INTRODUCTION
A urinary tract infection (UTI) is the most common bacterial infection in the ambulatory care setting in the USA, accounting for around 8.6 million visits in 2007 (Hooton, 2012; Schappert and Rechtsteiner, 2011). Complications of a UTI include recurrent infections, permanent kidney damage and scarring, premature delivery or low birth weight, urethral strictures, and sepsis (Alpay et al., 2018; Bahadi et al., 2010; Lugg-Widger et al., 2019). Antimicrobial resistance due to the incorrect selection of antibiotics, incorrect antibiotic dose regimen, and misuse of antibiotics that lead to resistant causative species of UTIs could lead to the development of such complications (Neal, 2008). Antimicrobial resistance is considered a major health problem worldwide; such resistance is caused by the excessive use of antibiotics with a lack of well-constructed programs that guide antibiotic use (Vivas et al., 2019). Around 700,000 patients die every year around the world due to antibiotic resistance (O’Neill, 2016). Earlier reports revealed a 40% prevalence of antibiotics’ self-medication (Nusair et al., 2021) and 87.8% of all deaths were due to secondary infections (Tseng et al., 2014) in Jordan. According to a hospital-based survey in the USA, 576 out of 1,941 therapy days with antibiotics were not essential due to the unnecessary prescribing length and using of antibiotics for nonbacterial causes (Hecker et al., 2003).
Several guidelines, such as those of the American Urological Association (AUA) and Infectious Diseases Society of America (IDSA), have been developed to optimize antibiotic use to minimize antibiotic resistance in UTI management. However, little is known about the extent of adherence to these guidelines for the management of hospitalized patients with UTIs in Jordan. Therefore, the current study is the first one that evaluated antibiotic prescription patterns among hospitalized patients with UTIs in Jordan.
AIM OF THE STUDY
The study’s aim was to evaluate the patterns and appropriateness of antibiotic selection among hospitalized patients with UTIs in Jordan.
METHOD
Study site and subjects
The largest hospital in the north of Jordan, King Abdullah University Hospital (KAUH), where this study was conducted, serves nearly a million people from the Irbid, Mafraq, Ajloun, and Jerash districts and represents a clinical training site for medical students at Jordan University of Science and Technology (JUST) (KAUH, 2018).
Data collection
Data were retrospectively collected by reviewing the electronic medical records of patients admitted between April and July 2019 to the Urology Department. The collected data included age, gender, height, weight, vital signs, white blood cell count, serum creatinine, the prescribed antibiotics and their dose regimens, microbiology reports, and sites of infection. Patients who were less than 18 years old, those who were hemodynamically unstable (e.g., ICU, cancer), and patients who were immunocompromised, had major illnesses, or had life-threatening infections were not included in the study. Antibiotics were classified according to the type of UTI. The AUA and the IDSA guidelines (AUA, 2021; IDSA, 2021) of UTI management were utilized to determine the appropriateness of antibiotic selection with regard to indication, dose, route of administration, and duration of therapy in UTI patients. In case of reporting inappropriate antibiotic use, the reason for the inappropriateness was reported and the suitable alternative therapy and its regimen were determined after referral to the AUA and IDSA guidelines. Both guidelines are complementary to each other. The IDSA guidelines are used to combat antibacterial resistance, and the AUA guidelines are used to assess the quality of urologic healthcare
The study was ethically approved by the Institutional Review Board Committee at KAUH/JUST in April 2019 and received Ethical Approval Number 22/104/2019.
Statistical analysis
Analytical and descriptive statistics and tables with summary data were created using percentage and mean to quantify and describe the demographics of the patients, the antibiotic prescription patterns according to the type of UTI, and the adherence to guidelines regarding appropriate antibiotic selection using the Excel program, 2015 version.
RESULTS
Table 1 shows some sociodemographics and the prevalence and distribution of UTI cases among the study participants. A total of 148 UTI patients were recruited for this study. The mean age of the participants was M = 55.29 years (SD = 9.94). More than half of the participants were female (54.1%) and under 65 years old (56.8%). Most of the participants (64.86%) were found to have complicated cystitis. A total of 68 patients had extended-spectrum β-lactamase (ESBL) +ve UTIs and 8 cases had multidrug resistant (MDR) UTIs. For patients who had ESBL +ve UTIs, 4 cases were ESBL +ve complicated pyelonephritis and 64 cases were ESBL +ve complicated cystitis. For patients who had MDR UTIs, four cases were MDR complicated cystitis and the other four cases were MDR complicated pyelonephritis. Table 2 presents antibiotics prescribed for ESBL +ve and MDR UTI cases.
A total of 168 intravenous (IV) antibiotic prescriptions were prescribed for 148 hospitalized UTI patients. As shown in Table 3, imipenem/cilastatin was the most commonly prescribed antibiotic, which was prescribed for 54.8% of the current study participants, followed by ceftriaxone, which was prescribed for 26.2% of the study participants. Other prescribed antibiotics included ertapenem, piperacillin/tazobactam, meropenem, metronidazole, amikacin, and lastly colistin. On the other hand, 10.8% of the patients received a combination therapy, such as colistimethate sodium and amikacin for MDR complicated cystitis; piperacillin/tazobactam, metronidazole, and meropenem for MDR complicated pyelonephritis; ceftriaxone and imipenem/cilastatin for patients with ESBL+ complicated cystitis; and imipenem/cilastatin and ceftriaxone for patients with uncomplicated pyelonephritis. With regard to the most commonly prescribed antibiotics (Table 4), imipenem/cilastatin was prescribed for 66.7% of the complicated cystitis cases and 57.1% of the complicated pyelonephritis cases. On the other hand, ceftriaxone was prescribed for 75% of the uncomplicated pyelonephritis cases. Imipenem/cilastatin and ceftriaxone were prescribed for 50% of the prostatitis cases.
 | Table 1. Demographics and UTI cases among the study participants (n = 148). [Click here to view] |
 | Table 2. Cases of ESBL* +ve and MDR** UTIs and the prescribed antibiotics. [Click here to view] |
 | Table 3. Patterns of antibiotic prescription among the study participants (n = 168). [Click here to view] |
The choice of antibiotics was inappropriate in 32.43% of the study participants.
The most common inappropriate antibiotic choice was prescribing IV ceftriaxone and IV imipenem/cilastatin in cases of uncomplicated pyelonephritis, complicated cystitis, and prostatitis, where guidelines recommend the administration of oral antibiotics such as ciprofloxacin or trimethoprim/sulfamethoxazole. Table 5 shows other examples of inappropriate antibiotic choice for the current study participants.
DISCUSSION
The current study is the first one that investigates the appropriateness of antibiotic prescription among patients with UTIs in Jordan. Considering the effects of improper antibiotic prescription procedures on raising antimicrobial resistance and poor health outcomes, it is crucial that medical professionals choose the right course of action for the treatment of UTIs.
According to the Centers for Disease Control and Prevention estimates, hospitals provide antibiotics in about 30% of the cases inappropriately or needlessly. This puts patients at risk of having negative outcomes and infections with antibiotic resistance (Bartlett et al., 2011). Antibiotic usage in cases when they are not necessary or protracted courses of therapy when shorter durations would be just as effective are two factors that contribute to antimicrobial resistance, which is seen as a major health issue globally (Dryden et al., 2011; Trémolières, 2002; Volluz et al., 2010). Therefore, for the prescription based on the patient’s history, antibiotics should be sensible, reasonable, and tailored to them, considering the prevalence of resistance, local practice patterns, and medication cost (Gupta et al., 2011). Utilizing the updated treatment guidelines for selecting the appropriate antibiotic and dosage regimen and avoiding unnecessary antibiotics could help improve clinical outcomes and decrease the potential toxicity and resistance (Abbo and Hooton, 2014).
The current study showed high prevalence of inappropriate antibiotic selection among hospitalized patients with UTIs. Clinical pharmacists in Jordan need to follow the updated UTI management guidelines of appropriate antibiotic selection in order to reduce the emergence of antibiotic resistance, reduce the cost of treatment, decrease the risk of hospitalization, and improve health outcomes.
The current study revealed that imipenem/cilastatin was the most commonly prescribed antibiotic for complicated UTI cases. In a prospective observational study conducted in Mumbai on patients with complicated UTIs, quinolones followed by β-lactamase inhibitors were the most commonly prescribed antibiotics (Dhodi et al., 2014). In a study conducted at the Institute of Nephrology at a tertiary care hospital for inpatients with complicated UTIs, the most commonly prescribed antibiotics were cephalosporins, followed by quinolones and penicillins (Muraraiah et al., 2012). An earlier prospective study showed that quinolones, including ofloxacin, ciprofloxacin, and levofloxacin, followed by ceftriaxone ± sulbactam and piperacillin + tazobactam, were the most commonly prescribed antibiotics for inpatients with complicated UTIs in India (Pallavi et al., 2015).
 | Table 4. Patterns of antibiotic prescription according to the type of UTI. [Click here to view] |
The current study showed that ceftriaxone was the most commonly prescribed antibiotic in cases of uncomplicated UTIs. An earlier study showed that amoxicillin and after that nitrofurantoin were the most commonly prescribed antibiotics for females with uncomplicated UTIs in Hong Kong (Wong et al., 2017). Another study showed that fluoroquinolones were the most commonly prescribed antibiotics for female outpatients with uncomplicated UTIs in the USA (Kobayashi et al., 2016). With regard to combination therapy, the current study participants received imipenem/cilastatin in combination with ceftriaxone, colistimethate sodium in combination with amikacin, and piperacillin/tazobactam in combination with metronidazole and meropenem. In comparison with a study conducted in a pediatric teaching hospital in Nepal, ceftriaxone with amikacin was the most common antibiotic combination prescribed (Panayappan et al., 2017). Variables including physicians’ experience, school of graduation, participation in training programs, and the impact of medical representatives could have influenced the antibiotic prescription patterns in different research studies.
The most commonly reported inappropriate antibiotic prescription was prescribing IV antibiotics such as ceftriaxone and imipenem/cilastatin in cases of mild to moderate infection for afebrile patients without or with slight elevation in WBC, who could be treated as outpatients with an oral antibiotic such as ciprofloxacin or trimethoprim/sulfamethoxazole. In a study conducted on patients with UTIs attending an academic emergency department, β-lactams were the most inappropriate antibiotic prescribed for adult patients (Chardavoyne and Kasmire, 2020).
The percentage of inappropriate antibiotic selection in the present study represents an average of the findings of earlier studies. Earlier research studies reported a significant variation regarding the extent of adherence to treatment guidelines of antibiotic prescription in patients with UTIs. A cross-sectional study conducted in the emergency departments of 10 hospitals in Spain found that 13.6% of the antibiotic prescriptions were inappropriate (Martínez et al., 2007). In a study conducted in a Swiss primary care center for the treatment of uncomplicated UTIs, fluoroquinolones were inappropriately prescribed for about 13.8% of the patients (Plate et al., 2020). Another retrospective study showed that antibiotic prescription was inappropriate in 12% of children and 32% of adults with cystitis and 19% of children and 54% of adults with pyelonephritis (Chardavoyne and Kasmire, 2020). A higher percentage of inappropriate antibiotic selection was reported in a study conducted on patients with UTIs, where 59% of the patients received inappropriate antibiotic treatment for UTI management at the emergency department and 68% of them received inappropriate antibiotics after hospital admission (Kiyatkin et al., 2016).
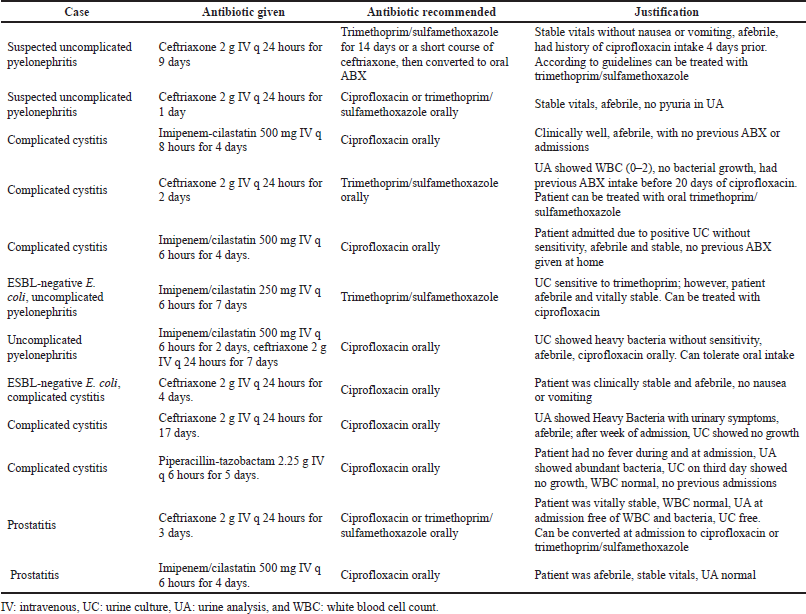 | Table 5. Selected cases of inappropriate antibiotic prescribing. [Click here to view] |
CONCLUSION
According to the results of the recent research, imipenem/cilastatin is the antibiotic combination that is administered most often to treat problematic UTI patients; ceftriaxone is the antibiotic that is prescribed to patients most often for treating UTIs that are not complicated; and the most often reported incorrect antibiotic prescription was IV antibiotics such ceftriaxone and imipenem/cilastatin given to patients with mild to severe infections.
Inappropriate antibiotic prescription for patients with UTIs in this study sheds light on the necessity to improve physicians and pharmacists’ antibiotic prescription patterns by adhering to the updated UTI management guidelines, with the aim of avoiding or minimizing antibiotic resistance and improving health outcomes among patients with UTIs.
ACKNOWLEDGMENTS
The authors thank the medical staff in the Urology Department at KAUH for facilitating the data collection process.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
The study was ethically approved by the Institutional Review Board Committee at KAUH/JUST in April 2019 and received Ethical Approval Number 22/104/2019.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics, 2014; 3(2):174–92. CrossRef
Alpay Y, Aykin N, Korkmaz P, Gulduren HM, Caglan FC. Urinary tract infections in the geriatric patients. Pak J Med Sci, 2018; 34:67–72. CrossRef
AUA. American Urological Association [Internet]. AUA, 2021. Available via https://www.auanet.org/?mod=article_inline (Accessed 9 January 2021).
Bahadi A, El Kabbaj D, Elfazazi H, Abbi R, Hafidi MR, Moussaoui R, Elouennass M, Dehayni M, Oualim Z. Urinary tract infection in pregnancy. Saudi J Kidney Dis Transpl, 2010; 21(2):342.
Bartlett JG, Auwaerter PG, Pham PA. Johns Hopkins ABX Guide 2012. Jones & Bartlett Publishers, Boston, MA, 2011.
Chardavoyne PC, Kasmire KE. Appropriateness of antibiotic prescriptions for urinary tract infections. West J Emerg Med, 2020; 21(3):633. CrossRef
Dhodi DK, Jaiswar S, Bhagat SB, Gambre RS. A study to evaluate prescribing pattern of antibiotics among patients of urinary tract infection with preexisting renal disorders in a tertiary care hospital. Int J Basic Clin Pharmacol, 2014; 3:687. CrossRef
Dryden M, Johnson AP, Ashiru-Oredope D, Sharland M. Using antibiotics responsibly: right drug, right time, right dose, right duration. J Antimicrob Chemother, 2011; 66(11):2441–3. CrossRef
Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis, 2011; 52(5):e103–20. CrossRef
Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med, 2003; 163(8):972–8. CrossRef
Hooton TM. Uncomplicated urinary tract infection. N Engl J Med, 2012; 366(11):1028–37. CrossRef
IDSA. Infectious Disease Society of America. 2021. Available via https://my.idsociety.org/home (Accessed 9 January 2021).
KAUH. King Abdullah University Hospital (KAUH) | LinkedIn [Internet]. 2018. Available via https://www.linkedin.com/company/kauh (Accessed 20 January 2018).
Kiyatkin D, Bessman E, McKenzie R. Impact of antibiotic choices made in the emergency department on appropriateness of antibiotic treatment of urinary tract infections in hospitalized patients. J Hosp Med, 2016; 11(3):181–4. CrossRef
Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. Outpatient antibiotic prescribing practices for uncomplicated urinary tract infection in women in the United States, 2002–2011. Open Forum Infect Dis, 2016; 3:ofw159. CrossRef
Lugg-Widger FV, Angel L, Cannings-John R, Jones H, Lau M, Butler C, Francis NA, Hay AD, Heginbothom M, Hood K, Paranjothy S, Vandervoort J, Hughes K. Long-term outcomes of urinary tract infection (UTI) in childhood (LUCI): protocol for an electronic record-linked cohort study. BMJ Open, 2019; 9(4):e024210. CrossRef
Martínez MA, Inglada L, Ochoa C, Villagrasa JR, Spanish Study Group On Antibiotic Treatments. Assessment of antibiotic prescription in acute urinary tract infections in adults. J Infect, 2007; 54(3):235–44. CrossRef
Muraraiah S, Rajarathna K, Rahman F, Jayanthi. Prescribing pattern in complicated urinary tract infections at tertiary care hospital. J Chem Pharm Res, 2012; 4(2):1222–30.
Neal DE Jr. Complicated urinary tract infections. Urol Clin North Am, 2008; 35(1):13–22. CrossRef
Nusair MB, Al-azzam S, Alhamad H, Momani MY. The prevalence and patterns of self-medication with antibiotics in Jordan: a community-based study. Int J Clin Pract, 2021; 75(1):e13665. CrossRef
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance, London, UK, 2016.
Pallavi D, Girish K, Pundarikaksha H, Pathapathi R, Kudagi B. A prospective study of the pattern of antimicrobial use in complicated urinary tract infections in inpatients in a tertiary care hospital. [Internet] Int J Pharm Bio Sci, 2015; 6(2):285–92.
Panayappan L, Babu A, Davis D, Joseph NM, Joshy N, Krishnakumar K. Urinary tract infection: prescribing pattern of antibiotics at a tertiary care hospital. Asian J Pharm Clin Res, 2017; 10:255–7. CrossRef
Plate A, Kronenberg A, Risch M, Mueller Y, Di Gangi S, Rosemann T, Senn O. Treatment of urinary tract infections in Swiss primary care: quality and determinants of antibiotic prescribing. BMC Fam Pract, 2020; 21(1):1–9. CrossRef
Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13, 2011; 169:1–38.
Trémolières F. Short or long course antibiotics. Is there a debate on the duration of treatment? Presse Med, 2002; 31(32):1495–501.
Tseng SH, Ke YF, Chang FY. National action plan to combat antimicrobial resistance in Taiwan. J Microbiol Immunol Infect, 2014; 47(3):167–70. CrossRef
Vivas R, Barbosa AAT, Dolabela SS, Jain S. Multidrug-resistant bacteria and alternative methods to control them: an overview. Microb Drug Resist, 2019; 25(6):890–908. CrossRef
Volluz D, Abbet P, Troillet N. What is the optimal duration of antibiotics in common infections? Rev Med Suisse, 2010; 6(266):1901–5.
Wong CKM, Kung K, Au-Doung PLW, Ip M, Lee N, Fung A, Wong SYS. Antibiotic resistance rates and physician antibiotic prescription patterns of uncomplicated urinary tract infections in southern Chinese primary care. PLoS One, 2017; 12(5):e0177266. CrossRef