INTRODUCTION
Polycystic ovary syndrome (PCOS) is a prevalent endocrinopathy in women during their reproductive age. Although the specific etiology of PCOS is unknown, hormonal imbalances such as hyperandrogenemia, insulin resistance (IR), and hyperinsulinemia might play a crucial role. Insulin affects the hypothalamus-hypophysis-ovary axis components, resulting in altered metabolic signaling in ovarian tissue. Increased androgen levels may cause IR through elevating the free fatty acid levels and changing the composition and functionality of muscle tissue, resulting in an IR-hyperinsulinemia-hyperandrogenemia cycle. Increased androgen levels are also linked to dermatological issues such as female pattern baldness, acne, and hirsutism (Rojas et al., 2014). PCOS is characterized by irregular ovulation, irregular menstrual cycles, delayed pregnancy or recurrent abortions, depression, anxiety, cardiovascular disease, diabetes mellitus type II, and other health complications (Janez and Jensterle, 2017).
Not only does hormone disruption play a part in the pathophysiology of this syndrome, but so do oxidative stress and elevated inflammatory cytokines, all of which contribute to a variety of consequences, including cell damage. Improving insulin intolerance, lowering androgen levels, maintaining normal menstrual cycles, and fertility are all part of PCOS treatment. Nonpharmacological treatments, such as proper physical exercise, body weight control, and nutritious meals, are also beneficial (Patel et al., 2020). Metformin is a drug that is used to treat diabetes mellitus type II and also used to increase insulin sensitivity in obese and PCOS patients. It has been linked to better menstrual cycles and ovulation and a decrease in testosterone levels (Elnashar, 2011). Lactic acidosis, vitamin B12 deficiency, diarrhea, nausea, vomiting, constipation, abdominal pain, and heartburn are some of the most common side effects of metformin (Aaoran and Caughey, 2018).
Oral contraceptives are also indicated in the management of PCOS, although they have side effects such as acne, hirsutism, irregular menstrual periods, increased body weight, nausea, bloating, breast tenderness, and the possibility of infertility (Rosenfield, 2015). For the prevention and treatment of some ailments, most people have used complementary medicine, which includes natural and herbal medicines, since ancient times.
Brassica oleracea flowers, often known as broccoli flowers, belong to the Brassicaceae family (mustard family). The blossoms are high in vitamins. It has many health benefits as the flowers contain vitamins A, E, C, K, B6, B12, niacin, riboflavin, and thiamin, as well as folic acid and minerals such as potassium, iron, zinc, selenium, and phosphorus. The plant’s principal active ingredients are glucosinolates, which are organosulfur compounds. Fibers, S-methyl cysteine sulfoxide, and phenolic acids, in addition to flavonoids such as kaempferol, isorhamnetin, and quercetin glucosides, were detected in the plant’s extract. All these constituents are responsible for the antioxidant activity of the broccoli flowers and the protection of the body cells from damage. In addition, broccoli flowers help to lower the blood glucose level, decrease weight, inhibit cancer cell development, treat osteoarthritis, prevent heart disease, and boost the immune system (Axelsson et al., 2017).
The Persea americana fruit, also known as avocado fruit, is a tropical fruit with a pear to round shape. It belongs to the family Lauraceae, and its origin is in South-Central Mexico. Vitamins like E, C, K, riboflavin, niacin, pantothenic acid, and pyridoxine are abundant in the fruit, making it incredibly beneficial to physiological functioning. Moreover, it includes minerals like magnesium, potassium, and copper. Persea americana is high in oleic acid, fibers, phenolic acids like gallic acid, and bioflavonoids like quercetin, all of which are monosaturated fatty acids. Avocado is said to help with eye problems since it contains carotenoids. As a result, it can protect against age-related eye illnesses, diabetes, inflammation, and cardiac disorders. Hypertension, overweight, and hypercholesterolemia can all be treated using P. americana. In addition, it reduces arthritis symptoms, prevents cancer formation, and provides immune system enhancement because of its fiber content (Dreher and Davenport, 2013).
Phyllanthus emblica is an Indian fruit, and it is commonly known as gooseberry or amla. Phyllanthus emblica contains several active constituents such as phenolic acids like gallic acid, amino acids, vitamins, tannins, and flavonoids including rutin, quercetin, and apigenin. Additionally, it contains fatty acids (lauric acid). Ayurvedic medicine uses P. emblica to treat a variety of ailments, including dyslipidemia, hypertension, diabetes mellitus, inflammation, and cancer. Because of its significant antioxidant activity, P. emblica is highly recommended in folk medicine as a therapeutic option for PCOS, which works by improving fertility and managing menstrual cycles (Variya et al., 2016).
Trigonella foenum-graecum L. (fenugreek) is extensively cultivated in countries such as India, Egypt, Turkey, Ethiopia, and Morocco. It has antioxidant effects due to its flavonoid content, such as quercetin, apigenin, and rutin. It has been found to contain alkaloids (carpaine, trigonelline, and gentianine). Trigonella foenum-graecum is frequently utilized in ayurvedic medicine for the management of hypoglycemia, hypercholesterolemia, hypertension, cancer, and obesity. Moreover, it has anti-inflammatory, antibacterial, and anthelmintic effects. Trigonella foenum-graecum is highly recommended for PCOS treatment as it significantly reduces ovarian cyst size and controls menstrual cycles (Aher et al., 2016).
Olive oil is extracted from the olive fruits which are known as Olea europaea L. using different physical as well as mechanical methods. Olea europaea is recognized to be one of the Mediterranean diets which is rich in dietary polyphenols, vitamins (vitamins K and E), monounsaturated fatty acids (e.g., oleic acid), and polyunsaturated fatty acids (such as linoleic acid). Previous studies revealed the effects of O. europaea in dyslipidemia and diabetes mellitus (Memon et al., 2018).
Tribulus arabicus (family Zygophyllaceae) has been used as a traditional herb for fertility. It is adapted to grow in dry climates (deserts). It possesses significant antioxidant, antihyperuricemic, and xanthine oxidase inhibitory activities (Abu-Gharbieh et al., 2018; Ksiksi et al., 2017). The main active constituent that was previously isolated is ursolic acid, which showed xanthine oxidase inhibitory activity (Abu-Gharbieh et al., 2018).
In this study, we aimed at investigating the effectiveness of some selected herbal remedies based on their folk medicine, e.g., P. emblica, P. americana, B. oleracea, T. foenum-graecum, the O. europaea oil, and the T. arabicus herb, as well as their published active constituents as alternative treatments of ovarian cysts to standardize the extracts of the plants and to correlate their biological activities with their active constituents.
MATERIALS AND METHODS
General
Chemicals and drugs
Methanol, ethanol, benzene, and ethyl acetate were obtained from Fisher Scientific (UK) 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, flavonoid standards, fatty acid standards, sodium carboxymethylcellulose (CMC), and the Folin-Ciocalteu reagent were purchased from Merck (Darmstadt, Germany).
Letrozole (Femara®, Cairo, Egypt) and metformin (Glucophage®, Dubai, UAE) were obtained from private retail pharmacies. Biochemical kits for triglycerides (TGs), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and glucose were obtained from DiaSys Diagnostic Systems GmbH, Holzheim, Germany (colorimetric assay kits). Progesterone and testosterone kits were purchased from ichromαTM and measured using the ichroma-II Immunoassay Analyzer, Boditech Med Inc. (Gan-won-do, Korea). Malondialdehyde (MDA), superoxide dismutase (SOD), and tumor necrosis factor-alpha (TNF-α) kits were ordered from Novus Biologicals LLC (CO, USA), Abnova (Taipei, Taiwan), and R&D Systems (MN, USA), respectively.
Plant material
The P. emblica fruits, T. foenum-graecum seeds, B. oleracea flowers, and P. americana fruits were obtained from local markets in Dubai, UAE, while the O. europaea oil (olive oil) was brought from Jordan. The T. arabicus plant was collected from the Muhaisnah desert, Dubai, UAE, in October 2019. The plants’ identities were confirmed by the staff members of the Department of Biology, Faculty of Science, UAE. Voucher specimens were kept at the Herbarium of the Pharmaceutical Chemistry Department, Dubai Pharmacy College for Girls, UAE (#12-10-19).
Phytochemical investigation
Extraction
The seeds of T. foenum-graecum were powdered, and the fresh plants of P. emblica, B. oleracea, P. americana, and T. arabicus were cut into small pieces.
The plants were exhaustively extracted by cold maceration in 50% alcohol (three times × 4 l). The alcoholic extract in each case was evaporated separately under reduced pressure at 50°C using a rotary evaporator. The remaining water was lyophilized using a lyophilizer (BIOBASE). The dried extracts were saved and used for both phytochemical investigation and biological evaluation. The weights of the different plants and the extractive yields are recorded in Table 1.
Standardization of the plant extracts
Measurement of the phenolic contents
A colorimetric assay was used to determine the amount of the phenolic and the flavonoid constituents using a spectrophotometer. All measurements were conducted in triplicate.
The Folin-Ciocalteu reagent was used to measure the amount of phenolic content (Shehab et al., 2015) (results were calculated as mg/g gallic acid dry weight of plant). Gallic acid was used to create the calibration curve. 9 ml of water and 1 ml of the Folin-Ciocalteu reagent were added to 1 ml of each sample and standard. Later, 10 ml of 7% sodium carbonate was added. All samples were kept for 90 minutes at room temperature, and then the absorbances were measured at 750 nm.
The AlCl3 method, described by Dewanto et al. (2002), was used to measure total flavonoid content. Quercetin, a flavonoid aglycone, was used to create the calibration curve. 0.1 ml from each extract was added to 0.3 ml of distilled water and 0.03 ml of sodium nitrite (5%). After 5 minutes, 0.03 ml of aluminum chloride (10%) was added; after another 5 minutes, 0.2 ml of 1 mM sodium hydroxide and 1 ml of distilled water were added to the tested samples. The absorbances of the yellow color produced were measured at 510 nm. The total flavonoid and phenolic percentage values are recorded in Table 2.
High-performance liquid chromatography (HPLC) analysis of the phenolic and flavonoid constituents
An Agilent 1100 HPLC apparatus, with a quaternary pump and a UV detector (280 nm), was used for the phenolic acid constituents. Both an Alltima C18 Column (particle size 5 mm, 150 × 4.6 mm) and an Alltima C18 Guard Column (5 mm) were used (Alltech, USA), while a Hypersil ODS C18 Column (particle size 5 μm, 4.6 × 250 mm) with a UV detector (325 nm) was used for the flavonoid constituents. Two solvents, A (methanol) and B [acetic acid in water (1:25 v/v)], were applied to the column to separate the phenolic acid constituents.
A methanol/water (50:50 v/v, pH 2.8) mixture was used (isocratic flow rate of 1.0 ml/minute) for the separation of the flavonoids. The HPLC analysis of the flavonoids and phenolic constituents is recorded in Tables 3 and 4 and Figures 1 and 2, respectively (Kuntic et al., 2007; Lin et al., 1996).
HPLC analysis of water-soluble vitamins
The Agilent 1100 HPLC (UV 220 nm) system was used for the identification of the vitamins. The Titan C18 Column (5 cm × 2.1 mm D) and a mixture of 20 mM potassium phosphate (pH 3.0) (A) and methanol (B) were used for the separation. Gradient elution was carried out according to Rokayya et al. (2014). The results of the water-soluble vitamins are recorded in Table 5 and Figure 3.
TLC analysis
All the extracts were analyzed for their triterpenoid contents using TLC and different solvent systems (benzene: ethyl acetate 86:14 v/v; chloroform: methanol 9:1 v/v). The spots were visualized under UV lamps 365 (with or without ammonia vapor). The chromatograms were sprayed with p-anisaldehyde (spray reagent) and then heated at 110°C.
Antioxidant activity
DPPH radical scavenging assay
In vitro, the antioxidant activities of the extracts and O. europaea oil were evaluated through hydrogen donating (radical scavenging ability) using the stable DPPH radical. The procedure was carried out according to Cheng et al. (2006). Moreover, the samples were shaken after the addition of the reagent and kept in the dark at 37°C for 30 minutes. The absorbance was measured at 517 nm using a UV microplate reader. The inhibition percentage of the DPPH radical (antioxidant activity) was calculated using the following equation:
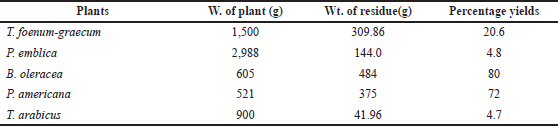 | Table 1. The weight of the plants and the extractive yield. [Click here to view] |
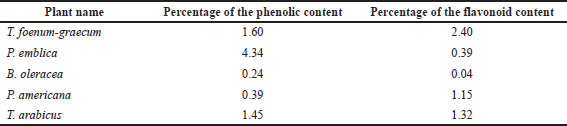 | Table 2. Percentages of the flavonoid and phenolic contents in different plant extracts. [Click here to view] |
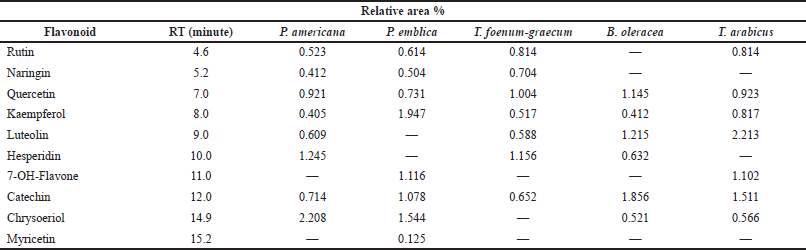 | Table 3. HPLC analysis of flavonoid content of the different plant extracts at wavelength 325 nm. [Click here to view] |
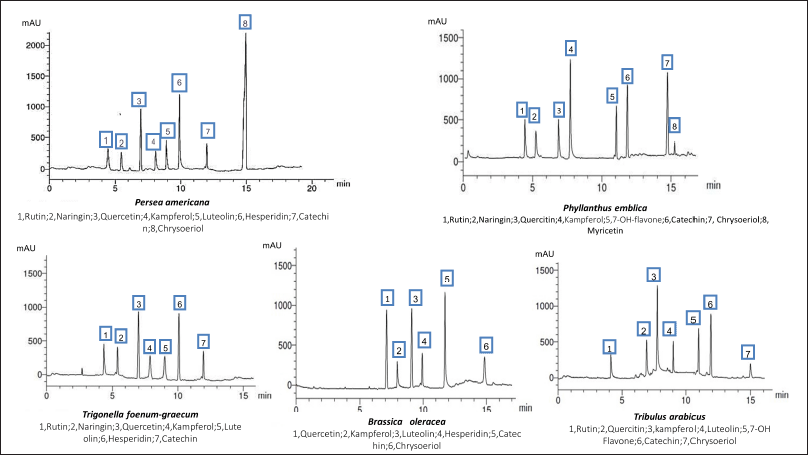 | Figure 1. HPLC analysis of the flavonoid constituents of the different plant extracts at wavelength 325 nm. [Click here to view] |
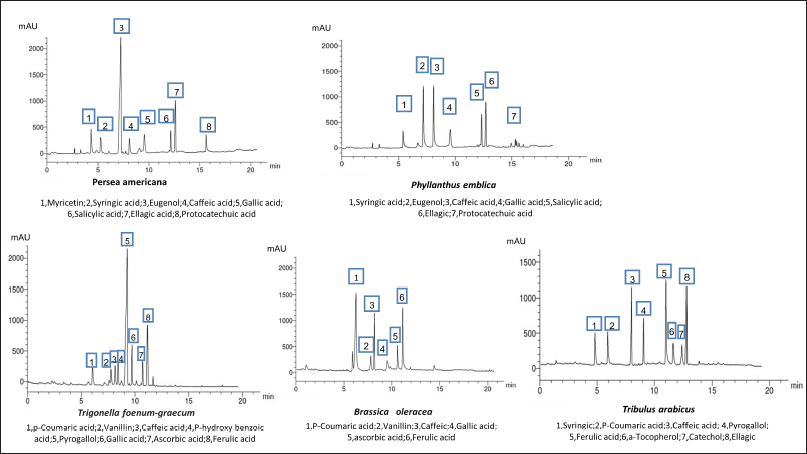 | Figure 2. HPLC analysis of the phenolic constituents of different plant extracts at wavelength 280 nm. [Click here to view] |
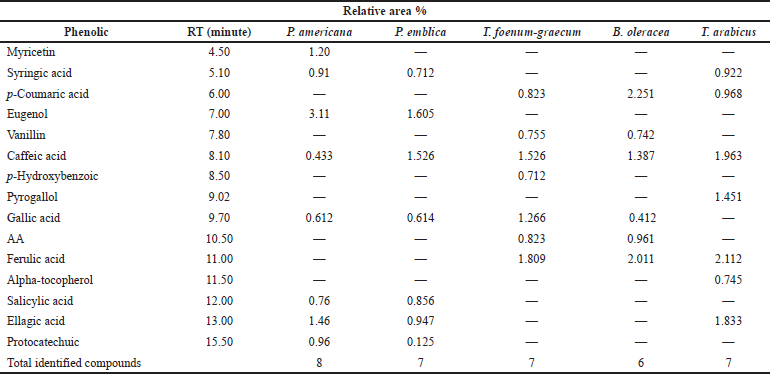 | Table 4. HPLC analysis of the phenolic constituents of the different plant extracts at wavelength 280 nm. [Click here to view] |
% inhibition = [A0 − (A1 − A2)]/A0 × 100%
where, A0 is the absorbance of the control, A1 is the absorbance of the sample + DPPH, and A2 is the absorbance of the sample without DPPH.
Ascorbic acid (AA) was used as a standard. The samples were analyzed in triplicate (Fig. 4).
 | Table 5. HPLC analysis of the vitamins of the different plant extracts at 220 nm. [Click here to view] |
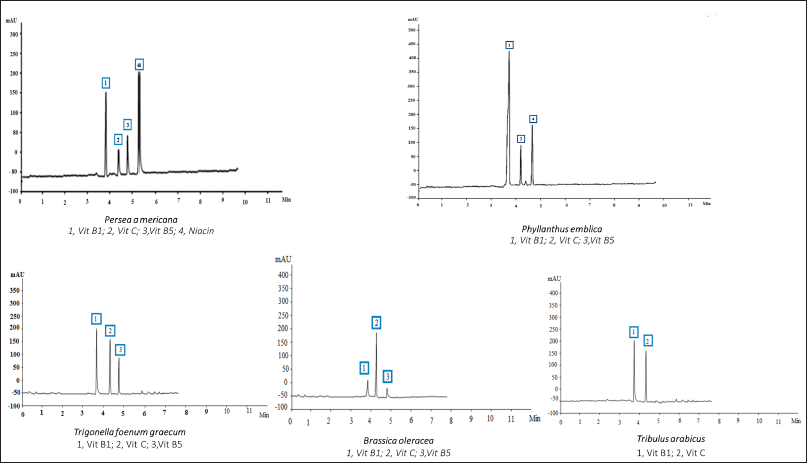 | Figure 3. HPLC analysis of the vitamins of the different plant extracts at 220 nm. [Click here to view] |
Biological study
Animals
Fifty-four mature female Wistar rats weighing 170–190 g, with 4–5 days of regular estrus cycles, were selected for this study and maintained in a controlled environment at a temperature of 25°C and a 12 hours light/dark cycle with free access to food and water. All the animal investigations were performed according to the ethical standards and upon approval of the Research and Ethical Committee of Dubai Pharmacy College for Girls, Dubai, UAE (REC-FD-2020-05).
Experimental design
All the animals except the control group were given letrozole (Femara®, Cairo, Egypt) orally at a dose of 1 mg/kg dissolved in 1% of CMC for 35 days to induce PCOS. This dose was selected according to previous studies on inducing cystic follicle formation (Karateke et al., 2018).
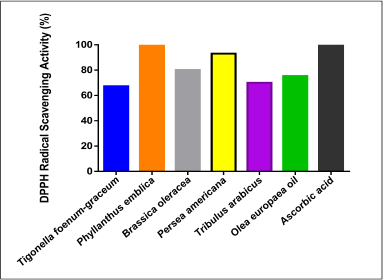 | Figure 4. DPPH radical scavenging inhibition activity of the plant extracts and AA. [Click here to view] |
During the study, the estrus cycle was evaluated microscopically by taking a vaginal smear and analyzing the relative proportion of epithelial cells, cornified cells, and leukocytes to confirm the PCOS model by the existence of the irregular estrus cycle (Fig. 5) (Zhou et al., 2019). After that, the animals were divided into nine groups of six rats per group: Group 1: control group received CMC only; Group 2: negative control group were administered letrozole only; Group 3: positive control group received letrozole and then metformin (70 mg/kg); and Groups 4–9: each group received letrozole and one of these plants’ extracts (500 mg/kg): T. arabicus, P. americana, P. emblica, T. foenum-graecum, B. oleracea, and O. europaea oil, respectively.
Termination of the procedure
On the 56th day, after 24 hours of receiving the last dose of the treatment, the rats were fasted overnight, and blood samples were collected from the retro-orbital plexus. Plasma was obtained by centrifugation for measurement of the biochemical markers, e.g., serum glucose, cholesterol, TGs, and LDL, in addition to progesterone and testosterone. The animals were weighed before dissection. The two ovaries were separated from each rat, detached from any surrounding tissue or fat, and weighed for the measurement of morphological changes in the ovaries of rats. The two ovaries were weighed, and the average ovary weight to the body weight for each animal in the group was calculated (Kafali et al., 2003; Mihanfar et al., 2021). The left ovaries were kept in 10% formalin for the histopathological measurements, and the right ones were used for the preparation of tissue homogenates using Wised instrument model HG-15D for the oxidative stress determinations, e.g., MDA, SOD, and TNF-α.
Measurement of circulating levels of progesterone and testosterone
Progesterone and testosterone levels were analyzed by fluorescence immunoassay using the ichroma™ II instrument for the quantitative determination of sex hormones in human plasma. Plasma (75 μl) was mixed with 30 μl of the displacing reagent; the mixture was shaken several times and then incubated at room temperature for 3 minutes. An aliquot of 75 μl of the prepared sample mixture was added to the sample well on the cartridge and was incubated for 15 minutes at room temperature, then scanned, and recorded in Table 6.
Measurement of circulating levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH)
Samples (50 μl) were added to the wells, and 50 μl of an horseradish peroxidase (HRP) conjugate was added to all wells except the blank. The plate was mixed and incubated for 1 hour at 37°C after the addition of a 50 μl antibody to all the wells. The washing step was performed three times. 50 μl of substrate A and 50 μl of substrate B were also introduced and mixed into all wells. The plate was incubated in a dark place for 15 minutes at 37°C, and then 50 μl of a stop solution was used to stop the reaction, and the optical density was determined at 450 nm within 10 minutes. The results are recorded in Figure 7.
Measurement of blood lipids and glucose levels
The levels of cholesterol, LDL, TGs, and serum glucose were determined using spectrophotometric techniques from the various blood samples.
Determination of serum cholesterol. Using commercial diagnostic kits from CHOD-PAP, the level of serum cholesterol was determined. 10 μl of the sample and 1 ml of the reagent (Good’s buffer phenol, 4-aminoantipyrine, cholesterol esterase, cholesterol oxidase, and peroxidase) were combined in the two vessels. After 10 minutes of incubation, the absorbance was measured at 546 nm against the reagent blank (Artiss et al., 1997).
Determination of serum LDL. According to Bairaktari et al. (2005), serum LDL-cholesterol was determined by a color-producing enzymatic reaction.
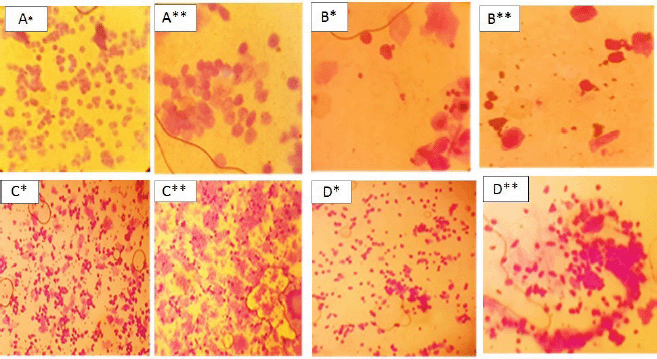 | Figure 5. Crystal violet-stained vaginal smear from normal* and PCOS group** showing proestrus (predominance of nucleated epithelial cells) A* and A**; estrus (consists of anucleated cornified cells) B* and B**; metestrus (equal proportion among leukocytes, cornified, and nucleated epithelial cells) C* and C**; and diestrus (leukocytes mainly) D*and D**. [Click here to view] |
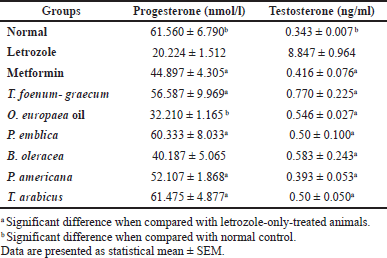 | Table 6. Effects of letrozole, metformin, and the plants’ extracts on progesterone and testosterone levels. [Click here to view] |
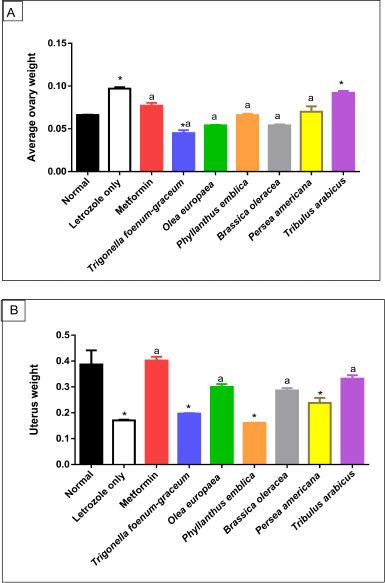 | Figure 6. Morphological changes in ovary (A) and uterus (B) weight of normal, letrozole, and different treatment groups of rats. * indicates significant difference when compared with normal control. a indicates significant difference when compared with letrozole-only-treated animals. Data are presented as statistical mean ± standard error of mean (SEM). [Click here to view] |
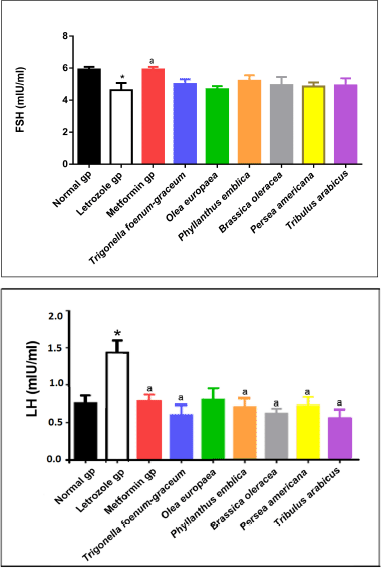 | Figure 7. LH and FSH levels for normal, letrozole, and different treatment groups of rats. * indicates significant difference when compared with normal control. a indicates significant difference when compared with letrozole-only-treated animals. [Click here to view] |
Determination of serum TGs. Glycerol-3-phosphate oxidase was used in the colorimetric enzymatic testing procedure to measure the amounts of TGs. As a blank, 10 μl of distilled water was used, and 1,000 μl of the reagent—buffer Good’s phenol, 4-aminoantipyrine, cholesterol esterase, cholesterol oxidase, and peroxidase—was added to the blank and sample solutions. For 10 minutes, the mixtures were incubated at 37°C. At 546 nm, the absorbance was measured in comparison to the reagent blank (Rifai et al., 1999).
Determination of serum glucose. Using the “GOD-PAP” enzymatic photometric test, the serum glucose level was determined. The reagent used in this assay was composed of phosphate buffer (250 mM), phenol (5 mM), 4-aminoantipyrine (0.5 mM), glucose oxidase (GOD, ≥10 kU/l), and peroxidase (≥1 kU/l). 10 μl of distilled water was used as a blank, and 1,000 μl of the reagent was added and mixed with the sample. After 20 minutes incubation at 20°C–25°C, absorbance against the blank within 60 minutes was obtained (Trinder, 1969). The results are recorded in Figure 8.
Measurement of oxidative stress markers
Measurement of MDA level. MDA level was investigated using the competitive ELISA method. A precoated microplate with MDA was used to compete with the sample or standard MDA for sites on the biotinylated detection Ab specific to MDA. After washing out the excess conjugate and unbound sample or standard, avidin conjugated to HRP was added to all the wells and incubated for 45 minutes. A TMB substrate solution was then added to all the wells, and then the stop solution (2 M H2SO4) was added to terminate the reaction. To measure the color intensity at a wavelength of 450 nm, the ROBONIK Readwell TOUCH ELISA Plate Analyzer (Ambarnath, India) was used, and MDA concentrations in the samples were measured (https://www.novusbio.com/support/support-by-application/elisa/direct-sandwich-protocol).
Measurement of SOD activity. To measure SOD activity, the colorimetric assay was employed. The WST-1 reagent was used as it produces a formazan dye upon reduction with superoxide anion. The rate of the reduction with a superoxide anion was linearly correlated with xanthine oxidase activity and was hampered by SOD. The results are recorded in Figure 9.
Measurement of TNF-α level. This was done utilizing the sandwich ELISA technique. Standards, control, and samples were added to wells precoated with monoclonal antibodies specific for rat TNF-α. TNF-α is bound to the immobilized antibody. After removal of the unbound substances by washing, the enzyme-linked polyclonal antibody specific for rat TNF-α was added and incubated for 2 hours. A substrate solution was added following the washing step producing a blue color after 30 minutes incubation. The termination step was done using the stop solution giving a yellow color whose intensity is proportional to the amount of TNF-α bound.
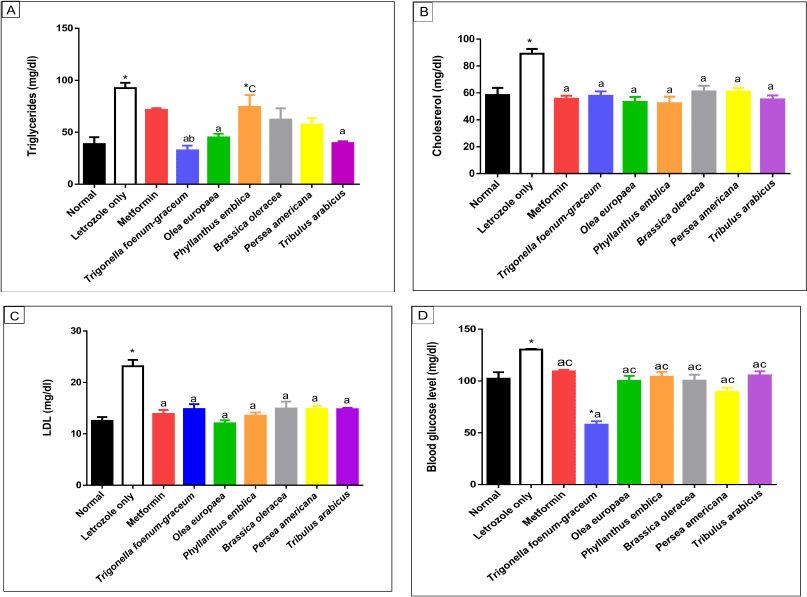 | Figure 8. Lipid profile including TGs (A), cholesterol (B), LDL (C), and blood glucose (D) levels, respectively, for normal, letrozole, and different treatment groups of rats. * indicates significant difference when compared with normal control. a indicates significant difference when compared with letrozole-only-treated animals. b indicates significant difference when compared with metformin. c indicates significant difference when compared with T. foenum-graecum. [Click here to view] |
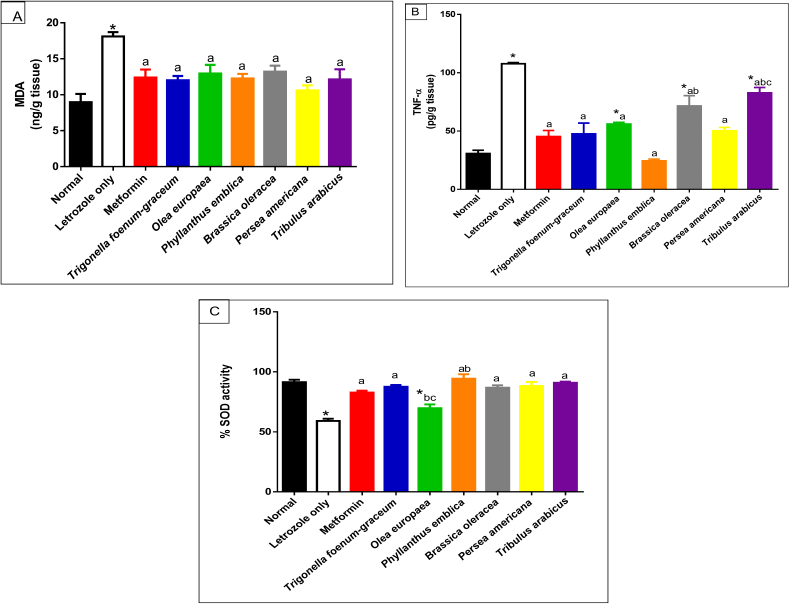 | Figure 9. Oxidative stress markers including MDA (A), TNF-α (B), and SOD (C) activity for normal, letrozole, and different treatment groups of rats.* indicates significant difference when compared with normal control. a indicates significant difference when compared with letrozole-only-treated animals. b indicates significant difference when compared with metformin. c indicates significant difference when compared with T. foenum-graecum. [Click here to view] |
Histopathological investigation
The left ovaries of the rats were excised and weighed before preparation. After cutting, the tissues were fixed in 10% formol saline for 24 hours. After that, all specimens were cleaned, gradually dehydrated using ascending grades of ethanol, and then embedded in paraffin at 56°C. Sections of 4–5 μ thickness were prepared using a microtome (Shandon Cat. No. 0525, England). On glass slides, the sections were collected, deparaffinized, and stained using hematoxylin and eosin. All slides were evaluated by a light microscope (Leica ICC50 E, MD 500, Germany). The follicles containing oocytes with nuclei were counted as healthy ones. The follicles were classified as follows: a follicle that is still growing, a preantral follicle with a single layer of cuboidal granulosa cells and an intact, expanded oocyte with a visible nucleus. Regardless of whether the cavity was visible or not, the antral follicle had two or more layers of granulosa cells. Degenerating oocytes or pyknotic granulosa cells are present in atretic follicles. The theca interna is thickened, and the granulosa cell layer of the cystic follicle is attenuated.
The numbers of developing atretic, cystic, and corpora lutea (CL) follicles in each group’s ovaries were counted. In the control group and PCOS groups, the thickness of the “tunica albuginea” capsule, hyperplasia of the theca interna, reduction in the number of CL, and subcapsular follicular cysts were examined.
Statistical analysis
Data were statistically analyzed using one-way analysis of variance followed by Tukey-Kramer’s multiple comparisons test. Statistical analyses were conducted using GraphPad Prism software (GraphPad Software Inc., V 4.03, San Diego, CA). The significance of the biochemical parameters was assumed at p < 0.05.
RESULTS
Phytochemical investigation
Extractive yields
As shown in Table 1, the B. oleracea extract yield showed the highest percentage (80%), followed by the yield of the P. americana extract (72%) among the other herbal remedies’ extracts.
Standardization of the remedies’ extracts
Measurement of the phenolic and flavonoid contents
Table 2 reveals that T. foenum-graecum seeds showed the highest flavonoid content (2.4%), followed by the T. arabicus herb (1.32%) and P. americana fruit (1.15%), while the least amount of flavonoids appeared in B. oleracea flowers (0.04%). On the other hand, the P. emblica fruit showed the highest phenolic content (4.34%), calculated as gallic acid, followed by the T. foenum-graecum seeds (1.6%) and T. arabicus herb (1.45%).
HPLC analysis of the flavonoid and phenolic acids
The HPLC analysis of the flavonoid constituents of the different plant extracts showed that quercetin aglycone, catechin, and kaempferol exist in all the plants under investigation at different concentrations. Luteolin was detected in all the plant extracts under investigation except P. emblica. Phyllanthus emblica showed the highest content of kaempferol (1.947) and 7-OH-flavone (1.116) among all the remedies. Meanwhile, P. americana showed the highest content of chrysoeriol (2.208) and hesperidin (1.245). Catechin was a predominant component in B. oleracea (1.856), while the highest content of rutin existed in T. arabicus and T. foenum-graecum (0.814). Myricetin was detected only in P. emblica (0.125). Hesperidin and quercetin were the predominant flavonoid constituents in T. foenum-graecum (1.156 and 1.004, respectively). Naringin was detected in all the plants except B. oleracea and T. arabicus.
As represented in Table 4, the HPLC analysis of the remedies’ extracts under investigation allowed the identification of various phenolic compounds. Seven components were identified in all extracts except P. americana and B. oleracea (eight and six, respectively). The percentages of the total identification were 9.445%, 6.385%, 7.714%, 7.764%, and 10.064% for P. americana, P. emblica, T. foenum-graecum, B. oleracea, and T. arabicus, respectively. The highest phenolic constituents were detected in T. arabicus, followed by P. americana, as shown in Table 4. Caffeic acid was detected in all the plant extracts under investigation, while gallic acid was detected in all the plant extracts except T. arabicus. Eugenol, a phenolic compound, was a predominant compound in P. americana, followed by ellagic acid and myricetin aglycone (3.11%, 1.46%, and 1.02%, respectively). However, AA was detected only in T. foenum-graecum seeds and B. oleracea flowers. Ferulic acid is a major phenolic acid in both T. foenum-graecum and T. arabicus. Brassica oleracea showed the highest content of p-coumaric acid (2.251%) among the other plants’ extracts.
Table 5 reveals that setting the detector at 220 nm and using another type of column allowed the detection of vitamin C and vitamin B1 in all the plant extracts at a different concentration, while niacin, a form of vitamin B, was only detected in P. americana. Furthermore, vitamin B5 was detected in all the plant extracts except T. arabicus. The highest vitamin content was explored in P. americana and P. emblica (4.446% and 3.03%, respectively). Moreover, P. emblica showed the highest amount of Vitamin B1 (1.76%), followed by P. americana (1.316%) and T. foenum-graecum (1.02%). Vitamin C was a prevalent component in B. oleracea, followed by T. arabicus (1.12% and 0.754%, respectively).
TLC screening of fatty acids and triterpenes of the different extracts
Preliminary screening of fatty acids and triterpenoidal constituents showed that O. europaea had palmitic, oleic, arachidonic, lauric, and stearic acids and alpha-amyrin. In addition, alpha-amyrin was detected in all the plants under investigation.
In vitro antioxidant activities of different plants
Figure 4 reveals that all the plants as well as the O. europaea oil showed high antioxidant activities. The highest DPPH free radical inhibition was shown in P. emblica (100%) and P. americana (93%) as compared to AA (100%).
Vaginal cytology
The types and number of cells from the vaginal smear of each rat were examined under the microscope daily to recognize the estrous cycle phases (Marcondes et al., 2002). It was noticed that the animals persisted in the diestrus stage in the letrozole-treated animals more than in the normal control group, and the vaginal smears were composed of cornified cells in all the phases of the estrous cycle in the PCOS group. This indicates the irregular cycle of the PCOS model if compared with the regular cycle of the normal group (Zhou et al., 2019).
Morphological changes in ovaries and uteri of rats
The average ovary weight increased significantly in the PCOS-negative control group compared to the normal group (p < 0.001). However, a marked reduction in uterus weight was recorded in the PCOS-negative control group compared to the normal group (p < 0.001). All the treated plant extracts were like metformin, except T. arabicus (p < 0.001), in decreasing the elevated ovaries’ weight to near-normal values and were superior to the effect of T. foenum-graecum (p < 0.001). On the other hand, T. arabicus (p < 0.001), the O. europaea oil (p < 0.01), and B. oleracea (p < 0.05) were superior to P. americana (p > 0.05), P. emblica (p > 0.05), and T. foenum-graecum (p > 0.05) in restoring uterus weight compared to the PCOS group, as shown in Figure 6.
Measurement of LH and FSH levels
A significant elevation in LH levels accompanied by a significant reduction in FSH levels was observed in rats with PCOS compared to the normal control group (p < 0.05). PCOS rats treated with metformin as well as the plants’ extracts showed significant improvement in LH and FSH levels compared to the PCOS untreated group (Fig. 7).
Measurement of circulating levels of progesterone and testosterone
As represented in Table 6, progesterone levels significantly decreased in the letrozole-treated groups compared to the normal control group (p < 0.001). Phyllanthus emblica (p < 0.001), P. americana (p < 0.01), and T. arabicus (p < 0.001), but not the O. europaea oil (p > 0.05), were superior to metformin (p > 0.05) in the elevation of progesterone levels compared to the letrozole-only group with no significant difference with the control group (p > 0.05) or T. foenum-graecum group (p > 0.05). The PCOS model without treatment (letrozole only) showed a remarkable increase in the level of the testosterone hormone compared to the control (p < 0.001) or the plants’ extracts (p < 0.001). All the tested extracts reduced the level to near the normal value (p > 0.05).
Measurement of lipid profile and glucose levels
The data of lipid profiles in the normal, letrozole-only, and different plant groups are provided in Table 7 and Figure 8. Significant elevation in TGs (p < 0.001), cholesterol (p < 0.001), and LDL levels was observed in the group treated with letrozole only in comparison to the normal group. The O. europaea and T. arabicus groups showed significant reduction of the TG levels to near the normal value (p > 0.05) compared to the letrozole group (p < 0.01) and superior to the metformin group (p > 0.05 with letrozole). Brassica oleracea and P. americana showed similar results to those of the metformin group compared to the normal and letrozole groups (p > 0.05), as shown in Table 7 and Figure 8A.
The natural plants used successfully decreased the elevated LDL and cholesterol levels to near the normal value (p > 0.05) compared to the letrozole group [all, p < 0.001, B. oleracea in cholesterol (p < 0.01)], as represented in Figure 8B and C.
The blood glucose level increased significantly in the PCOS group (p < 0.01) compared to the normal group. Trigonella foenum-graecum demonstrated the most significant effect in lowering the blood glucose level compared to metformin (p < 0.001), the plants’ extracts (p < 0.001), and even the normal control group (p < 0.001). The O. europaea oil (p < 0.001), B. oleracea (p < 0.01), P. americana (p < 0.001), P. emblica (p < 0.01), and T. arabicus (p < 0.01) significantly decreased the blood glucose level compared to rats with PCOS and near the normal value (p > 0.05), as represented in Table 7 and Figure 8D.
Measurement of oxidative stress markers
The letrozole-only-treated group showed a marked increase in MDA level compared to the normal group (p < 0.001). Treatment with P. emblica, P. americana, and T. arabicus significantly reduced the elevated lipid peroxidation level compared to the letrozole group (p < 0.01). The O. europaea oil- and B. oleracea-treated groups (p < 0.05) showed comparable results, as represented in Figure 9A.
The letrozole-treated groups showed a significant elevation of the TNF-α cytokine level. All the plant extracts used in the current study reduced the elevated TNF-α level compared to the group that received letrozole only. Persea americana and P. emblica succeeded in decreasing the level to be close to the normal group (p > 0.05), as represented in Figure 9B.
On the other hand, SOD activity was significantly decreased in the letrozole group compared to the normal rats (p < 0.001). Trigonella foenum-graecum, P. emblica, B. oleracea, P. americana, and T. arabicus increased the SOD activity to near the normal ratio, as illustrated in Figure 9C.
Histopathological examination
The ovaries of the negative control animals showed a thin covering ovarian capsule and follicles (F) in various stages of development, as well as fresh CL and corpus albicans (CA). Some of the follicles were antral while others were preantral. The Graafian follicle (GF) was clear, as well as the thin covering capsule (C) (Fig. 10A and A*). The ovarian sections of the animals treated with letrozole showed thickening of the ovarian capsule, the appearance of many large subcapsular cysts (Cy), and very few CL, but a greater number of degenerated ovarian follicles (F) were noticed. The subcapsular cysts revealed follicular debris and were lined with a thin layer of granulosa cells (GC) with hyperplasia of the theca interna cells. The ovarian medulla showed dilated congested blood vessels (BVs) (Fig. 10B and B*).
The ovaries of the animals treated with metformin were covered with a thin capsule. They revealed a suitable number of CL and several ovarian follicles in different stages of development. Some of the CL showed vacuolated (V) granulosa lutein cells. Other corpora revealed many BVs in the surrounding theca cells layer. There were no observed subcapsular cysts. The medulla showed dilated congested BVs (Fig. 10C and C*).
When the rats with PCOS were treated with T. foenum-graecum, their ovarian sections revealed developing ovarian follicles (F), a GF, and a degenerated follicle (DF). Many CL with vacuoles were seen, and the capsule appeared thin. The medulla (M) had dilated congested BVs (Fig. 10D and D*).
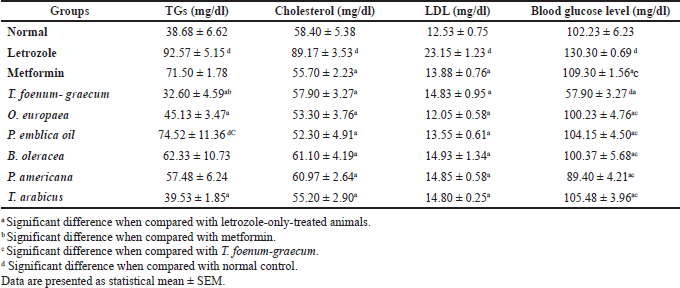 | Table 7. Effect of letrozole, metformin, and the plants’ extracts on lipid profile as well as blood glucose levels. [Click here to view] |
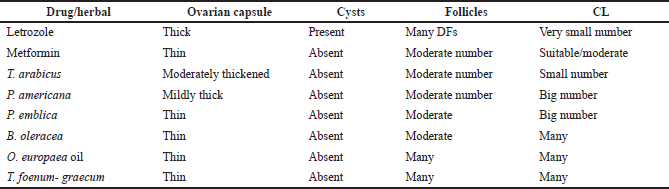 | Table 8. Histopathological examination of the extracts-, letrozole-, and metformin-treated groups. [Click here to view] |
The treatment of PCOS induced in the animals with T. arabicus showed moderately thickened covering ovarian capsules (C). There were ovarian follicles (F) in different stages of development, but there were no subcapsular cysts. The number of CL was smaller and showed vacuolation of the granulosa lutein cells. The medulla (M) revealed dilated congested BVs (Fig. 10Eand E*).
When PCOS rats were treated with P. americana, their ovarian sections revealed some ovarian follicles (F) in different stages of development, some DFs, and numerous CL with vacuolations (V). The capsule (C) was mildly thick with no subcapsular cysts. The medulla (M) revealed dilated congested BVs (Fig. 10F and F*).
The ovarian sections of the PCOS animals treated with P. emblica showed that the ovaries were full of CL and some developing ovarian follicles (F) while no subcapsular cysts were seen. The covering capsule (C) was thin. The medulla (M) revealed dilated and congested BVs (Fig. 10G and G*).
The treatment of PCOS animals with B. oleracea revealed some developing ovarian follicles (F) and many CL with vacuoles (V). The capsule appeared thin with no subcapsular cysts (Fig. 10H and H*).
The ovarian sections of the animals treated with the O. europaea oil showed many ovarian follicles (F) in different stages of development as well as many CL. The corpora showed BVs in the center (corpora hemorrhagicum), and the capsule (C) was thin. The medulla (M) revealed dilated and congested BVs. There were no subcapsular ovarian cysts seen (Fig. 10I and I*).
DISCUSSION
This study investigated the effect of some plant extracts in the PCOS model. A woman with PCOS needs long-term treatment because PCOS is a lifelong endocrinopathy. PCOS is a hormonal and metabolic disorder that may lead to infertility in many women during their reproductive age (Salehpour et al., 2016). Many synthetic drugs such as oral contraceptives and insulin sensitizers, e.g., metformin, are used to control PCOS (Dehghan, 2012). However, the adverse effects of such drugs shift the research interest toward the use of plants as safer alternatives for women with PCOS (Kurzthaler et al., 2014).
Letrozole was used in some studies to induce the PCOS model in experimental animals to resemble that of humans (Demirel et al., 2016). As evidence of the induction of PCOS, a marked elevation in testosterone and LH levels with reduction of the progesterone and FSH levels was observed. In addition, an irregular estrus cycle including elongation of both the diestrus and estrus phases and persistent cornified cells in the vaginal smears was observed (Marcondes et al., 2002). The blood glucose level increased in the rats with PCOS, which indicated IR (Glintborg and Andersen, 2010). The etiology of IR in PCOS is still unclear to date. The elevated blood glucose level may be due to a defect in insulin binding, glucose transporters, or glucose metabolism. The lipid profile was elevated compared to the normal group; this dyslipidemia with prominent levels of TGs, TC, and LDL along with the reduction in progesterone level and increase of testosterone may be due to hyperandrogenemia, which is characteristic of the PCOS model (Andersson et al., 2002).
Oxidative stress is the imbalance between the overproduction of reactive oxygen species (ROS) and the limited antioxidant defenses. In PCOS, there is an increase in free radical damage causing an elevated MDA level in ovary tissue with the reduction in the SOD activity, which protects the tissues from the harmful effects of superoxide radicals (Papalou et al., 2016). TNF-α is markedly increased in our study in accordance with other reports (Mohammadi et al., 2017).
In this study, we selected plants based on their folk medicine used for the treatment of PCOS, their reported active constituents, and their antioxidant activities. The study compared the activities of the selected plant extracts for PCOS treatment by both synthetic and natural drugs. Currently, metformin and fenugreek are present as products in the market for the treatment of PCOS (metformin and fenugreek, respectively).
Before performing the biological study, standardization of the plant extracts was carried out by the investigation of their constituents via HPLC analysis as well as determination of their phenolic and flavonoid contents and evaluation of their in vitro antioxidant activities. The highest flavonoid content was detected in the T. arabicus herb and P. americana fruit, while the P. emblica fruit showed the highest phenolic content compared to the standard used which is T. foenum-graecum seeds. All the plant extracts under investigation showed variable concentrations from the flavonoid, phenolic, and vitamin constituents. The highest content of rutin existed in T. arabicus, while P. americana showed the highest content of chrysoeriol and hesperidin. On the other hand, P. emblica showed the highest content of kaempferol. In addition, the highest phenolic constituents were detected in T. arabicus followed by P. americana. AA and vitamin B1 were detected in all plant extracts at different concentrations while niacin, a form of vitamin B, was only detected in P. americana.
In the current study, treatment with metformin improved most of the studied parameters in accordance with other studies (Weerakiet et al., 2004). This can be attributed to its insulin-sensitizing and steroidogenic effects. Metformin is a synthetic drug whose use is accompanied by many side effects, such as lactic acidosis, dizziness, GI pain, diarrhea, nausea, or vomiting. In addition, some females complain of drowsiness, muscle pain, breathing difficulty, and irregular heartbeats (Nasri et al., 2014). Therefore, herbal alternatives with fewer side effects are superior to metformin and oral contraceptives.
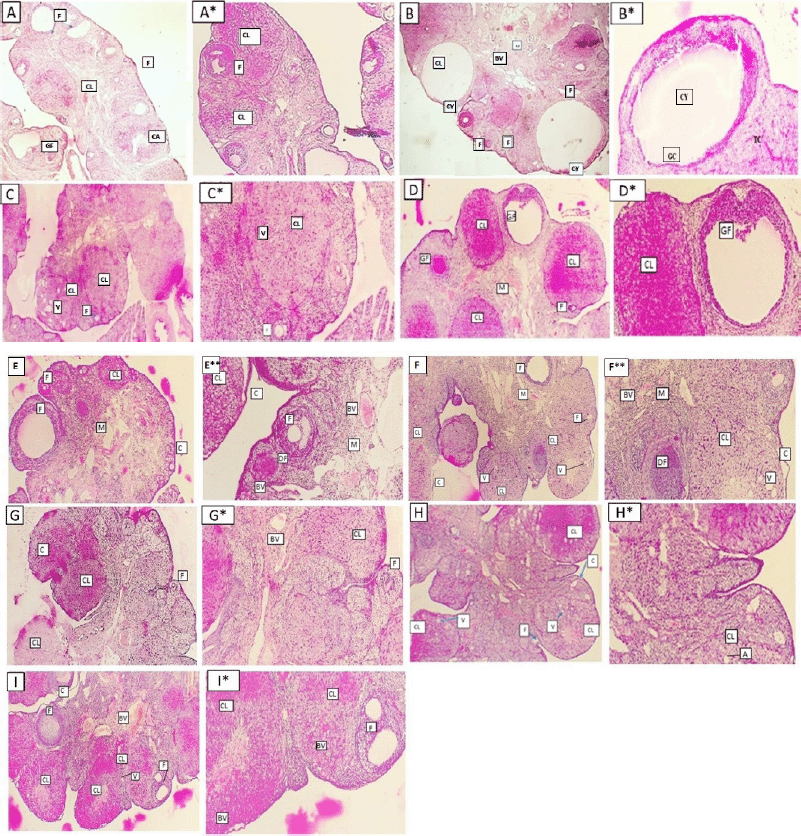 | Figure 10. Ovarian sections of different groups of rats (A and A*: normal group, B and B*: letrozole group, C and C*: letrozole and metformin, D and D*: letrozole and T. foenum-graecum, E and E*: letrozole and T. arabicus, F and F*: letrozole and P. americana, G and G*: letrozole and P. emblica, H and H*: letrozole and B. oleracea, and I and I*: letrozole and O. europaea oil treated groups). Some of them showing ovarian follicles (F), corpora lutea (CL), vacuoles (V), corpus albicans (CA), Graafian follicle (GF), capsule (C), subcapsular ovarian cysts (Cy), the medulla (M), and blood vessels (BV) (A: I, H&E ×40) (A*: I*, H&E ×100). [Click here to view] |
Trigonella foenum-graecum (fenugreek) was reported as an alternative to synthetic drugs for PCOS management due to its antidiabetogenic as well as antiandrogenic outcomes (Swaroo et al., 2015). However, T. foenum-graecum should be averted in females with peanut and chickpeas hypersensitive reactions as it might lead to cross-reactivity and asthma. It has been considered a potentially emerging allergen and as a herb is not favorable by all people (Dutau et al., 2013).
The doses of herbal remedies, as well as olive oil, were chosen according to their LD50 that were mentioned in previously published articles. The plant extracts used in our studies showed relevant contents of flavonoids known for their antioxidant and anti-inflammatory activities. This explained the reduction of lipid peroxidation and TNF-α activity with the increase in the antioxidant effect by increasing the SOD level due to the free radical scavenging activities of the plants’ extracts. Quercetin, kaempferol, myricetin, and rutin, which were detected in most of the plants under investigation, are known to possess antioxidant activities and exhibit anti-inflammatory effects (Narayana et al., 2001).
The scavenging activity of the flavonoids is in the order myricetin > quercetin > naringenin > catechin > kaempferol > flavone, which may explain the slight difference between the plant extracts used in the reduction of oxidative stress. Phyllanthus emblica showed a high concentration of kaempferol, and this may explain the normalization of the oxidative stress markers (MDA and TNF-α) and the elevation of the antioxidant and SOD levels, as shown in the present study. In addition, rutin was reported to decrease the size of polycystic ovaries (Ratty and Das, 1988; Sarwat et al., 2016), which explains the current finding related to the effect of T. arabicus to improve PCOS in the animal model as it showed the highest content of rutin.
Flavonoid contents of the tested plant extracts have also been reported in Noro et al. (1983), to have hormone-regulatory activity by binding to both 17β-hydroxysteroid dehydrogenases and 3β-hydroxysteroid dehydrogenase. Binding to these enzymes regulates estrogen and androgen in addition to progestin and androgen levels in humans, respectively. Tribulus arabicus in addition to P. americana revealed the highest content of flavonoids that led to a significant improvement in the testosterone and progesterone levels in our study model. The reduction in testosterone level can clarify the restoration of the lipid profile as it was mentioned in another report that testosterone has an antilipolytic activity by its selective inhibition of catecholamine-induced lipolysis (Noro et al., 1983), which might be the explanation for the improvement in the lipid profile by T. arabicus. The flavanone, naringenin, which had the highest concentration in P. emblica modifies steroidogenic enzyme activity in rats with letrozole-induced PCOS (Hong et al., 2019). Moreover, quercetin that was detected in most of the plant extracts promotes the proliferation of pancreatic β-cells and enhances glucose metabolism and insulin secretion (Tabrizi et al., 2020). This may clarify the reduction in the blood glucose level shown in this research. Furthermore, hesperidin can improve the follicular development of the isolated ovarian follicles of mice (Shoorei et al., 2019), which was proved by our histopathological study, where ovarian sections of P. americana showed ovarian follicles in various stages of development compared to PCOS.
Nonflavonoid phenolic compounds such as caffeic acid that was detected in all plant extracts with the highest concentration in T. arabicus were also documented in several studies to possess antioxidant effects through inactivation of ROS and inhibiting lipid peroxidation (Patil and Masand, 2018), while gallic acid was reported to modify the sex hormones’ level in PCOS rats due to its antioxidant activity. Both P. americana and P. emblica contain high concentrations of gallic acid, which gives more interpretation of the current findings. Most of the tested plant extracts contain vitamins B1, B5, and C. Vitamin C is known as an antioxidant and potentiates the antioxidant effects of the plant extracts (Mazloom et al., 2017). Niacin was detected in P. americana, which is known for its effect in the treatment of lipid disorders (Kamanna et al., 2013). This provides more clarification of the improvement in the lipid profile in the group treated with P. americana in addition to the previously mentioned effect due to testosterone reduction. All of that adds to its usefulness in women with PCOS to be not only a treatment but also a source rich in vitamins.
Many reports encourage people to increase the consumption of olive oil because of both health benefits and its effect on cardiovascular disorders, diabetes, many types of cancer, inflammation, and metabolic disorders. The antioxidant activity of olive oil is due to its constituents such as phenolic compounds, tocopherols, and sterols (Gamze et al., 2021).
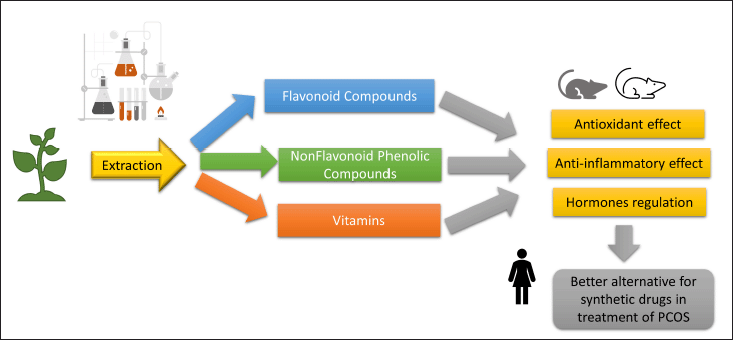 | Figure 11. Summary of plant extract constituents and their effects on PCOS model. [Click here to view] |
These explanations of our results together with the histopathological findings confirmed the potential, useful effects of the tested natural extracts in the treatment of PCOS. Figure 11 summarizes the constituents of the tested plant extracts and their potential effects on the PCOS model.
CONCLUSION
The extracts of T. arabicus, P. americana, and P. emblica were investigated as potential alternatives to metformin for the treatment of PCOS. These natural extracts were found to be rich in flavonoids, phenols, and vitamins. They decreased the elevated lipid profile, reduced oxidative stress, regulated hormonal levels, and improved the histopathological findings of ovaries in the in vivo model of letrozole-induced PCOS. These plant extracts can be promising candidates for the treatment of PCOS.
LIST OF ABBREVIATIONS
CMC: Carboxymethylcellulose; CL: Corpora lutea; DF: Degenerated follicle; DPPH: 2,2-Diphenyl-1-picrylhydrazyl; FSH: Follicle-stimulating hormone; GF: Graafian follicle; HPLC: High-performance liquid chromatography; PCOS: Polycystic ovary syndrome; LDL: Low-density lipoproteins; LH: Luteinizing hormone; MDA: Malondialdehyde; ROS: Reactive oxygen species; SOD: Superoxide dismutase; TNF-α: Tumor necrosis factor-alpha; TGs: Triglycerides.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Saeed Ahmed Khan, Dean of Dubai Pharmacy College for Girls, Dubai, United Arab Emirates, for his continuous support in all the research steps. They are also grateful to Dubai Pharmacy College for Girls for the financial support of this study.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Naglaa Shehab contributed to conceptualization, methodology, formal analysis, original draft, project administration, and review and editing. Hanan S. Anbar contributed to methodology, formal analysis, original draft, and review and editing. Nadia Mahmoud Alrouby contributed to methodology and original draft. Aya Abouelalamin contributed to investigation and original draft. Lama Lutfi contributed to investigation and original draft. Israa Tyseer Allo contributed to investigation and original draft. Salma Mohamed Elayoty contributed to investigation and original draft.
ETHICAL APPROVALS
The approval of the Research and Ethical Committee of Dubai Pharmacy College for Girls, Dubai, UAE (REC-FD-2020-05).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aaoran B, Caughey T. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol, 2018; 131:49–64. CrossRef
Abu-Gharbieh E, Shehab G, Almasri M, Bustanji Y. Antihyperuricemic and xanthine oxidase inhibitory activities of Tribulus arabicus and its isolated compound, ursolic acid: in vitro and in vivo investigation and docking simulations. PLoS One, 2018; 13:e0202572; doi:10.1371/journal.pone.0202572 CrossRef
Aher R, Belge A, Kadam R, KHarade S, Misal V, Yeole T. Therapeutic importance of fenugreek (Trigonella foenum-graecum L). A review. J Plant Sci Res, 2016; 3:152.
Andersson L, McTernan P, Hart A, Barnett A, Kumar S. The regulation of HSL and LPL expression by DHT and flutamide in human sebaceous adipose tissue. Diabetes Obes Metab, 2002; 4:209–13; doi:10.1046/j.1463-1326.2002.00214.x CrossRef
Artiss D, Yang C, Harake B, Capellari E, Kretch C, Eisenbrey B, Zak B. Application of a sensitive and specific reagent for the determination of serum iron to the Bayer DAX48. Am J Clin Pathol, 1997; 108:269–74; doi:10.1093/ajcp/108.3.269 CrossRef
Axelsson A, Tubbs E, Mecham B, Chacko S, Nenonen H, Tang Y, Fahey J, Derry J, Wollheim C, Wierup N, Haymond M, Friend S, Mulder H, Rosengren A. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med, 2017; 9:eaah 4477; doi:10.1126/scitranslmed.aah4477 CrossRef
Bairaktari E, Seferiadis I, Elisaf M. Evaluation of methods for the measurement of low-density lipoprotein cholesterol. J Cardiovasc Pharmacol Ther, 2005; 10:45–54; doi:10.1177/107424840501000106 CrossRef
Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem, 2006; 54:7429–36; doi:10.1021/jf0611668 CrossRef
Dehghan A, Esfandiari A, Momeni Bigdeli S. Alternative treatment of ovarian cysts with Tribulus terrestris extract: a rat model. Reprod Domest Anim, 2012; 47:12–5; doi:10.1111/j.1439-0531.2011.01877.x CrossRef
Demirel M, Ilhan M, Suntar I, Keles H, Akkol K. Activity of Corylus avellana seed oil in letrozole-induced polycystic ovary syndrome model in rats. Rev Bras Farmacogn, 2016; 26:83–8; doi:10.1016/j.bjp.2015.09.009 CrossRef
Dewanto V, Wu X, Adom K, Liu H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem, 2002; 50:3010–4; doi:10.1021/jf0115589 CrossRef
Dreher M, Davenport A. Has avocado composition and potential health effects. Crit Rev Food Sci Nutr, 2013; 53:738–50; doi:10.1080/10408398.2011.556759 CrossRef
Dutau G, Lavaud F. Le fenugrec: un allergène émergent? Fenugreek: an emerging allergen. Rev Fr Allergol, 2013; 53:613–4; doi:10.1016/j.reval.2013.11.002 CrossRef
Elnashar A. The role of metformin in ovulation induction: current status. Middle East Fertil Soc J, 2011; 16:175–81; doi:10.1016/j.mefs.2010.10.003 CrossRef
Gamze G, Hasim K, Serkan S. Antioxidant activity in olive oils. Olives and olive oil in health and disease prevention. 2nd edition, Academic Press, Cambridge, MA, pp 313–25, 2021. CrossRef
Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecol Endocrinol, 2010; 26:281–96; doi:10.3109/09513590903247873 CrossRef
Hong Y, Yin Y, Tan Y, Hong K, Zhou H. The flavanone, naringenin, modifies antioxidant and steroidogenic enzyme activity in a rat model of letrozole-induced polycystic ovary syndrome. Med Sci Monit, 2019; 25:395–401; doi:10.12659/MSM.912341 CrossRef
Janez A, Jensterle M. Potential new pharmacological approaches in obese women with polycystic ovary syndrome. J Diabetes Obes, 2017; 4:1–3; doi:10.15436/2376-0494.17.1246 CrossRef
Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res, 2004; 35(2):103–8; doi:10.1016/j.arcmed.2003.10.005 CrossRef
Kamanna V, Ganji SH, Kashyap M. Recent advances in niacin and lipid metabolism. Curr Opin Lipidol, 2013; 24(3):239–45; doi:10.1097/MOL.0b013e3283613a68 CrossRef
Karateke A, Dokuyucu R, Dogan H, Ozgur T, Tas Z, Tutuk O, Agturk G, Tumer C. Investigation of therapeutic effects of erdosteine on polycystic ovary syndrome in a rat model. Med Princ Pract, 2018; 27:515–22; doi:10.1159/000494300 CrossRef
Ksiksi T, Palakkott A, Ppoyil B. Tribulus arabicus and Tribulus macropterus are comparable to Tribulus arabicus terrestris: an antioxidant assessment. Curr Bioact Compd, 2017; 13:82–7; doi:10.2174/1573407212666161014130546 CrossRef
Kuntic V, Pejic N, Ivkovic B. Isocratic RP-HPLC method for rutin determination in solid oral dosage forms. J Pharm Biomed Anal, 2007; 43:718–72; doi:10.1016/j.jpba.2006.07.019 CrossRef
Kurzthaler D, Hadziomerovic D, Wildt L, Seeber B. Metformin induces a prompt decrease in LH-stimulated testosterone response in women with PCOS independent of its insulin-sensitizing effects. Reprod Biol Endocrinol, 2014; 12:98; doi:10.1186/1477-7827-12-98 CrossRef
Lin Y, Chen Y, Liang Y, Lin J. Composition of polyphenols in fresh tea leaves and associations of their oxygen radical absorbing capacity with antiproliferative actions in fibroblast cells. J Agric Food Chem, 1996; 44:1387–94; doi:10.1021/jf950652k CrossRef
Marcondes F, Bianchi F, Tanno A. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol, 2002; 62:609–14; doi:10.1590/s1519-69842002000400008 CrossRef
Mazloom B, Edalatmanesh M, Hosseini SE. The effect of gallic acid on pituitary- ovary axis and oxidative stress in rat model of polycystic ovary syndrome. Rep Health Care, 2017; 3:41–7.
Memon A, Ghanghro A, Shaikh I, Qazi N, Ghanghro I, Shaikh U. Effects of olive oil and garlic on serum cholesterol and triglycerides levels in the patients of type–II diabetes mellitus. J Liaquat Uni Med Health Sci, 2018; 17:101–5; doi:10.22442/jlumhs.181720559 CrossRef
Mihanfar A, Nouri M, Roshangar L, Khadem-Ansari MH. Therapeutic potential of quercetin in an animal model of PCOS: possible involvement of AMPK/SIRT-1 axis. Eur J Pharmacol, 2021; 900:174062; doi:10.1016/j.ejphar.2021.174062 CrossRef
Mohammadi S, Kayedpoor P, Karimzadeh-Bardei L, Nabiuni M. The effect of curcumin on TNF-α, IL-6 and CRP expression in a model of polycystic ovary syndrome as an inflammation state. J Reprod Infertil, 2017; 18:352–60.
Narayana K, Reddy M, Chaluvadi M, Devarakonda K. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol, 2001; 33:2–16.
Nasri H, Behradmanesh S, Maghsoudi A, Ahmadi A, Nasri P, Rafieian-Kopaei M. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type2 diabetes mellitus: a randomized double blind clinical trial. J Ren Inj Prev, 2014; 3:31–4; doi:10.12861/jrip.2014.10
Noro T, Oda Y, Miyasa T, Ueno A, Fukushim S. Inhibition of adinosine deaminase activity of aortic endothelial cells by selected flavonoids. Chem Pharm Bull, 1983; 31:3984–91. CrossRef
Papalou O, Victor VM, Diamanti-Kandarakis E. Oxidative stress in polycystic ovary syndrome. Curr Pharm Des, 2016; 22(18):2709–22; doi:10.2174/1381612822666160216151852 CrossRef
Patel R, Tiwari A, Chouhan S. Poly cystic ovarian syndrome: an updated review. J Appl Pharm Sci Res, 2020; 3:7–10; doi:10.31069/japsr.v3i1.2 CrossRef
Patil V, Masand N. Anticancer potential of flavonoids: chemistry, biological activities, and future perspectives. Stud Nat Prod Chem, 2018; 59:401–30; doi:10.1016/B978-0-444-64179-3.00012-8 CrossRef
Ratty A, Das NP. Effects of flavonoids on nonenzymatic lipid peroxidation: structure-activity relationship. Biochem Med Metabol Biol, 1988; 39:67–79; doi:10.1016/0885-4505(88)90060-6 CrossRef
Rifai N, Bachorik P, Albers JJ. Lipids, lipoproteins and apolipoproteins. In: Burtis CA, Ashwood ER (eds.). Tietz textbook of clinical chemistry, WB Saunders, Philadelphia, PA, pp 809–61, 1999.
Rokayya S, Li Y, Qi B, Wang S, Zhang Q, Han F, Ma Y, Jing J, Jiang L. HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-carotene) of Okra (Abelmoschus esculentus). J Chem, 2014; 2014:831357. CrossRef
Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, Calvo M, Bermúdez V. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med, 2014; 2014:719050; doi:10.1155/2014/719050 CrossRef
Rosenfield R. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics, 2015; 136(6):1154–65; doi:10.1542/peds.2015-1430 CrossRef
Salehpour S, Nazari L, Hoseini S, Saharkhiz N, Ghazi F, Sohrabi M. A potential therapeutic role of myoinositol in the metabolic and cardiovascular profile of PCOS Iranian women aged between 30 and 40 years. Int J Endocrinol, 2016; 2016:1–5; doi:10.1155/2016/7493147 CrossRef
Sarwat J, Faryal M, Suhail R, Anam M, Quart U, Hizb U, Tayyaba A, Ghazala S, Ali A. Ameliorative effects of Rutin against metabolic, biochemical and hormonal disturbances in polycystic ovary syndrome in rats. J Ovarian Res, 2016; 9:86; doi:10.1186/s13048-016-0295-y CrossRef
Shehab N, Abu-Gharbieh E, Bayoumi F. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complement Altern Med, 2015; 15:401; doi:10.1186/s12906-015-0919-6 CrossRef
Shoorei H, Banimohammad M, Kebria M, Afshar M, Taheri M, Shokoohi M, Farashah MS, Eftekharzadeh M, Akhiani O, Gaspar R, Pazoki-Toroudi H. Hesperidin improves the follicular development in 3D culture of isolated preantral ovarian follicles of mice. Exp Biol Med (Maywood), 2019; 244(5):352–61; doi:10.1177/1535370219831615 CrossRef
Swaroo A, Jaipuriar A, Gupta S, Bagchi M, Kumar P, Preuss H, Bagchi D. Efficacy of a novel fenugreek seed extract (Trigonella foenum-graecum, Furocyst) in polycystic ovary syndrome (PCOS). Int J Med Sci, 2015; 12(10):825–31; doi:10.7150/ijms.13024 CrossRef
Tabrizi F, Hajizadeh D, Vaezi M, Jafari H, Alizadeh M, Maleki V. Quercetin and polycystic ovary syndrome, current evidence and future directions: a systematic review. J Ovarian Res, 2020; 13:11; doi:10.1186/s13048-020-0616-z CrossRef
Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem, 1969; 6(1):24–7; doi:10.1177/000456326900600108 CrossRef
Variya B, Bakrania A, Patel S. Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol Res, 2016; 111:180–200; doi:10.1016/j.phrs.2016.06.013 CrossRef
Weerakiet S, Srisombut C, Rojanasakul A, Panburana P, Thakkinstian A, Herabutya Y. Prevalence of gestational diabetes mellitus and pregnancy outcomes in Asian women with polycystic ovary syndrome. Gynecol Endocrinol, 2004; 19:134–41; doi:10.1080/09513590400007242 CrossRef
Zhou Y, Lv L, Liu Q, Song J. Total flavonoids extracted from Nervilia fordii function in polycystic ovary syndrome through IL-6 mediated JAK2/STAT3 signaling pathway. Biosci Rep, 2019; 39(1):BSR20181380; doi:10.1042/BSR20181380 CrossRef