INTRODUCTION
Phytosterols are important constituents that strengthen the plant’s biological cell membranes (Ayaz et al., 2019). Phytosterols have been reported to have more than 250 different compounds that are isolated from various plants (Bolaños-Carrillo et al., 2015). Since phytosterols are not synthesized in the animal or human body, they must be obtained from the food of the plant derivate. The main dietary sources of these compounds are cereals, vegetable oils, nuts, fruits, and other vegetables (Gylling and Simonen, 2015). Phytosterols have been reported to have several potential bioactivities such as antioxidant, antibacterial, antidiabetic, lipid-lowering, and anticancer effects (Ferlay et al., 2010; Jones and Abumweis, 2009; Misawa et al., 2012; Ramprasath and Awad, 2015). Phytosterols have been shown to have important low-density lipoproteins (LDL) reducing abilities and thus have the potential to be developed as a future drug (Salehi et al., 2021). Phytosterol clinical studies have reported that these compounds significantly reduce LDL levels without any prominent side effects (Zhang et al., 2020). Consumption of dietary phytosterols can provide protection and reduction of the risk of cancer by 20% (Shahzad et al., 2017). Furthermore, experimental evidence proposes that phytosterols are helpful for treating some cancers, such as breast, lung, colon, and cervix cancers (Miras-Moreno et al., 2016).
The plant cell membranes are naturally occurring of phytosterols. The phytosterol structure similar to the animal sterol, both sterols have a steroid main structure and a hydroxyl group at C-3.
The important variation between phytosterol and cholesterol is the side chain, while phytosterols have different substitutions at C-24 compared to cholesterol. (Moreau et al., 2018). However, the main difference between phytosterols and cholesterol is the side chain at C-17; phytosterols have different substitutions at C-24 compared to cholesterol (Shahzad et al., 2017). Campesterol, stigmasterol, and β-sitosterol are the most common phytosterols, and ergosterols are less abundantly found in plants (Miras-Moreno et al., 2016). Our previous study reported that phytosterols are the main secondary metabolites isolated from R. apiculata’s root bark (Kurniawan et al., 2021). Rhizophora apiculata, known as bakau minyak in Coastal South Lampung (Dwiputra et al., 2020), is used as a traditional local medicine to treat several diseases, such as asthma, scabies, and rheumatism (Patra and Thatoi, 2011; Sachithanandam et al., 2019; Wu et al., 2008). Bakau minyak is used to treat genital warts by the people of South Lampung. The roots are dried and powdered, coconut oil is added, and then the mixture is applied to the warts (Kurniawan et al., 2021). This plant has tannin-rich and high-density wood, which is mainly used for manufacturing and creating charcoal (Getzner and Islam, 2020). The root bark of bakau minyak has a tolerance to high-salt seawater content. Phytosterols are related to the defense response of plants to various types of stress, both abiotic and biotic tension (Miras-Moreno et al., 2016). The root bark of bakau minyak directly interacts with seawater, which is mainly reported to accommodate steroids and tannin content, representing that it has important sources of phytosterols (Dwiputra et al., 2019; Kurniawan et al., 2021).
Rhizophora apiculata has an important role in traditional medicine in many tropical countries, such as Indonesia, but studies investigating the bioactivity of this plant scientifically may be limited. Therefore, this study aimed to investigate the cytotoxic activity of bioactive phytosterols from the root bark of R. apiculata. Three phytosterols have been isolated by extensive chromatographic techniques. Herein, isolation, structure elucidation, and cytotoxic evaluation by the [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (MTT) method against the lung, breast, and cervix cancer cell lines of these phytosterols are presented.
MATERIALS AND METHODS
General procedures
Extraction of the samples was carried out using maceration by methanol. The extract was concentrated by rotary evaporation (Heidolph). Chromatographic columns were initiated with silica gel 7734 (0.063–0.2 mm) and Sephadex presented from Merck. Thin-layer chromatography was carried out on PF254 plates, and the spots were monitored using UV light (366 and 254 nm) and by interacting with staining vapor. 13C and 1H NMR spectra were determined on a Brucker spectrometer 500 MHz in acetone-d6 and using tetramethyl silane as the zero-point standard. infrared (IR) spectra were recorded on a PerkinElmer FTIR. The MS spectra were obtained using the MS HP 5973 model configurated with JEOL JMS AX-500.
Plant samples
The root barks of R. apiculata were collected in August 2021 in the Lampung Sea Culture Fisheries Center (South Lampung, Indonesia), latitude: −5.527850327554739, longitude: 105.24841361893142. The herbarium specimen is stored at Herbarium Bogoriense, Bogor, under Specimen Code IT.IS.507389.
Isolation of compounds
The dried powder (610 g) from the root barks of R. apiculata was macerated using 1.5 l MeOH four times at 30°C under treated pressure to get a crude extract (41.0 g) of the methanolic extract. The extractions were carried out for about 72 hours to remove the solvent in a rotary evaporator at a temperature of 40°C. The methanol extract was partitioned in 400 ml (three times) of each solvent to yield an 8.8 g hexane fraction, a 12.7 g ethyl acetate fraction, and a 4.6 g acetone fraction. 10 g of the ethyl acetate fraction was chromatographed using a silica gel column, eluted with a solvent gradient by increasing polarity (using 1 l of hexane and ethyl acetate and 300 ml of methanol), giving 32 fractions. Fractions 8–11 (754 mg) were rechromatographed using Sephadex LH-20 and eluted using methanol: chloroform (1:1, v:v) (using 350 ml of both solvents). Of the 29 fractions obtained, fractions 7–10 were puri?ed on a Sephadex LH-20 column to obtain 14.1 mg of compound (1) and 10.5 mg of compound (2). Fractions 18–24 (143.2 mg) were chromatographed over a silica gel column yielding 14 fractions. Fraction 8–10 provided 6.7 mg of compound (3). Fraction 14, presented by recrystallization using methanol, gave compound (1) (12.8 mg).
Preparation of phytosterols
The phytosterols from the root bark of R. apiculata were dissolved using dimethyl sulfoxide (DMSO) to prepare a 4 mg/ml sample solution stock. The sample solution stock was presented in 96-well plates (Falcon, USA) to a final nontoxic DMSO solution concentration of about 0.1%.
Culturing of cancer cell lines
The cytotoxic activity assay of the phytosterols from the root bark of R. apiculata was carried out against three human cell lines: human cervix epithelioid [HeLa, European Collection of Authenticated Cell Culture (ECACC) 93021013], human Caucasian breast adenocarcinoma (MCF7, ECACC 86012803), and human Caucasian lung carcinoma (A549 ECACC 86012804). The cells were cultured following the standard protocol by the ECACC procedures: cells were grown in Dulbecco’s Modified Eagle Media (DMEM, Sigma) supplemented with 10% fetal bovine serum (Sigma) and 1× antimycotic solution (Sigma) and stored at 37°C with 5% CO2 in an incubator (95% humidity). The cells were under 80%–90% confluent, the old medium was disposed of, the cultures were washed using phosphate-buffered saline (PBS, pH 7.4, Sigma), and the trypsinized cultures were treated with 0.05% Trypsin-Ethylenediaminetetraacetic acid (Gibco, USA), and cells were passaged using a new fresh DMEM medium. Cell confluences were presented by the standard trypan blue exclusion method using a 0.4% Trypan Blue Solution (Gibco, USA). Cells were cultured in 100 μl aliquots onto 96-well plates using a final cell confluence of 1 × 104 cells/well. The plates were further incubated overnight at 37°C with 5% CO2 in an incubator until inoculating density was reached.
Cytotoxic assay
The prepared plates were determined by MTT, known as the thiazole blue method assay. The serial concentration of each compound was added to cell plates. After 48 hours incubation, each well was washed using PBS and 100 μl of a 5 mg/ml MTT (Thermo) solution was added to each well and incubated for 4 hours at 37°C. After dismissing the waste solution, the formazan crystal formed was dissolved by adding 100 μl/well of DMSO. Absorbance was measured at 540 nm using a microplate enzyme-linked immunosorbent assay reader. The cell viability was calculated using the following equation:
% viability cell = Abs of treated cell/Abs of nontreated cell.
All data are presented as a mean value by triplicate experiments (mean ± SE), and data analysis was performed by analysis of variance supported by Dunnett’s comparison test p < 0.05. The graphical data were analyzed using OriginLab (MA, USA).
RESULTS AND DISCUSSION
The phytosterols obtained were 7.1 mg of compound (1), 4.5 mg of compound (2), and 15.7 mg of compound (3). Compounds (1), (2), and (3) were directly identified as β-sitosterol, stigmasterol, and brassicasterol, respectively, by comparing their spectroscopy data (Table 1) with published literature. Compound (1) was shown as a white needle-sharp crystal. It has a melting point at 140.8°C–141.2°C, and the IR spectrum band at 3,435.34 cm−1 presented O–H stretching for the hydroxyl group. Its compound has a C29H50O molecular formula, which was confirmed by Electron Ionization Mass Spectroscopy (EIMS) at m/z 414 [M]+. The comparison of 1H and 13C NMR data (Table 2) showed decent relationships between compounds (1), (2), and (3); these constitute the main structure of the sterol compound, presenting the same ring formation of the A, B, C, and D regular ring system for phytosterols. Compound (2) was obtained as a white amorphous powder and the melting point at 161.4°C–162.2°C. The IR spectrum showed a hydroxyl group band at 3,463.55 cm−1. Compound (2) has the molecular formula C29H48O, which was assigned by EIMS at m/z 412 [M]+. Compound (3) was obtained as a colorless crystal, and it displayed a melting point at 152.6°C–153.4°C. The hydroxyl group band appeared at 3,383.78 cm−1. It has the molecular formula C28H46O, which was assigned by EIMS at m/z 398 [M]+.
In accordance with the isolated phytosterols and the tetracyclic terpenoid ring system of phytosterols, the variety of the isolated compounds is mainly qualified by the groups related by C-17 in the ring system. Based on the different aliphatic group at C-24, a chiral center carbon, phytosterols are distributed into 24-methylsterol and 24-ethylsterol. Brassicasterol has a methyl substituent at C-24 and a π bond at C-22. While stigmasterol and β-sitosterol have an ethyl group at C-24, respectively, stigmasterol has an additional π bond between C-22 and C-23 in comparison with β-sitosterol.
Considering the phytosterol ability and the background of R. apiculata, the bioactive phytosterol content from the root bark of R. apiculata was first investigated. The cytotoxicity of R. apiculata plants against three cancer cell lines, MCF-7, A549, and HeLa, was reported. Results (Table 3) show that the stigmasterol showed potential cytotoxicity against the three cancer cells and Cmb3 showed significant cytotoxicity against MCF-7 with an IC50 value of 28.48 ± 0.9 μM. The weak cytotoxicity of β-sitosterol was observed in the three cancer cell lines (Table 3). This trend may be due to the presence of double bonds at C-22 and C-23 of the sterol aliphatic chain; the activity of β-sitosterol may be lowered. Furthermore, the most important feature of the isolated phytosterol is a hydroxyl group at C-3 and different types of substitution by R1 at the C-17 position.
Compound (1), (2), (3) and integrated phytosterol were also studied their cytotoxicity against three cancer cell (Fig. 1, Table 3) compared by control positive for assay using 5-flouracil.All samples except Cmb3 showed moderate-weak cytotoxicity in cancer cells (Fig. 2). An introductory activity-structure relationship (Fig. 2, Table 1) shows that phytosterols with double bounds at C-22 and C-23 exhibited a stronger effect than those without these units, which was reasoned from the outcomes that compounds (2) and (3), but not compound (1), showed relatively higher cytotoxicity. When comparing the activities of compounds (1), (2), and (3) with the same effect, it is obvious that the binding site of the hydroxy at the C-3 moiety may not play the most significant role in the efficiency. In addition, the evaluation of the IC50 values of the Cmb1 and Cmb3 samples indicated that the saturated aliphatic chain at R1 may provide negative evidence in sterol cytotoxicity. In addition, compound (2), with a 24-methylsterol moiety, showed stronger cytotoxicity than compound (3), with a 24-methylsterol moiety. This suggests that the 24-ethylsterol substituent may be involved in the biological action of compound (3). Taken together, stigmasterol and brassicasterol integrated are newly effective, demonstrating improvement in their cytotoxicity in cancer cells.
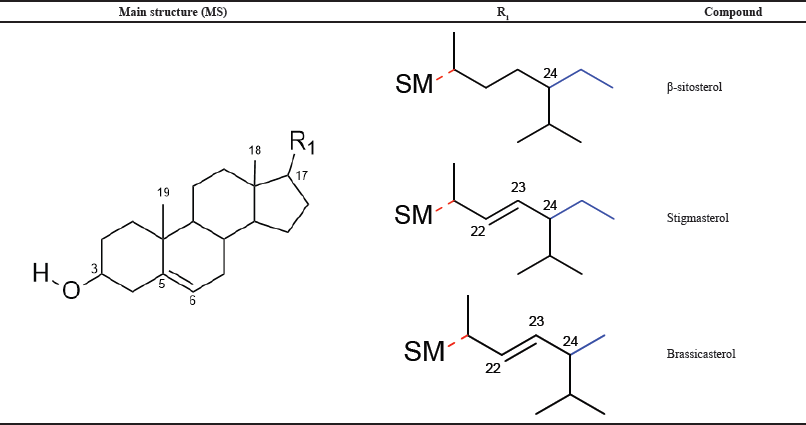 | Table 1. Bioactive phytosterols from root bark of R. apiculata: different types of substitution by R1 at C-17 position. [Click here to view] |
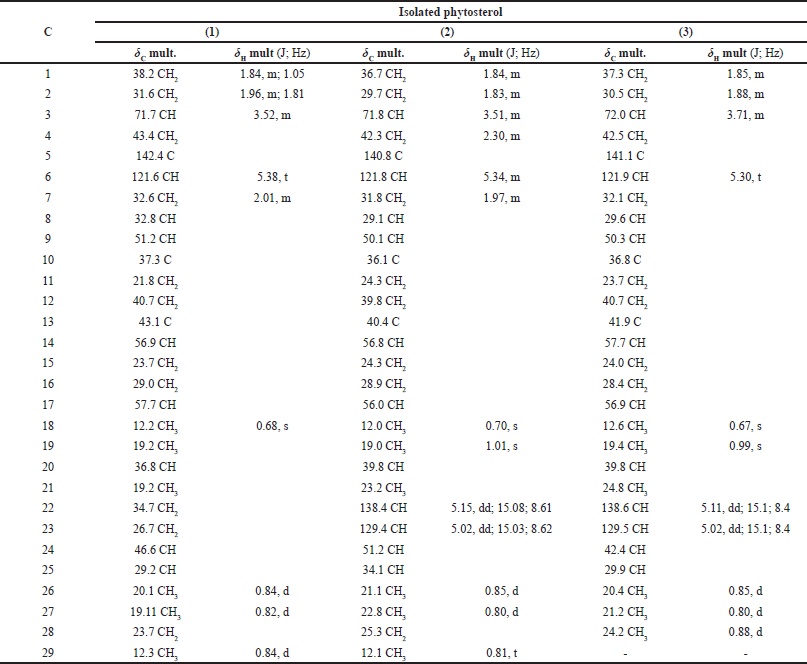 | Table 2. 13C NMR and 1H NMR data of isolated phytosterol. [Click here to view] |
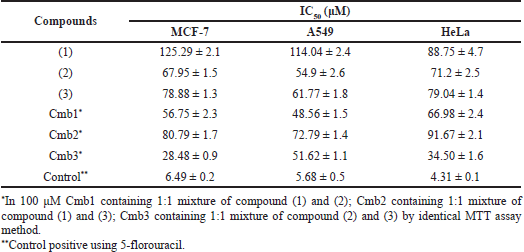 | Table 3. Cytotoxic activity of isolated phytosterol from root bark of R. apiculata. [Click here to view] |
Based on a study of the literature, phytosterol compounds have been the foremost chemical constituents reported from the root bark of R. apiculata until now (Kurniawan et al., 2021). They have various biological activities, such as anti-inflammatory, anticancer, antioxidant, antihyperglycemic, antifungal, and antibacterial. In most published papers, significant pharmacological activity was observed in the R1 aliphatic chain. Moreover, it should be noted that these phytosterols are present in several plant parts, such as edible fruit, leaves, and stems, and they play an important role in the corresponding declared biological activity. These data suggest that plant sterols could potentially be advanced to lead compounds due to their lower cell toxicity. Additionally, it has been discovered in the literature that phytosterols have a good effect in lowering LDL, and this bioactivity indicates the important ability of phytosterols to be delivered into the blood. This showed that these types of triterpenoids can enter the bloodstream and apply their effects and have certain therapeutic properties. However, only few studies have described detailed mechanisms of their anticancer action in vitro and in vivo. Therefore, additional pharmacological studies of phytosterol are needed.
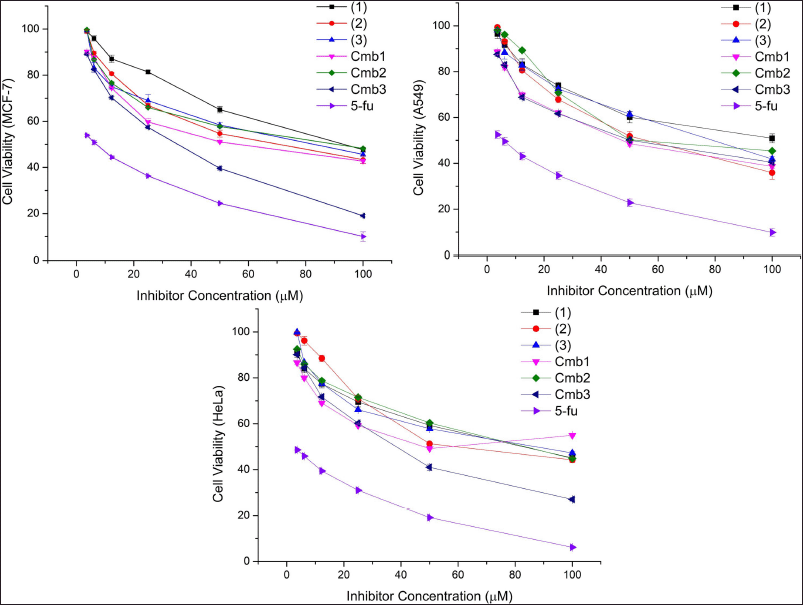 | Figure 1. Cell viability chart of bioactive phytosterol and integrated phytosterol from root bark of R. apiculata. [Click here to view] |
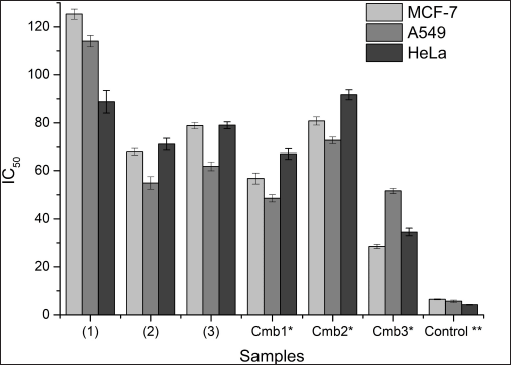 | Figure 2. Cytotoxicity of bioactive phytosterol and integrated phytosterol from root bark of R. apiculata. [Click here to view] |
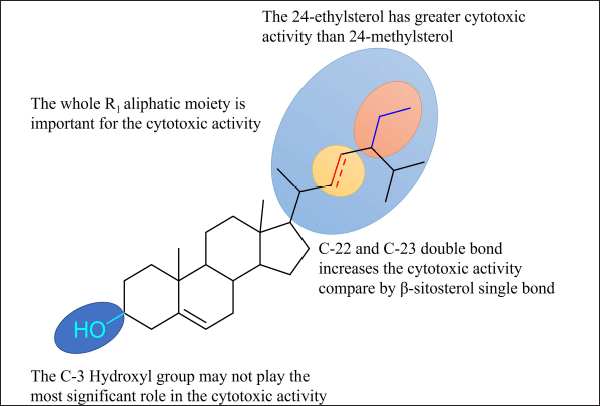 | Figure 3. Structure-activity relationships of phytosterol from root bark of R. apiculata. [Click here to view] |
CONCLUSION
The cytotoxicity of three phytosterols isolated from the root bark of R. apiculata was reported for the first time. All phytosterols and their integrated mixtures were tested for cytotoxicity against the MCF-7, A549, and HeLa cancer cells. The integrated phytosterol mixtures showed increasing cytotoxicity compared to the original compound. The structure-activity relationship of compounds (1), (2), and (3) and the mixture of all the integrated phytosterols indicated that the 24-ethylsterol chain showed a stronger effect than the 24-methylsterol moiety; the double bonds between C-22 and C-23 played an important role in phytosterol cytotoxicity. This study indicates that the root bark of R. apiculata is an important source of phytosterols, which may offer potential support for the application of traditional South Lampung medicine. Therefore, in our further investigation, an in vivo study will be required for a more extensive evaluation of the anticancer influence of phytosterols incorporated in the root bark of R. apiculata.
FUNDING
This research was supported by LPPM ITERA GBU45 Research Grant 2021 (No. B/503/IT9.C/PT.01.03/2021) and the Research and Innovation Center for Biological and Natural Materials.
CONFLICTS OF INTEREST
All authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ayaz M, Sadiq A, Wadood A, Junaid M, Ullah F, Zaman KN. Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids, 2019; 141:30–5; doi:10.1016/j.steroids.2018.11.005 CrossRef
Bolaños-Carrillo MA, Ventura-Gallegos J, Saldivar-Jiménez AD, Zentella-Dehesa A, Martínez-Vázquez M. Effect of sterols isolated from Myrtillocactus geometrizans on growth inhibition of colon and breast cancer cells. Evid Based Complement Altern Med, 2015; 589350:9; doi:10.1155/2015/589350 CrossRef
Dwiputra MA, Kurnia R, Rian, E. Penggunaan Data Citra Landsat Multitemporal untuk Monitoring Kondisi Ekosistem Mangrove di Teluk Kulisusu Kabupaten Buton Utara. J Sci Appl Technol, 2019; 3(1):1; doi:10.35472 CrossRef
Dwiputra MA, Mustofa A, Prasetyo BA. Aplikasi Sistem Informasi Geografis untuk Kajian Perencanaan Rehabilitasi Hutan Mangrove di Kecamatan Punduh Pedada, Lampung. J Sci Appl Technol, 2020; 4(2):67; doi:10.35472 CrossRef
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer, 2010; 127(12):2893–917; doi:10.1002/ijc.25516 CrossRef
Getzner M, Islam MS. Ecosystem services of mangrove forests: results of a meta-analysis of economic values. Int J Environ Res Publ Health, 2020; 17(16):1–13; doi:10.3390/ijerph17165830 CrossRef
Gylling H, Simonen P. Phytosterols, phytostanols, and lipoprotein metabolism. Nutrients, 2015; 7(9):7965–77; doi:10.3390/nu7095374 CrossRef
Jones, PJH, Abumweis SS. Phytosterols as functional food ingredients: linkages to cardiovascular disease and cancer. Curr Opin Clin Nutr Metab Care, 2009; 12(2):147–51; doi:10.1097/MCO.0b013e328326770f CrossRef
Kurniawan R, Suhartati T, Yandri AS, Meriyanti D, Sukrasno S. Potential antibacterial activity of bioactive β-sitosterol from root bark of Rhizophora apiculata from Lampung Coastal. J Kim Sains Apl, 2021; 24(4):114–9; doi:10.14710/jksa.24.4.114-119 CrossRef
Miras-Moreno B, Sabater-Jara AB, Pedreno MA, Almagro L. Bioactivity of phytosterols and their production in plant in vitro cultures. J Agric Food Chem, 2016; 64(38):7049–58; doi:10.1021/acs.jafc.6b02345 CrossRef
Misawa E, Tanaka M, Nomaguchi K, Nabeshima K, Yamada, Toida T, Iwatsuki K. Oral ingestion of Aloe vera phytosterols alters hepatic gene. J Agric Food Chem, 2012; 60:2799–806. CrossRef
Moreau RA, Nyströ L, Whitaker BD, Winkler-Moser JK, Baer DJ, Gebauer SK, Hicks KB. Phytosterols and their derivatives: structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog Lipid Res, 2018; 70(207):35–61; doi:10.1016/j.plipres.2018.04.001 CrossRef
Patra JK, Thatoi HN. Metabolic diversity and bioactivity screening of mangrove plants: a review. Acta Physiol Plant, 2011; 33(4):1051–61; doi:10.1007/s11738-010-0667-7 CrossRef
Ramprasath VR, Awad AB. Role of phytosterols in cancer prevention and treatment. J AOAC Int, 2015; 98(3):735–8; doi:10.5740/jaoacint CrossRef
Sachithanandam V, Lalitha P, Parthiban A, Mageswaran T, Manmadhan K, Sridhar R. A review on antidiabetic properties of Indian mangrove plants with reference to island ecosystem. Evid Based Complement Altern Med, 2019; 10(1155):24–31. doi:10.1155/2019/4305148 CrossRef
Salehi B, Quispe C, Sharifi-Rad J, Cruz-Martins N, Nigam M, Mishra AP, Konovalov DA, Orobinskaya V, Abu-Reidah IM, Zam W, Sharopov F, Venneri T, Capasso R, Kukula-Koch W, Wawruszak A, Koch W. Phytosterols: from preclinical evidence to potential clinical applications. Front Pharmacol, 2021; 11(January); doi:10.3389/fphar.2020.599959 CrossRef
Shahzad N, Khan WMD, Ali A, Saluja SS, Sharma S, Al-Allaf FA, Abduljaleel Z, Ibrahim IAA, Abdel-Wahab AF, Afify MA, Al-Ghamdi SS. Phytosterols as a natural anticancer agent: current status and future perspective. Biomed Pharmacother, 2017; 88:786–94; doi:10.1016/j.biopha.2017.01.068 CrossRef
Wu J, Xiao Q, Xu J, Li MY, Pan JY, Yang M. Natural products from true mangrove flora: source, chemistry and bioactivities. Nat Prod Rep, 2008; 25(5):955–81; doi:10.1039/b807365a CrossRef
Zhang X, Lin K, Li Y. Highlights to phytosterols accumulation and equilibrium in plants: biosynthetic pathway and feedback regulation. Plant Physiol Biochem, 2020; 155(August):637–49; doi:10.1016/j.plaphy.2020.08.021 CrossRef