response rate of 51% and longer median TTF with afatinib, with 14.3 months versus 9.8 months, respectively. It was slightly higher in Asian populations with 15.7 months and 10.4 months, respectively [57].
BM EGFR mutation
EGFR TKIs plus concurrent whole-brain radiotherapy (WBRT) could effectively control intracranial lesions and significantly improve OS in patients with BM. In combination, gefitinib had the longest OS compared to icotinib and erlotinib, but the benefit comparison is not statistically significant [47]. Instead, a multivariable analysis from a Japanese study showed that in the absence of BM before EGFR TKI therapy, erlotinib had a significant favorable PFS effect on central nervous system (CNS) progression compared to gefitinib (HR 0.321; 95% CI: 0.114–0.903; p = 0.031) (BM vs. no BM HR 2.54; 95% CI: 1.131–5.702; p = 0.024). In common mutation patients with BM, erlotinib more prolonged PFS compared with gefitinib, while the median PFS was longer in the gefitinib group in patients without BM [48]. Another study in Taiwan revealed that afatinib prolonged PFS and OS in relatively younger patients without BM [54].
First-generation versus second-generation EGFR TKIs
Pooling of first-generation TKI data in two studies in Taiwanese [38,42] showed no significant difference in PFS compared to afatinib (p = 0.163 and p = 0.360), but a significant difference in OS (p = 0.334 vs. p = 0.016). The results showed that the treatment with afatinib was 0.7 times more likely to improve PFS with 0.72 and 0.54 times higher likelihood of improving OS, respectively [38,42].
Median TTF in Taiwanese with the exon 19 deletion was significantly longer in the afatinib group compared to gefitinib and erlotinib, with 18.2 months versus 11.1 months versus 11.9 months (log-rank test, p = 0.003). Conversely, in patients with the L858R mutation, no differences were observed: afatinib 16.1 months versus gefitinib 12.0 months versus erlotinib 11.4 months (log-rank test, p = 0.187) [39]. On the other hand, the risk factor analysis of L858R versus e19del in Taiwanese NSCLC treated with EGFR TKIs which was not statistically significant revealed that L858R had a 6% lower risk of treatment failure and a 21% lower risk of progression than e19del, with HR 0.94 (0.80–1.10; p = 0.413) and 0.79 (0.47–1.34; p = 0.381), respectively. And those had the same risk of survival, HR 1.02 (0.61–1.69; p = 0.951) [39,42].
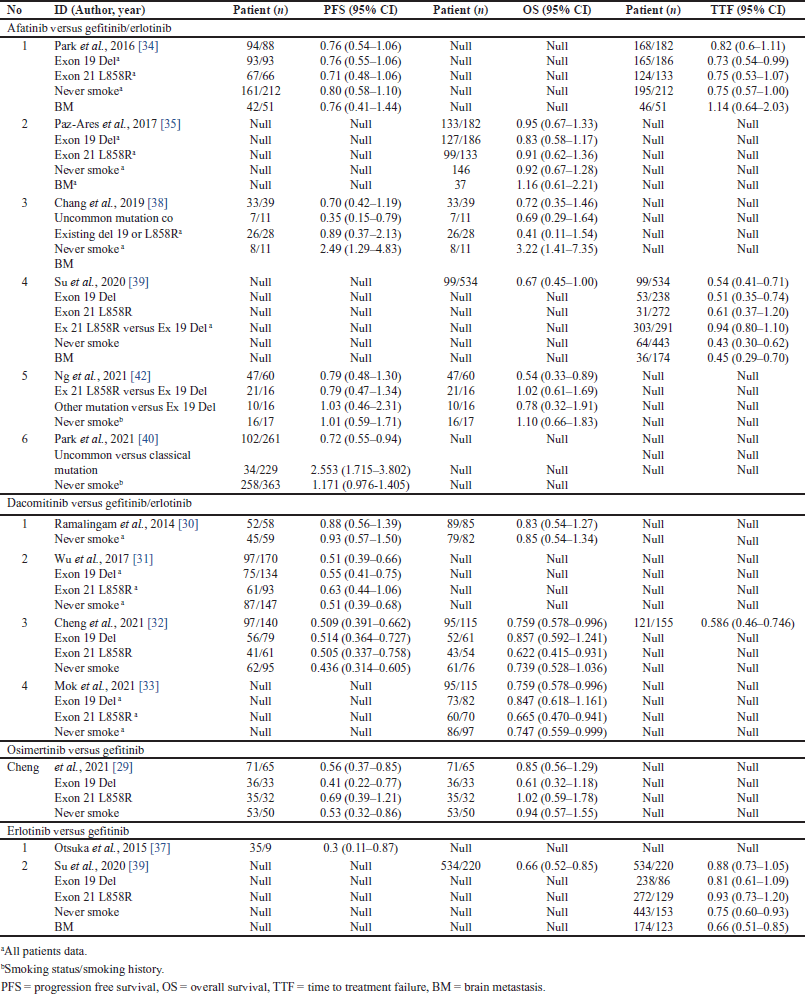 | Table 3. The comparative effectiveness of EGFR TKI included in the meta-analysis. [Click here to view] |
The LUX lung 7 trial was the only RCT comparing afatinib with first-generation TKI. It showed that the median PFS of afatinib and gefitinib was 11.0 months with an HR of 0.76. The median TTF of afatinib and gefitinib were 13.2 and 11.4 months with HR 0.82. The OS comparing afatinib and gefitinib was 0.95 [34,35]. Afatinib was significantly associated with longer PFS and showed consistent efficacy across all EGFR mutation types, although a recent multivariate analysis showed that a specific TKI regimen was not associated with better PFS in a specific EGFR mutation type [40].
Four studies in Indonesia showed efficacy between first- and second-generation EGFR TKIs. Their different results prompted further investigation. There was no difference in efficacy between gefitinib and erlotinib [50] and both have similar efficacy [53]. Afatinib tends to be associated with longer PFS [53]. Compared to gefitinib, afatinib had a significantly longer median PFS but did not have a significantly longer OS [59]. In contrast, Fitri et al. [55], revealed that gefitinib had a longer median PFS than erlotinib and afatinib.
In the same way, a comparison of the irreversible second-generation TKIs, dacomitinib versus gefitinib, showed that dacomitinib’s OS advantage persisted with longer follow-up and dose reduction (HR = 0.759, p = 0.0457). A significant improvement in OS was observed in patients with the L858R (HR = 0.622, p = 0.0203). In patients with e9del, no significance was reached (p = 0.3021), although longer OS was observed (HR = 0.857) [32,33]. Dacomitinib treatment significantly improved PFS over gefitinib, 0.43 times higher (95% CI: 0.32–0.59) [31]. Dacomitinib was not superior to erlotinib in unselected advanced NSCLC (HR 0.941, p = 0.229) or KRAS wild-type tumors (HR 1.022, p = 0.587) [30].
First-generation versus third-generation EGFR TKIs
As with the reversible second-generation TKI, osimertinib showed a clinically meaningful PFS benefit compared to gefitinib, HR 0.56 (95% CI: 0.37–0.85). Subgroup analyses revealed significant PFS in e19del (HR 0.41 95% CI: 0.22–0.77) and not significant in L858R (HR 0.69 95% CI: 0.39–1.21). However, OS was not significant in common aberrations, HR 0.61 95% CI: 0.32–1.18 and HR 1.02 95% CI: 0.59–1.78, respectively [29].
The HR data representing the comparative effectiveness of first-, second-, and third-generation EGFR TKIs are presented in Table 3.
Meta-analysis of first-generation versus second-generation EGFR TKIs
The forest plot and reference table of CER between afatinib and gefitinib/erlotinib are presented in Figure 2 which favors afatinib in PFS, OS, and TTF. Uncertainty was found due to the upper bound of the pooled HR 0.66 (0.44–1.00) for afatinib in TTF as data from two studies, while the evidence for PFS and OS were more robust when compared to TTF. Treatment with afatinib was associated with a 26% lower risk of progression and a 27% lower risk of death, with HR 0.74 (0.61–0.88) and 0.73 (0.57–0.94), respectively.
Moreover, the CER of dacomitinib, with no data in TTF is shown in Figure 3 which favors dacomitinib in PFS and OS. There was no variation in effect estimates beyond change found in OS due to significant association (p = 0.004) and nonsubstantial heterogeneity (p = 0.93; I2 0%).
Meta-analysis results of clinical outcomes comparing afatinib and dacomitinib toward gefitinib/erlotinib are presented in Table 4. It showed that the pooled HR of the second generation of EGFR TKIs versus gefitinib/erlotinib in PFS and OS had a significant overall effect (p < 0.05). A substantial heterogeneity (I2>50%) was observed for TTF in afatinib versus gefitinib/erlotinib and PFS in dacomitinib versus gefitinib/erlotinib. For all survival outcomes, the comparative effectiveness results favor the second generation.
We used a random effects model for subgroup analysis of EGFR mutation type to see if the comparative effect of comparison would vary and show similarity in characteristics, including smoking, BM, and ethnicity to explore the source of substantial heterogeneity. The results of the subgroup analysis are presented in Table 5.
Although there was no significant overall effect (p = 0.42) in the efficacy comparison results between afatinib and the first-generation EGFR TKIs in terms of OS. Interestingly, our results showed that patients with BM had significant heterogeneity (p = 0.06; I2 73%). Therefore, there is a significant overall effect in terms of PFS (p = 0.02) and TTF (p < 0.00001) with also significant heterogeneity in patients with BM (p = 0.010; I2 85%) and (p = 0.01; I2 84%), respectively. Potential sample overlap in the ARCHER 1050 study [31–33] may have contributed to the nonsubstantial heterogeneity in the subgroup analysis of the overall comparison of dacomitinib versus gefitinib/erlotinib on PFS and OS, (p = 0.97; I2 0%) and (p = 0.51; I2 0%), respectively.
As detailed in Table 5, patients with BM had a 37% higher risk of progression and an 87% higher risk of death when treated with afatinib compared to gefitinib/erlotinib. However, remarkably, there was a 30% lower risk of TTF with afatinib compared to gefitinib/erlotinib.
As a result, as shown in Table 5, the meta-analysis revealed that in never-smoking NSCLC EGFRm+ patients, there was significant heterogeneity in the comparative efficacy results between afatinib and the first-generation EGFR TKIs only for TTF (p = 0.02; I2 83%). In addition, there was heterogeneity only in PFS (p = 0.04; I2 69%) in the comparative efficacy results between dacomitinib and the first-generation EGFR TKIs. These patients treated with afatinib had a 0.42-fold lower risk of TTF and a 0.08-fold longer OS, but an equal risk of PFS. When treated with dacomitinib, they had a 0.4-fold lower risk of progression and a 0.3-fold lower risk of death compared to gefitinib/erlotinib.
In addition, in Table 5, the meta-analysis showed that patients with exon 19 deletion or exon 21 substitution aberrations benefited more from the second generation of EGFR TKIs than gefitinib/erlotinib. There was significant heterogeneity (I2 53%) only in the efficacy comparison results between afatinib and the first-generation EGFR TKIs for TTF in patients with e19del. Afatinib had a 0.3-fold lower risk of treatment failure compared to gefitinib/erlotinib (HR 0.62; 95% CI: 0.44–0.88; p = 0.008) (HR 0.7; 95% CI: 0.53–0.93; p = 0.01) in patients with e19del and L858R, respectively. Dacomitinib had a half lower risk of progression compared to gefitinib/erlotinib in both patients with e19del and L858R. Dacomitinib had a lower risk of death in both e19del and L858R patients, with a 0.15-fold lower risk and a 0.35-fold lower risk, respectively, compared to gefitinib/erlotinib. As tabulated in Table 3, they had a 0.3-fold lower risk of progression when treated with afatinib compared with gefitinib/erlotinib, (HR 0.76; 95% CI: 0.55–1.06) (HR 0.71; 95% CI: 0.48–1.06) in e19del and L858R patients, respectively [34]. In addition, OS was 0.3-fold longer in patients with an uncommon mutation with coexisting e19del or L858R [38]. It should be noted that afatinib treatment in uncommon mutation versus common mutation patients had a 2.5-fold higher risk of progression [40].
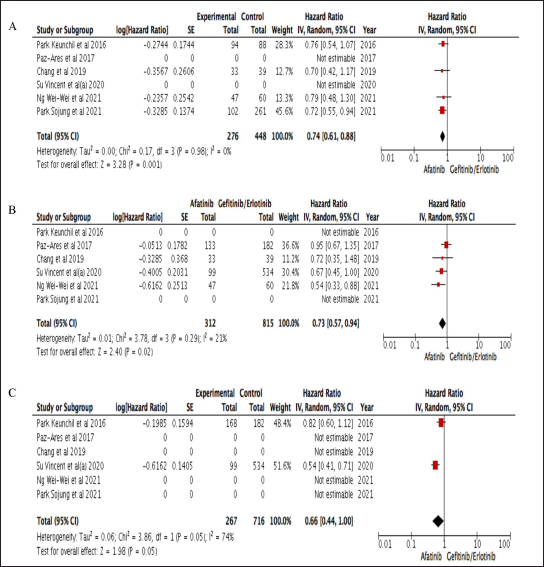 | Figure 2. Table and Forest plot of comparative effectiveness results of afatinib and gefitinib/erlotinib survival outcome within studies conducted in Asia. A. PFS; B. OS; C. TTF. [Click here to view] |
Furthermore, there was no significant overall effect (p = 0.25) between the Korean and Taiwanese populations comparing afatinib and the first-generation EGFR TKIs, pooled HR 0.84 (95% CI: 0.62–1.13) as presented in Table 5. Interestingly, our results revealed significant statistical heterogeneity in overall effect (p = 0.003; I2 72%) and subgroup differences (p = 0.002; I2 83.5%). Although afatinib had significantly a 0.4-fold lower risk of death in Korean and Taiwanese (HR 0.62; 95% CI: 0.45–0.84; p = 0.002), there was no significant heterogeneity (I2 0%, p = 0.5).
DISCUSSION
In this systematic review, we present all previously published EGFR TKI studies in lung oncology. We identified a large qualified number of published CER studies. However, one-third of these studies varied in their overall quality score measured with the GRACE checklist, but there was no statistically significant difference in mean GRACE score between multicenter and single-center studies. Therefore, the center design did not significantly affect the quality of this CER study.
Only one-sixth of the identified results could be further analyzed as an effectivity comparison of EGFR TKIs. Since there is a clear difference in EGFR mutation frequencies between Asian and non-Asian NSCLC patients [16–20], we compared the efficacy of EGFR TKI as a first-line therapy in Asian populations with common and uncommon mutations.
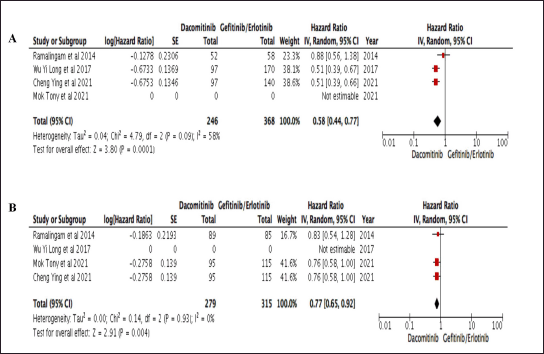 | Figure 3. Table and Forest plot of comparative effectiveness results of dacomitinib and gefitinib/erlotinib survival outcome within studies conducted in Asia. A. PFS; B. OS. [Click here to view] |
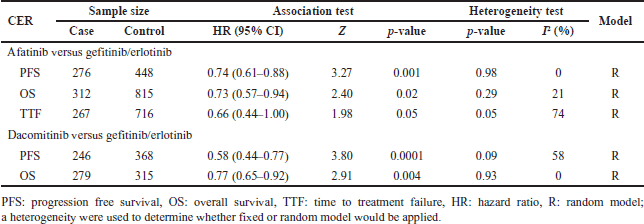 | Table 4. Meta-analysis result of effectiveness comparison of afatinib and dacomitinib for gefitinib/erlotinib. [Click here to view] |
The Chinese and Korean ethnic groups were the most involved in this result, and gefitinib was the most compared to other EGFR TKIs.
A narrative review found that Japanese patients are strongly affected by second-generation EGFR-TKIs, and more Asian studies are needed to confirm whether osimertinib is best used in first- or second-line treatment [10]. The Chinese Thoracic Oncology Group (CTONG) 0901 study revealed that patients with e19del had better outcomes than those with L858R. Equally important in terms of safety, a meta-analysis revealed that the overall incidence of adverse events with afatinib was similar to erlotinib, but higher than with gefitinib. Afatinib caused more diarrhea than gefitinib or erlotinib. Afatinib compared to gefitinib caused more rash but less liver dysfunction, although afatinib compared to erlotinib caused more stomatitis but less rash [60].
The activity of EGFR TKIs varies by EGFR type of mutation. Uncommon EGFR mutations are heterogeneous and represent a distinct subset of classic mutations with variable responses to EGFR TKIs. Uncommon EGFR mutations such as G719X, L861Q, and S768I are highly heterogeneous in both composition and sensitivity. Mixed mutations are common in patients with EGFRm+ NSCLC. In a real-world setting, EGFR TKI was the preferred treatment option in patients with EGFR aberrations. The use of second- and first-generation TKIs in patients with EGFRm+ should be used with caution based on mutation identification results. An appropriate sequencing of EGFR TKI will maximize clinical outcome benefit [61]. The risk for progression was 2.55 times higher for patients with uncommon mutations on afatinib treatment compared with the first generation of TKIs [40]. Treatment with afatinib compared to pooled first-generation EGFR TKIs in uncommon mutation with co-existing e19del or L858R had a 0.65-fold lower risk of progression than without co-existing mutations and the survival had a 0.3-fold lower risk [38]. Again, the survival of the e19del mutation group was 0.2-fold lower than the other EGFR mutations [42]. A narrative review revealed a similar result, showing a favorable outcome of afatinib compared to first-generation TKIs. While afatinib was active against L861Q, S768I, or G719X, there were no significant differences between gefitinib and erlotinib in OS, but patients had a much longer median PFS. These patients had a median PFS similar to those with L858R, but shorter than those with e19del [62]. A recent systematic review identified a need for improvement in the detection and reporting of EGFR mutations due to the heterogeneity of uncommon mutations and differential sensitivity to afatinib [63].
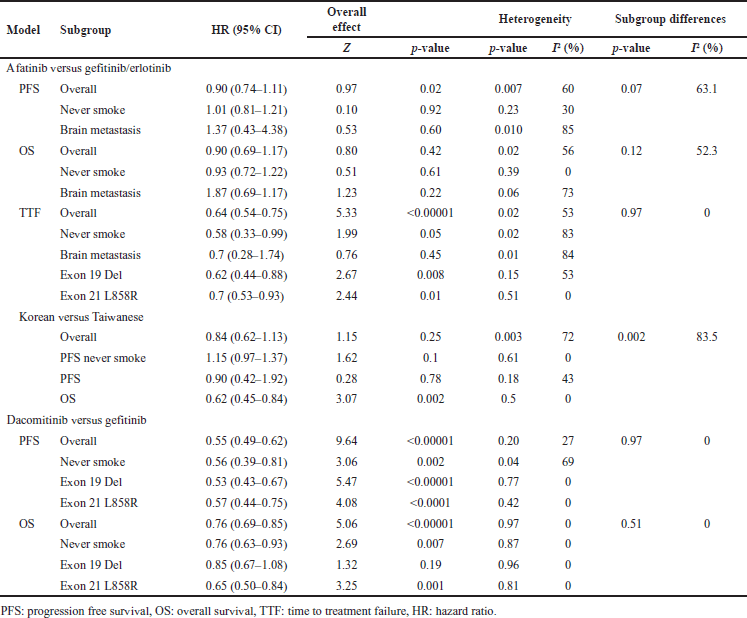 | Table 5. Subgroup analysis of effectiveness comparison (using random model effect). [Click here to view] |
Furthermore, this meta-analysis showed that L858R has a higher risk of short survival, PFS, and OS than e19del when treated with second generation compared with gefitinib/erlotinib. A recent systematic review found that e19del patients have a better OS than L858R patients due to the higher incidence of EGFR T790M in NSCLC after progression on first- or second-generation EGFR TKIs [64]. In addition, afatinib had a 0.04–0.08-fold higher risk of treatment failure, and dacomitinib had a 0.05–0.2-fold lower risk of survival (progression and death) than first generation. This is similar to the Taiwanese finding that L858R patients had a 0.2-fold longer time to progression (HR 0.79; 95% CI: 0.47–1.34) but similar OS (HR 1.02; 95% CI: 0.61–1.69) compared to e19del when treated with afatinib than with gefitinib or erlotinib [42]. However, erlotinib had a 0.1-fold difference lower risk of TTF compared with gefitinib in patients with e19del or L858R, (HR 0.51; 95% CI: 0.35–0.74) (HR 0.61; 95% CI: 0.37–1.20), respectively [39]. A review of Chinese patients with EGFRm+ NSCLC revealed that afatinib was effective and well-tolerated as a first-line treatment, suggesting osimertinib as a second-line option [61]. Narrative reviews in the Asian population suggested that efficient molecular diagnostic services were paramount to consider sequential therapy of osimertinib because it found that e19del mutation had positive testing for the T790M [40,62].
In never-smoking EGFRm+ NSCLC patients, second-generation EGFR TKIs conferred longer survival compared with first-generation EGFR TKIs. Conversely, in patients with BM, our results showed that first-generation EGFR TKIs conferred a lower risk of disease progression and death but a higher risk of TTF, regardless of EGFR mutation type. Concurrent EGFR TKI therapy and WBRT effectively controlled intracranial lesions.
In comparison to other published Asian reviews [10,61,62], this review conducted a meta-analysis that assessed the comparative efficacy outcome of EGFR TKI generation as a first-line therapy with EGFR aberrant among the Asian population. The two narrative reviews published in 2021 [10,62] collated data and did not provide qualitative or quantitative analysis. Notably, the clinical outcomes of PFS, OS, and as well as TTF were reported. A review published in 2020 was limited to the Chinese population [61]. This current systematic review has several strengths. First, it summarized the direct evidence concerning the comparative effectiveness results of EGFR TKIs from the majority of studies conducted with an RCT method design, that had appraised the data quantitatively. Second, it is a systematic search result of several databases and a comprehensive dataset, thus allowing us to obtain precise estimates and conduct meta-analysis. Third, it showed statistically there was heterogeneity between studies in comparing afatinib and first generation of EGFR TKIs, with p-values of 0.007, 0.02, and 0.02 on overall PFS, OS, and TTF, respectively.
The main limitation of this study is the unavailability of complete clinical outcomes according to the study criteria to compare efficacy between second- and first-generation agents. This prevents us from drawing a firm survival conclusion on the comparative efficacy of osimertinib, among first-generation, and common-uncommon comparison. Another limitation of the present work is the lack of data on adverse events, mainly because most of the included studies did not report them separately. Our findings due to the limited number of included studies comparing the efficacy of EGFR TKIs in Asian ethnicities should be clarified by other studies that include the number of studies and more diverse ethnic results.
CONCLUSION
In summary, we conclude that patients with common mutations survive longer on EGFR TKIs than those with uncommon. From the forest plot and survival risk meta-analysis, second-generation TKIs offer more favorable survival outcomes than first-generation agents, particularly in patients with e19del or L858R mutations.
L858R has a higher risk of survival, progression-free survival, and overall survival than e19del on second-generation agents. Afatinib treatment in uncommon mutations with coexisting e19del or L858R was associated with longer progression than without coexistence. There should be a comparison between ethnic groups in the Asian population with regard to EGFR TKIs CER.
AUTHOR CONTRIBUTIONS
EK conceived CER as a systematic review. MW performed the study search and got data extraction with reference tracking then approved by EK. MW calculated the data for the meta-analysis, furthermore, the results were checked by RD and confirmed by DE. MW prepared this manuscript. EK, SH, and DE revised the manuscript critically for important intellectual content and gave final approval for the version to be published. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
ACKNOWLEDGMENTS
The authors are thankful to the reviewers for their thoughtful comments on improving this review.
FINANCIAL SUPPORT
The authors thank the Indonesian Endowment Fund for Education (LPDP) for funding the publication.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
LIST OF ABBREVIATIONS
BM, brain metastases; CI, confidence interval; EGFR TKIs, epidermal growth factor receptor tyrosine kinase inhibitors; EGFRm+, epidermal growth factor receptor mutation-positive; GRACE, good research for comparative effectiveness; HR, hazard ratio; LC, lung cancer; NCCN, national comprehensive cancer network; NSCLC, nonsmall cell lung cancer; OS, overall survival; PICO, population intervention comparison outcome; PFS, progression-free survival; SD, standard deviation; TTF, time to treatment failure.
REFERENCES
1. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer [Internet]. 2021 Aug 15;149(4):778–89. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ijc.33588
2. Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol [Internet]. 2005 May 10;23(14):3243–56. Available from: http://ascopubs.org/doi/10.1200/JCO.2005.18.853
3. Tam K, Daly M, Kelly K. Treatment of locally advanced non-small cell lung cancer. Hematol Oncol Clin North Am [Internet]. 2017 Feb;31(1):45–57. CrossRef
4. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol [Internet]. 2015 Apr;16(4):e165–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204514711805
5. Sato M, Shames DS, Girard L, Gazdar AF, Minna JD. Molecular basis of lung cancer. In: Mendelsohn J, Howley PM, Thompson CB, editors. The molecular basis of cancer [Internet]. Amsterdam, Netherlands: Elsevier; 2008. pp. 397–407. CrossRef
6. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. CrossRef
7. Ng IW, Tey JCS, Chia DWT, Yee CM, Cheo TST. A review of whole brain radiotherapy outcomes in a high epidermal growth factor receptor mutation rate population: does QUARTZ apply in Asia? Asia Pac J Clin Oncol [Internet]. 2019 Dec;15(6):353–7. CrossRef
8. Cooper WA, Lam DCL, Toole SAO, Minna JD. Review Article Molecular biology of lung cancer. J Thorac Dis. 2013;5 (S5):S479–90. CrossRef
9. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res [Internet]. 2015;5(9):2892–911. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4633915/
10. Kim ES, Melosky B, Park K, Yamamoto N, Yang JCH. EGFR tyrosine kinase inhibitors for EGFR mutation-positive non-small-cell lung cancer: outcomes in Asian populations. Futur Oncol [Internet]. 2021 Jun;17(18):2395–408. Available from: https://www.futuremedicine.com/doi/10.2217/fon-2021-0195
11. Hsu R, Herrmann A, Gaur K, Xia B, Nieva JJ. Evaluating real world mutational differences between Hispanics and Asians in NSCLC at a large academic institution in Los Angeles. Clin Lung Cancer [Internet]. 2022 Nov;23(7):e443–52. CrossRef
12. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer NCCN evidence blocks [Internet]. 2023 [cited 2023 Jun 8]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf
13. Amelia T, Kartasasmita RE, Ohwada T, Tjahjono DH. Structural insight and development of EGFR tyrosine kinase inhibitors. Molecules [Internet]. 2022 Jan 26;27(3):819. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128094006000032
14. Peng LX, Jie GL, Li AN, Liu SY, Sun H, Zheng MM, et al. MET amplification identified by next-generation sequencing and its clinical relevance for MET inhibitors. Exp Hematol Oncol [Internet]. 2021 Dec 10;10(1):52. Available from: https://ehoonline.biomedcentral.com/articles/10.1186/s40164-021-00245-y
15. Chen R, Manochakian R, James L, Azzouqa AG, Shi H, Zhang Y, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. 2020;13(1):1–23. CrossRef
16. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget [Internet]. 2016 Nov 29;7(48):78985–93. Available from: https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.12587
17. Graham RP, Treece AL, Lindeman NI, Vasalos P, Shan, M, Jennings LJ, et al. Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med [Internet]. 2018 Feb 1;142(2):163–7. CrossRef
18. Winfree KB, Molife C, Peterson PM, Chen Y, Visseren-Grul CM, Leusch MS, et al. Real-world characteristics and outcomes of advanced non-small-cell lung cancer patients with EGFR exon 19 deletions or exon 21 mutations. Futur Oncol [Internet]. 2021 Aug;17(22):2867–81. Available from: https://www.futuremedicine.com/doi/10.2217/fon-2021-0218
19. Sutiman N, Tan SW, Tan EH, Lim WT, Kanesvaran R, Ng QS, et al. EGFR mutation subtypes influence survival outcomes following first-line gefitinib therapy in advanced Asian NSCLC patients. J Thorac Oncol. 2017 Mar;12(3):529–38. CrossRef
20. Elisna ARHS, Laksmi W, Nunuk Sri M, Ana R, Noni S, Sabrina E, et al. Uncommon EGFR mutations in cytological specimens of 1,874 newly diagnosed Indonesian lung cancer patients. Lung Cancer Targets Ther [Internet]. 2018 Mar;9:25–34. Available from: https://www.dovepress.com/uncommon-egfr-mutations-in-cytological-specimens-of-1874--newly-diagno-peer-reviewed-article-LCTT
21. Karachaliou N, Fernandez-Bruno M, Bracht JWP, Rosell R. EGFR first- and second-generation TKIs—there is still place for them in EGFR-mutant NSCLC patients. Transl Cancer Res [Internet]. 2018 Jan;8(S1):S23–47. Available from: http://tcr.amegroups.com/article/view/24920/19756
22. Peters BJM, Janssen VEMT, Schramel FM, van de Garde EMW. Quantitative and qualitative assessment of real world data comparative effectiveness research of systemic therapies in lung oncology: a systematic review. Cancer Epidemiol [Internet]. 2016 Oct;44:5–15. CrossRef
23. Qi Y, Xia X, Shao L, Guo L, Dong Y, Tian J, et al. An updated network meta-analysis of EGFR-TKIs and combination therapy in the first-line treatment of advanced EGFR mutation positive non-small cell lung cancer. Front Oncol [Internet]. 2022 Aug 1;12:1–14. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2022.616546/full
24. Farris M, Larkin-Kaiser KA, Scory T, Boyne D, Wilner KD, Pastel M, et al. Network meta analysis of first-line therapy for advanced EGFR mutation positive non-small-cell lung cancer: updated overall survival. Futur Oncol [Internet]. 2020 Dec;16(36):3107–16. Available from: https://www.futuremedicine.com/doi/10.2217/fon-2020-0541
25. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med [Internet]. 2009 Jul 21;6(7):e1000100. CrossRef
26. Dreyer NA, Velentgas P, Westrich K, Dubois R. The GRACE checklist for rating the quality of observational studies of comparative effectiveness: a tale of hope and caution. J Manag Care Pharm [Internet]. 2014 Mar;20(3):301–8. Available from: http://www.jmcp.org/doi/10.18553/jmcp.2014.20.3.301
27. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials [Internet]. 1996 Feb;17(1):1–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/0197245695001344
28. Lunny C, Pieper D, Thabet P, Kanji S. Managing overlap of primary study results across systematic reviews: practical considerations for authors of overviews of reviews. BMC Med Res Methodol [Internet]. 2021 Dec 7;21(1):140. Available from: https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-021-01269-y
29. Cheng Y, He Y, Li W, Zhang HL, Zhou Q, Wang B, et al. Osimertinib versus comparator EGFR TKI as first-line treatment for EGFR-mutated advanced NSCLC: FLAURA China, a randomized study. Target Oncol [Internet]. 2021 Mar 5;16(2):165–76. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02245630/full
30. Ramalingam SS, Jänne PA, Mok T, O’Byrne K, Boyer MJ, Von Pawel J, et al. Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase 3 trial. Lancet Oncol [Internet]. 2014 Nov;15(12):1369–78. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204514704528
31. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol [Internet]. 2017 Nov;18(11):1454–66. CrossRef
32. Cheng Y, Mok TS, Zhou X, Lu S, Zhou Q, Zhou J, et al. Safety and efficacy of first-line dacomitinib in Asian patients with EGFR mutation-positive non-small cell lung cancer: results from a randomized, open-label, phase 3 trial (ARCHER 1050). Lung Cancer. 2021 Apr;154:176–85. CrossRef
33. Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Updated overall survival in a randomized study comparing dacomitinib with gefitinib as first-line treatment in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. Drugs [Internet]. 2021 Feb 17;81(2):257–66. Available from: http://link.springer.com/10.1007/s40265-020-01441-6
34. Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–89. CrossRef
35. Paz-Ares L, Tan EH, O’Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28(2):270–7. CrossRef
36. Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol [Internet]. 2013 Sep;14(10):953–61. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00962866/full
37. Otsuka T, Mori M, Yano Y, Uchida J, Nishino K, Kaji R, et al. Effectiveness of tyrosine kinase inhibitors in Japanese patients with non-small cell lung cancer harboring minor epidermal growth factor receptor mutations: results from a multicenter retrospective study (HANSHIN Oncology Group 0212). Anticancer Res [Internet]. 2015 Jul;35(7):3885–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26124334
38. Chang LC, Lim CK, Chang LY, Chen KY, Shih JY, Yu CJ. Non-small cell lung cancer harbouring non-resistant uncommon EGFR mutations: mutation patterns, effectiveness of epidermal growth factor receptor-tyrosine kinase inhibitors and prognostic factors. Eur J Cancer [Internet]. 2019 Sep;119:77–86. Available from: https://www.sciencedirect.com/science/article/pii/S0959804919304174
39. Su VYF, Yang KY, Huang TY, Hsu CC, Chen YM, Yen JC, et al. The efficacy of first-line tyrosine kinase inhibitors combined with co-medications in Asian patients with EGFR mutation non-small cell lung cancer. Sci Rep [Internet]. 2020 Dec 11;10(1):14965. Available from: https://www.nature.com/articles/s41598-020-71583-w
40. Park S, Lee SY, Kim D, Sim YS, Ryu JS, Choi J, et al. Comparison of epidermal growth factor receptor tyrosine kinase inhibitors for patients with lung adenocarcinoma harboring different epidermal growth factor receptor mutation types. BMC Cancer [Internet]. 2021 Dec 11;21(1):52. Available from: https://www.proquest.com/working-papers/comparison-epidermal-growth-factor-receptor/docview/2539348898/se-2
41. Sun JM, Won YW, Kim ST, Kim JH, Choi YL, Lee J, et al. The different efficacy of gefitinib or erlotinib according to epidermal growth factor receptor exon 19 and exon 21 mutations in Korean non-small cell lung cancer patients. J Cancer Res Clin Oncol [Internet]. 2011 Apr 16;137(4):687–94. CrossRef
42. Ng WW, Lin CC, Cheng CY, Jiang JS, Kao SJ, Yeh DY. Real-world outcomes of first- and second-generation tyrosine kinase inhibitors first-line in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer: a retrospective observational cohort study. PLoS One [Internet]. 2021 Jun 24;16(6):e0253335. CrossRef
43. Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol [Internet]. 2014 Aug 6;19(4):594–600. CrossRef
44. Nakao M, Muramatsu H, Sone K, Aoki S, Akiko H, Kagawa Y, et al. Epidermal growth factor receptor-tyrosine kinase inhibitors for non-small-cell lung cancer patients aged 80 years or older: a retrospective analysis. Mol Clin Oncol [Internet]. 2015 Mar;3(2):403–7. CrossRef
45. Yang X, Chen H, Zhang H, Duan J, An T, Zhao J, et al. [Effectiveness of tyrosine kinase inhibitors on uncommon epidermal growth factor receptor mutations in non-small cell lung cancer]. Chinese J Lung Cancer [Internet]. 2015 Aug;18(8):493–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26302346
46. Hirano R, Uchino J, Ueno M, Fujita M, Watanabe K. Low-dose epidermal growth factor receptor (EGFR)-tyrosine kinase inhibition of EGFR mutation-positive lung cancer: therapeutic benefits and associations between dosage, efficacy and body surface area. Asian Pacific J Cancer Prev [Internet]. 2016 Mar 7;17(2):785–9. Available from: http://koreascience.or.kr/journal/view.jsp?kj=POCPA9&py=2016&vnc=v17n2&sp=785
47. Lu Y, Fan Y. Combined action of EGFR tyrosine kinase inhibitors and whole-brain radiotherapy on EGFR-mutated non-small-cell lung cancer patients with brain metastasis. Onco Targets Ther [Internet]. 2016 Mar 9;9:1135. CrossRef
48. Aiko N, Shimokawa T, Miyazaki K, Misumi Y, Agemi Y, Ishii M, et al. Comparison of the efficacies of first-generation epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in patients with advanced non-small-cell lung cancer harboring EGFR mutations. BMC Cancer [Internet]. 2018 Dec 22;18(1):1012. CrossRef
49. Zhu QQ, Wang C, Chen YY, Ding ZY. Impaired liver function implied shorter progression free survival for EGFR tyrosine kinase inhibitors. Asian Pac J Cancer Prev [Internet]. 2018 Aug 24;19(8):2177–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30139222
50. Laitupa A, Wulanari L. Efficacy of gefitinib and erlotinib in non-small-cell lung carcinoma. New Armen Med J [Internet]. 2019;13(3):4–10. Available from: https://ysmu.am/website/documentation/files/838e2c50.pdf
51. Matsumoto Y, Sawa K, Fukui M, Oyanagi J, Izumi M, Ogawa K, et al. Impact of tumor microenvironment on the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with EGFR-mutant non-small cell lung cancer. Cancer Sci [Internet]. 2019 Oct 30;110(10):3244–54. CrossRef
52. Ni Y, Liu B, Ye X, Fan W, Bi J, Yang X, et al. Local thermal ablation with continuous EGFR tyrosine kinase inhibitors for EGFR-mutant non-small cell lung cancers that developed extra-central nervous system (CNS) oligoprogressive disease. Cardiovasc Intervent Radiol [Internet]. 2019 May 30;42(5):693–9. CrossRef
53. Sutandyo N, Hanafi A, Jayusman M. Comparison of effectiveness of gefitinib, erlotinib, and afatinib in advanced non-small cell lung cancer patients with EGFR mutation positive in Indonesian population]. Zhongguo Fei Ai Za Zhi [Internet]. 2019;22(9):562–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31526459
54. Chang CY, Chen CY, Chang SC, Lai YC, Wei YF. Efficacy and prognosis of first-line egfr-tyrosine kinase inhibitor treatment in older adults including poor performance status patients with egfr-mutated non-small-cell lung cancer. Cancer Manag Res. 2021;13:7187–201. CrossRef
55. Fitri N, Yusi A, Elisna S, Hesty Utami R, Tri K. Cost-effectiveness analysis of tyrosine kinase inhibitors (erlotinib vs. gefitinib vs. afatinib) in non-small-cell lung cancer. J Appl Pharm Sci [Internet]. 2021 Apr 5;11(4):88–95. Available from: https://japsonline.com/abstract.php?article_id=3340&sts=2
56. Liu J, Itchins M, Nagrial A, Cooper WA, De Silva M, Barnet M, et al. Relationship between PD-L1 expression and outcome in EGFR-mutant lung cancer patients treated with EGFR tyrosine kinase inhibitors. Lung Cancer [Internet]. 2021 May;155:28–33. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169500221001008
57. Popat S, Hsia TC, Hung JY, Jung HA, Shih JY, Park CK, et al. Tyrosine kinase inhibitor activity in patients with NSCLC harboring uncommon EGFR mutations: a retrospective international cohort study (UpSwinG). Oncologist [Internet]. 2022 Apr 5;27(4):255–65. CrossRef
58. Sari S, Andayani TM, Endarti D, Widayati K. Cost-effectiveness analysis of afatinib versus gefitinib in non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutation in Indonesia: observational studies with retrospectives. Res J Pharm Technol [Internet]. 2022 Apr 23;15(4):1598–602. Available from: https://www.rjptonline.org/AbstractView.aspx?PID=2022-15-4-33
59. Sari S, Andayani TM, Endarti D, Widayati K. Effectiveness of afatinib and gefitinib in non-small cell lung cancer (NSCLC) epidermal growth factor receptor (EGFR) mutations in Indonesia: observational studies with retrospectives. Int J Res Pharm Sci. 2019;10(4):3516–22. CrossRef
60. Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer [Internet]. 2017 Feb 19;116(5):568–74. Available from: http://www.nature.com/articles/bjc2016456
61. Tu HY, Wu YL. Afatinib for the first-line treatment of EGFR mutation-positive NSCLC in China: a review of clinical data. Future Oncol. 2020 Nov;16(31):2569–86. CrossRef
62. Lu S, Shih JY, Jang TW, Liam CK, Yu Y. Afatinib as first-line treatment in Asian patients with EGFR mutation-positive NSCLC: a narrative review of real-world evidence. Adv Ther [Internet]. 2021 May 17;38(5):2038–53. Available from: https://link.springer.com/10.1007/s12325-021-01696-9
63. Yang JCH, Schuler M, Popat S, Miura S, Park K, Passaro A, et al. Afatinib for the treatment of non-small cell lung cancer harboring uncommon EGFR mutations: an updated database of 1023 cases brief report. Front Oncol [Internet]. 2022 Apr 28;12:834704. CrossRef
64. Huang LT, Zhang SL, Han CB, Ma JT. Impact of EGFR exon 19 deletion subtypes on clinical outcomes in EGFR-TKI-treated advanced non-small-cell lung cancer. Lung Cancer [Internet]. 2022 Apr;166:9–16. CrossRef
APPENDIX
Appendix can be downloaded from the journal’s website