INTRODUCTION
The oral route of drug administration is often considered a favorite route for intake of active pharmaceutical ingredients. It is associated with several patient-related benefits like patient compliance, administration convenience and economic. From the pharmaceutical research perspective, the associated advantages are formulation stability, dosage accuracy, storage, the option of sterile facilities, and reduced chances of acute drug reactions. Therefore, the development of solid oral dosage forms is the first choice for pharmaceutical scientists when making a dosage form decision. The success of oral drug delivery mainly depends on the aqueous solubility of the drug as it governs dissolution rate and bioavailability (Alqahtani et al., 2021). As a result of limited solubility in gastrointestinal fluids, poorly soluble pharmaceuticals frequently result in lower oral bioavailability. Therefore, a number of techniques have been used for solubility enhancement of chemical actives like micronization, nanonization, salt preparation, formulating dispersions, pH adjustment, complexation, etc. (Batrisyia et al., 2021).
Docetaxel (DOCE), an anticancer drug, belonging to the “taxanes” class, is used as a chemotherapeutic agent for various types of cancer, such as head, neck, lung, breast, ovarian, prostate, and gastric cancers. This antineoplastic drug disrupts the cell cycle by blocking its progression from G2 phase to M phase. Currently, the drug is administered to patients via the IV route in a dose range of 60–100 mg/m2 at a 1-hour infusion every 3 weeks. This procedure is “hospital-dependent” and hence cumbersome for patients. Furthermore, few cases receiving docetaxel show febrile neutropenia, which further necessitates hospitalization of patients along with IV antibiotics. Neuropathy, another major side effect of docetaxel, is cumulative, dose-related, and hence can halt therapy midway. Hence, to achieve the intended therapeutic effect and improve patient compliance, an appropriate oral docetaxel administration system must be developed (Lim et al., 2015). This task is challenging since docetaxel is a BCS class IV drug, representing poor solubility and poor permeability. The drug has a log P value of 4.1 and a pka value of 10.97, indicating its poor aqueous solubility, which is 0.025 μg/ml (Muhammad et al., 2018).
A number of approaches have been utilized to enhance aqueous solubility of docetaxel such as liposomes, PEGylated liposomes, polymeric, metallic, and hybrid nanoparticles, self-emulsifying formulations, self-assembled nanomicells, dendrimers, etc. However, all these formulations have a limitation in terms of cost, stability, and scale-up (Alshamrani et al., 2022; Campani et al., 2022; Muhammad et al., 2018; Vakili-Ghartavol et al., 2020; Zawilska et al., 2021). Therefore, a well-established, complexation technique with cyclodextrin for solubility enhancement of docetaxel is chosen for the present work. Cyclodextrins, the known and capable pharmaceutical excipients, act as the host and can entrap the guest molecules and pharmaceutical actives, forming a binary complex with it. This inclusion complexation results in enhanced aqueous solubility of drug molecules as the lipophilic portion of the drug molecule is trapped in the cavity of β-cyclodextrin (Saokham et al., 2018). The formed complexes also have a tendency to self-assemble and form complex aggregates, which further enhances the drug solubility (Jansook et al., 2010). When a hydrophilic polymer is added to cyclodextrin and the drug, a ternary inclusion complex is formed, which improves the drug’s solubility even more (Miranda et al., 2011). The water-soluble polymers stabilize the complex aggregates and, in turn, enhance drug solubilization (Messner et al., 2010). The formation of binary and ternary complexes of a drug with cyclodextrin and a polymer can be seen in Figure 1. Addition of hydrophilic polymers also benefits in reducing the manufacturing cost of a dosage form by decreasing the amount of cyclodextrin needed for solubilization of the drug dose (Savolainen et al., 1998).
The current study was carried out with the aim of increasing the anticancer activity of docetaxel by forming a ternary inclusion complex with β-cyclodextrin and a suitable hydrophilic polymer. The developed formulation will have pharmaceutical advantages in terms of aqueous solubility, drug release, and stability. Both the binary and ternary complexes of drug were prepared by the kneading and freeze-drying methods. The formed complexes were evaluated for their solubility and dissolution enhancement as compared with the free drug. These complexes were also characterized by Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), powder X-ray diffractometry (PXRD), and scanning electron microscopic (SEM) methods to study their solid-state properties at the molecular level. Finally, the anticancer activity of binary and ternary complexes was estimated on MCF-7 cell line to determine their minimum inhibitory concentrations.
MATERIALS AND METHODS
Materials
Docetaxel was obtained as a generous gift sample from Fresenius-Kabi Oncology Ltd., Gurgaon, Haryana, India. Anhydrous β-cyclodextrin, hydroxypropyl methylcellulose (HPMC) E5, PEG 4000, and PVP K-30 were procured from Himedia Laboratories Pvt. Ltd., Mumbai, India. Deionized water was used for all the experiments.
Phase solubility analysis
This study was performed as per Higuchi and Connors’ method with β-cyclodextrin (β-CD) to estimate stability constant and complexation efficiency of DOCE with it (Alshehri et al., 2020). An excess of DOCE was added to 5 ml aqueous solution of β-CD (0–10 mM) in a 10 ml stoppered vial. The generated suspension was vortexed for 1 minute before being placed in shaker baths at room temperature for 48 hours. Afterward, the suspension in vials was withdrawn and filtered (0.22 μm pores size). The filtrate was then analyzed by UV-Visible spectroscopy (Schimadzu, UV-1800) at 230 nm for estimation of DOCE content (Alshamrani et al., 2022). A phase solubility diagram was then plotted between β-CD concentration and DOCE concentration and the values of stability constant (Ks) and complexation efficiency (CE) were calculated from obtained curve using Equation (1) and (2).
where Ks is the stability constant and S0 is the intrinsic aqueous solubility of DOCE (mM).
Effect of hydrophilic polymers on complexation
The hydrophilic polymers selected for this study include hydroxypropyl methylcellulose (HPMC E-5), polyvinylpyrrolidone (PVP K-30), and polyethylene glycol 4000 (PEG 4000) since they have a high synergistic impact with β-CD. To pick the best polymer from the available ones, again phase solubility analysis was performed, where excess DOCE was added into 5 ml of the aqueous β-CD solution (1–10 mM) containing 0.5% w/v of each polymer in a 10 ml stoppered vial (Alshehri et al., 2020; Giri et al., 2021). Further processing of these suspensions was carried out as per the method described in Section Phase solubility analysis.
Preparation of the solid inclusion complexes: binary and ternary
The phase solubility analysis revealed the formation of AL type of complex; therefore, a binary complex of DOCE was prepared using an equimolar ratio of it with β-CD. The ternary complex was prepared using 0.5% w/v of HPMC E5 and an equimolar ratio of DOCE and β-CD by the freeze-drying method. The complexes were prepared by the freeze-drying method. The physical mixtures in the same ratio were prepared as described in Taupitz et al. (2013).
Preparation of physical binary and ternary mixtures
The physical mixtures (PM) of DOCE-β-CD and DOCE-β-CD-HPMC were made by uniformly mixing sieved (# 100 m sieve) DOCE, β-CD, and HPMC in a pestle mortar at a 1:1 molar ratio (Taupitz et al., 2013).
Preparation of freeze-dried (FD) binary and ternary complexes
The binary FD complex was prepared by dissolving DOCE and β-CD in a 1:1 mM ratio. At room temperature, the solution was agitated for 48 hours using a magnetic stirrer. The resultant solution was lyophilized using Martin Christ, Alpha 2-4 LSC freeze dryer. The process was carried out for 6 hours at a vacuum of 0.3 mbar. Similarly, ternary complex was prepared using 0.5% v/v HPMC, DOCE, and β-CD in a 1:1mM ratio by the same procedure.
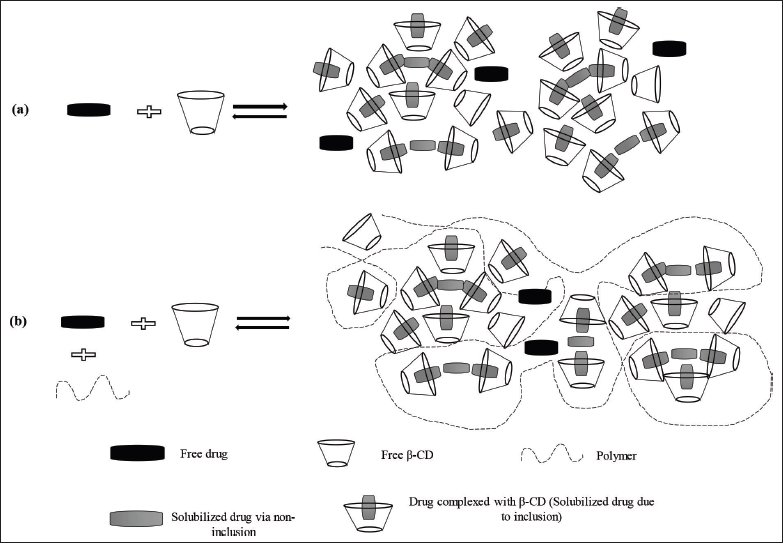 | Figure 1. Schematic of the formation of binary and ternary complexes of the drug with cyclodextrin and polymer. [Click here to view] |
Differential scanning calorimetry (DSC)
The prepared complexes were characterized by various techniques like DSC, XRD, FTIR, and SEM. The thermograms of DOCE, β-CD, HPMC, DOCE-β-CD-BC, and DOCE-β-CD-HPMC-TC were recorded using differential scanning calorimetry, Mettler-Toledo GmbH, Switzerland. Initially, the system was calibrated for the temperature axis and cell constant using indium. Then, about 1–10 mg of the sample was weighed and analyzed in sealed pin holed aluminum pans at heating rates of 10°C/min, over a temperature range of 100–300°C under nitrogen purging of 50 ml/min. The results obtained were analyzed by STAR SW 12.10 software.
X-ray diffractometry
PXRD patterns of API, carrier, polymer, and prepared complexes were recorded on an X-ray powder diffractometer using Bruker’s D8 advance diffractometer using Cu Kα radiation (1.54 Ao) at 40 kV, with 40 mA passing through a nickel filter. Data were collected in a continuous scan mode with a step size of 0.01 and a step time of 0.1 sec over an angular range of 5°–85° 2θ. The recording was made with the rotation of a sample holder in order to nullify the orientation effect. The obtained diffractograms were analyzed with DIFFRACplus EVA version 9.0 diffraction software.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of all the abovementioned samples were recorded using about 2 mg of each sample on a Perkin-Elmer Sp.2 spectrometer. The samples were scanned from 450 to 4,000 cm−1. The obtained spectra were analyzed using Spectrum 10 software.
Scanning electron microscopy
The surface morphology of binary and ternary complexes of DOCE was examined using scanning electron microscopy (Nova NanoSEM). Every sample was analyzed by sticking its powder on to a double-sided adhesive tape pasted over the sample stubs and sputter-coated with gold using an ion sputter under a vacuum of 0.000139 Pascal. The samples were scanned with an electron beam of an acceleration potential of 10 kV under the scanning electron microscope, and the images were collected as the secondary electron mode at different magnifications.
Assay and solubility determination of complexes
The lyophilized complexes were assayed for DOCE content. DOCE equivalent to 2 mg in complex (calculated theoretically for a 1:1 complex) was dissolved in 5 ml of dichloromethane. The contents were vortexed until a clear solution was formed, filtered through a 0.22 m porous filter, and the DOCE content was determined using UV-Visible spectroscopy at 230 nm. To determine the solubilizing potential of β-CD and HPMC, the binary and ternary complexes were added in excess to 10 ml of water in a 15 ml stoppered vial. The vials were kept at 37°C for 48 hours in a shaker water bath (Lab Tech), filtered, and the DOCE was measured using UV-Visible spectroscopy. The study was conducted in triplicate (Giri et al., 2021).
Dissolution study
Dissolution studies were conducted for DOCE, PM of DOCE and β-CD, DOCE-β-CD-BC, and DOCE-β-CD-HPMC-TC using USP-II, paddle-type dissolution test apparatus (Electrolab, India) at 37°C ± 0.5°C at 55 rpm. The dissolution medium used was 900 ml of 3% polysorbate 80 in phosphate buffer of pH 7.4. Aliquots from samples containing 50 mg of DOCE or its equivalent in PMs and binary and ternary complexes were withdrawn after 2, 4, 6, 8, 12, 18, 24, and 48 hours and the equivalent amount of fresh dissolution medium was added to maintain the sink conditions. The aliquots were filtered (0.22 μm pore size) and DOCE content was determined by UV-Visible spectroscopy. Data were expressed as percentage of DOCE released. All the studies were performed in triplicate (Li et al., 2009).
In-vitro anticancer activity
In order to evaluate the effect of prepared complexes on cell toxicity, the MCF-7 cell line was chosen and the effect of different concentrations of samples on inhibition of cell growth was studied. Samples of β-CD, HPMC, DOCE, DOCE-β-CD-BC, and DOCE-β-CD-HPMC-TC were investigated at concentrations of 10, 25, 50, 75, and 100 μg/ml, respectively. These samples were diluted in a culture medium (DMEM with high glucose, FBS, and antimycotic 100× solution) and were filtered. A suspension of MCF-7 cells was sown in a 96-well plate at a density of 1 x 104 cells per well. These cells were then exposed to all samples of selected concentrations. The control group was pure culture media, and 5-fluorouracil was taken as the standard. All samples were incubated in triplicate for 24 hours at 37°C and 5% CO2 in a CO2 incubator (Thermo Scientific BB150). After 24 hours, the culture media was replaced with 20 μl (3-[4-dimethylthiazol-2-yl]-2, 5- diphenyltetrazolium bromide) (MTT) and 80 μl fresh media, and cell incubation procedure was carried out further for 4 hours. Afterward, 200 μl of DMSO was added to each well and incubated for 10 minutes until formazan crystal reaction occurred. The yellowish MTT was reduced to a dark colored formazan by viable cells only, so every well was observed under a microscope to check cell survival. The absorbance of every sample was measured by a microplate reader (Benesphera E21) at 550 nm. From the obtained values of optical density, %cell inhibition and IC50 values were calculated by graphical means (Alshehri et al., 2020). Since the experiments were performed in triplicate, the average value was used in the calculation of %cell inhibition.
RESULTS AND DISCUSSION
Phase solubility analysis
A phase solubility analysis was carried out to establish the interaction strength and stoichiometry between docetaxel and β-CD molecules. The graph of the phase solubility analysis is shown in Figure 2. The diagram clearly demonstrates the rise in DOCE concentration with an increase in β-CD concentration in a linear manner, indicating attainment of the AL type of curve. The slope is likewise less than unity, indicating the formation of a stochiometric complex in a 1:1 ratio between the two (Kicuntod et al., 2018). The stability constant and complexation efficiency values were found to be 1164.16 M−1 and 0.0178, respectively, implying the formation of a stable complex. The intrinsic solubility of DOCE (S0) in water was found to be 0.0153 mM. A Ks value of more than 100 M−1 suggests the formation of stable complexes. Thus, it can be stated that β-CD is efficient in improving the solubility of docetaxel. An earlier complexation study performed using sulfobutyl ether β-cyclodextrin showed that docetaxel also formed a stable inclusion complex with it, which also showcased a magnificent increase in water solubility (Ren et al., 2020). This solubility has been further enhanced by the addition of the hydrophilic polymer.
Effect of hydrophilic polymers on complexation
The effects of the polymers, hydroxypropyl methylcellulose (HPMC E-5), polyvinylpyrrolidone (PVP K-30), and polyethylene glycol (PEG 4000) on the solubility, stability constant, and complexation efficiency of β-CD with docetaxel were investigated. Every polymer showcased synergistic action with β-CD, with HPMC E-5 being the highest, followed by PVP K-30 and PEG 4000, as shown in Figure 3. At 10 mM concentration of β-CD, the solubility enhancement was 21.30 times greater than the pure drug and with 0.5% w/v of polymer added, the solubility was enhanced 41.56, 39.73, and 26.53 times with HPMC E5, PVP K-30, and PEG 4000, respectively. The use of polymers increased the values of Ks and CE, implying the creation of a ternary complex between the drug, CD, and polymer. These values are summarized in Table 1. Since HPMC E-5 displayed a higher solubilization enhancement potential, Ks as well as CE, it was selected for further studies.
Assay and solubility determination of complexes
Docetaxel formed a 1:1 complex with β-cyclodextrin as evident from the phase solubility studies. The addition of hydrophilic polymers increased the complexation tendency; the highest improvement was attained with HPMC E5 from the screened polymers. So, the binary and ternary complexes were prepared by mixing 1 mM of each DOCE, β-CD, and 0.5% w/v of HPMC E5 for the ternary complex. After freeze drying, the complexes were assayed for DOCE content. The UV analysis of the binary complex (DOCE-β-CD-BC) revealed the assay of DOCE to be 78.12% and that of the ternary complex (DOCE-β-CD-HPMC-TC) to be 81.34%. The saturation solubility studies in water dictated notable solubility enhancement with the ternary complex as compared to the binary complex one, as shown in Figure 4. The ternary and binary complexes enhanced solubility of DOCE in water by 12.77 and 17.02 times, respectively. Thus, a significant rise in the solubility of DOCE was attained with the complexation technique. Alkylenediamine-modified β-CDs have also been studied for complexation with DOCE in order to enhance its water solubility, and the trial resulted in efficient solubility enhancement (Chen et al., 2021). However, since the research has selected modified β-CDs, limitations regarding GRAS-listed ingredients come into the picture. Dimethyl β-CD has also been found to tremendously enhance the aqueous solubility of DOCE. However, the safety status of concerned CD is questionable. Similarly, hydroxypropyl-β-CD was efficiently complexed with DOCE in another study, with a remarkable rise in the solubility of API (Sadaquat et al., 2020). However, all the reported studies have employed the crystalline form of DOCE and the research discussed in the current paper has used an amorphous form of API, so the values of Ks and solubility obtained were also notably high.
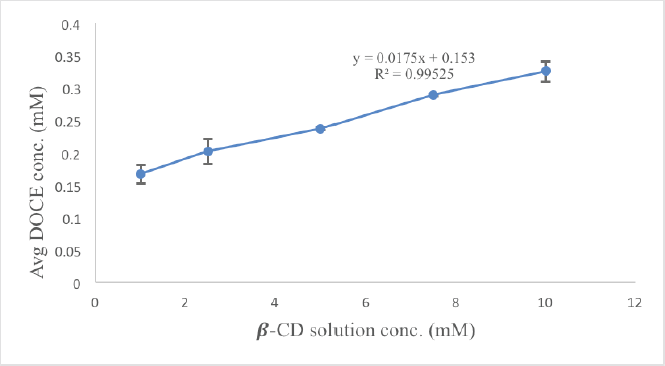 | Figure 2. Phase solubility study of DOCE in the presence of different concentrations (1–10 mM) of β-CD. [Click here to view] |
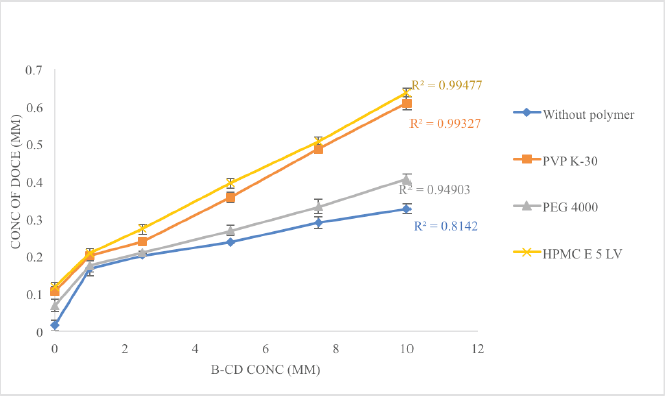 | Figure 3. The comparative solubility profile of DOCE in the presence of the hydrophilic polymer. [Click here to view] |
 | Table 1. The complexation efficiency of γ-CD in the presence of 0.5% w/v of various polymers. [Click here to view] |
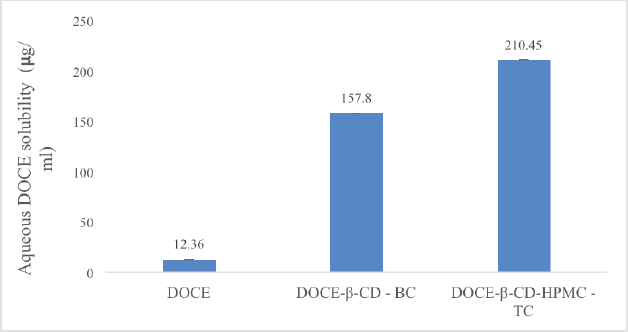 | Figure 4. The saturation solubility data of DOCE, freeze-dried binary complex (DOCE-β-CD-BC), and freeze-dried ternary complex (DOCE-β-CD-HPMC-TC). [Click here to view] |
Dissolution study
The percentage of drug dissolved against the time interval is used to understand the findings of dissolution investigations. Figure 5 shows the dissolution profiles of the drug and its carriers. The dissolution profile of DOCE in phosphate buffer with polysorbate 80 revealed a drug release of 11.05% at the first time point of 2 hours and it reached only 18.32% at 48 hours. The physical mixture released 14.29% DOCE at 2 hours and 27.69% at 48 hours, indicating a wettability effect of β-cyclodextrin on the drug release. The process of freeze drying resulted in the complexation of DOCE in the cavity of β-CD and, therefore, enhanced drug release was seen with DOCE-β-CD-BC and DOCE-β-CD-HPMC-TC formulations. A 20.79% drug release was attained at 2 hours in a binary complex formulation, which is 1.88 times higher than pure DOCE. Similarly, 22.63% drug release was seen with a ternary formulation that is 2.04 times greater than DOCE. After 48 hours, the drug releases from DOCE-β-CD-BC and DOCE-β-CD-HPMC-TC formulations were 40.76% and 45.44%, respectively, representing 2.22 and 2.48 times rise in %drug release of DOCE. The higher drug release with ternary complex is attributed to HPMC E5, which enhanced the inclusion of DOCE in β-CD. Thus, the prepared complexes of docetaxel with β-CD resulted in higher inclusion, complexation, solubility, and dissolution. All these parameters were further enhanced with the addition of a hydrophilic agent, HPMC E5.
Differential scanning calorimetry (DSC)
The DSC patterns of all the components are shown in Figure 6. DSC thermogram of β-CD shows a broad endotherm in a temperature range of 107°C–139°C due to dehydration. Amorphous docetaxel depicts a broad exothermic peak corresponding to its degradation centering at 228.49°C, starting from 220°C and ending at 239.89°C (Lim et al., 2015). The enthalpy of this exotherm is 33.81 J/g. Since the amorphous form of API was involved, the melting peak/ endotherm is completely absent. The DSC patterns of binary and ternary complexes, DOCE-β-CD-BC and DOCE-β-CD-HPMC-TC, also portrayed exothermic peaks due to degradation at 235.15°C (ΔHf = 18.49 J/g) and 239.19°C (ΔHf = 21.67 J/g), respectively. This small change in the Tpeak of exotherm demonstrates that DOCE interacts with β-CD and HPMC E5. However, significant changes in enthalpy values confirm the inclusion of DOCE in the bucket of β-CD. The amorphous character of the drug was maintained in the complexed form since there was no appearance of new thermal events in the thermograms of binary and ternary complexes. The solid-state nature of all these components is further confirmed with PXRD studies.
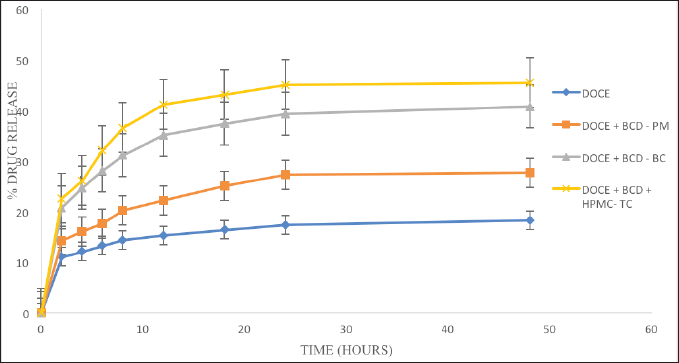 | Figure 5. The dissolution profiles of DOCE, PM, and binary and ternary complexes. [Click here to view] |
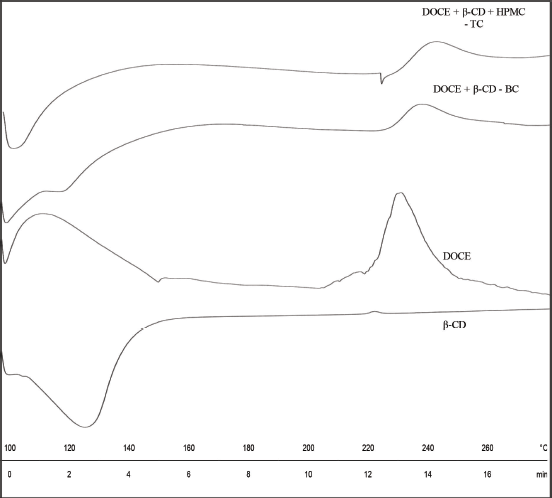 | Figure 6. DSC thermograms of β-CD, DOCE, binary complex (DOCE-β-CD-BC) and ternary complex (DOCE-β- CD-?HPMC-TC). [Click here to view] |
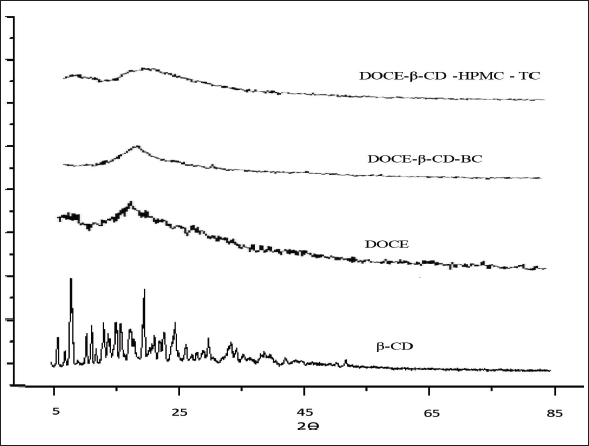 | Figure 7. The X-ray diffractograms of β-CD, DOCE, binary complex (DOCE-β-CD-BC) and ternary complex (DOCE-β- ?CD-HPMC-TC). [Click here to view] |
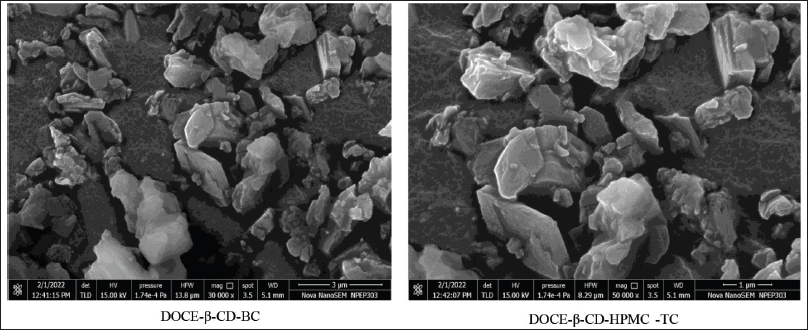 | Figure 8. SEM photomicrographs of docetaxel’s binary and ternary formulations: DOCE-β-CD-BC and DOCE-β-CD-HPMC-TC. [Click here to view] |
Powder X-ray Diffraction (PXRD)
As shown in Figure 7, the PXRD pattern of β-CD presented few sharp peaks at 2?θ value of 5.1, 7.3, 17.6, and 23.2, indicating its partially crystalline nature, and X-ray diffractogram of DOCE exhibited a complete diffused pattern displaying its amorphous character. The FD binary complex, DOCE-β-CD-BC, also displayed a complete diffused pattern confirming the amorphous nature of the formulation. Similarly, a broad halo pattern was obtained in the diffractogram of the ternary lyophilized complex, DOCE-β-CD-HPMC-TC, again suggesting its amorphous nature. The limited sharp peaks associated with β-CD were also totally absent in both formulations signifying its altered solid state with DOCE during the freeze drying. The results of the PXRD studies are thus in agreement with DSC studies, confirming the amorphous nature of the prepared DOCE formulations.
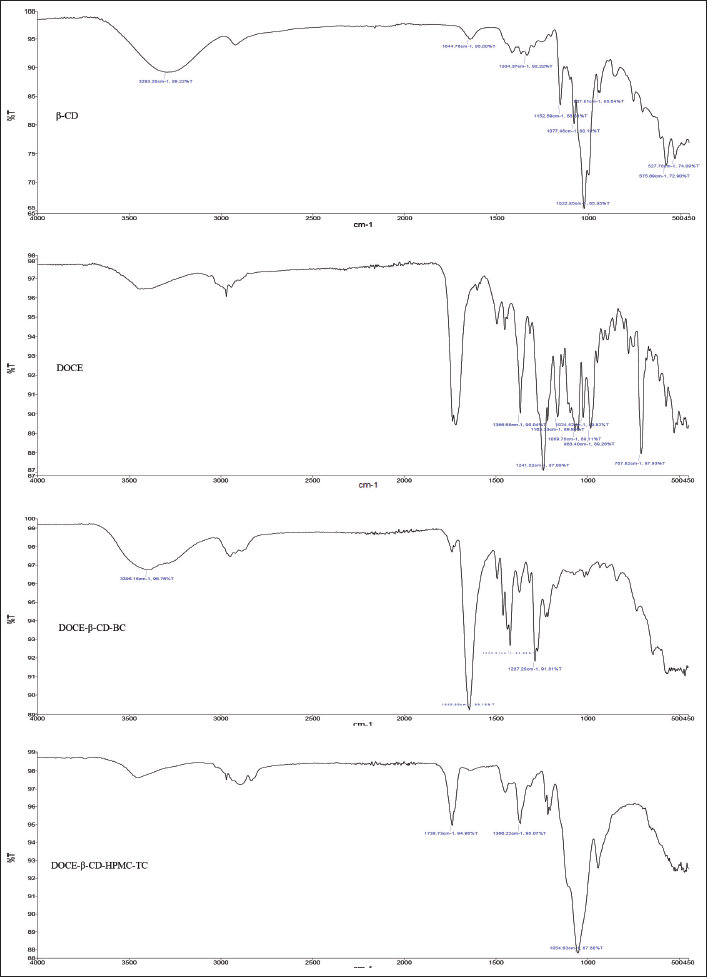 | Figure 9. FTIR spectra of (a) β-CD, (b) DOCE, (c) binary complex (DOCE-β-CD-BC), and (d) ternary complex (DOCE-β-CD-HPMC-TC). [Click here to view] |
Scanning electron microscopy
The SEM microphotographs of binary and ternary complexes of docetaxel are shown in Figure 8. The surface morphology of the binary DOCE-β-CD formulation showed slightly porous irregularly shaped soft FD particles of size 3 μm. Crystalline morphology of any particle was not visible, indicating the amorphous solid-state nature of formulation. The ternary lyophilized complex, DOCE-β-CD-HPMC-TC, presented porous amorphous structures, with no separate component in the complex being discrete. The amorphous particles have a size of 1 μm, almost similar to the binary formulation. Reduced particle size, amorphous nature of prepared formulations, and absence of both distinct β-CD or DOCE particles in SEM photographs of formulations denote encapsulation of DOCE in the void of β-CD.
FTIR Spectroscopy
The FTIR spectrum of β-CD revealed the vibration of free –OH groups between 3,300 and 3,500 cm−1 and for –CH stretching at 2,926.23 cm−1. Docetaxel showed characteristic IR absorption peaks at 3,369, 2,982, 1,717, 1,437, 1,241, and 707 cm−1 attributed to -OH, -CH asymmetric stretch, C=O stretch, C=C stretch, C-O stretch, and C-H bend, respectively (Lim et al., 2015; Alshamrani et al., 2022). The peaks corresponding to C=C and C-O stretching vibrations were absent in the binary complex, DOCE-β-CD-BC, emphasizing the complexation of N-tert-butyl group of DOCE in β-CD cavity. Since other explicit peaks of DOCE were visible, it can be predicted that weaker interaction was taking place in the duo, DOCE and β-CD. The ternary complex, DOCE-β-CD-HPMC-TC, efficiently masked the typical peaks of DOCE, pointing a significant structural entry of the drug in the β-CD cavity and involvement of carbonyl group and alkene group in complexation phenomenon. The presence of hydrophilic HPMC E5 might have promoted the access of lipophilic groups of DOCE in the hydrophobic interior of β-CD. Altogether, it can be stated that β-CD can efficiently complex with docetaxel with or without a third agent since major changes were observed in the FTIR spectra of both complexes. However, the addition of a ternary agent is definitely beneficial as it increased the complexation efficiency, by enhancing the interaction between the drug and β-CD. All the discussed spectra can be seen in Figure 9.
In-vitro anticancer activity
The percentage inhibition of β-CD, HPMC, DOCE, DOCE-β-CD-BC, and DOCE-β-CD-HPMC-TC on the MCF-7 cells at concentrations of 10–100 μg/ml were studied and the obtained results are shown in Figure 10. Table 2 depicts the %cell inhibition values at all the concentrations along with the standard deviation. Inclusion complexes of docetaxel showed noticeable %inhibition with IC50 values of 27.52 μg/ml and 22.08 μg/ml, respectively, for binary and ternary complexes, as compared to API, DOCE with IC50 value of 36.93 μg/ml. This superior cell growth inhibition, with inclusion complexes as compared to pure drug, is the result of enhanced drug availability due to its increased drug solubility, attributed to API’s complexation with β-CD. Furthermore, the ternary complex has higher solubility, so its IC50 value as compared to binary complex is further less which showed its supremacy in terms of in-vitro anticancer activity. Carriers β-CD and HPMC exhibited poor cell inhibition with IC50 values of 423.76 μg/ml and 1585.81 μg/ml, respectively, indicating their low cell toxicity.
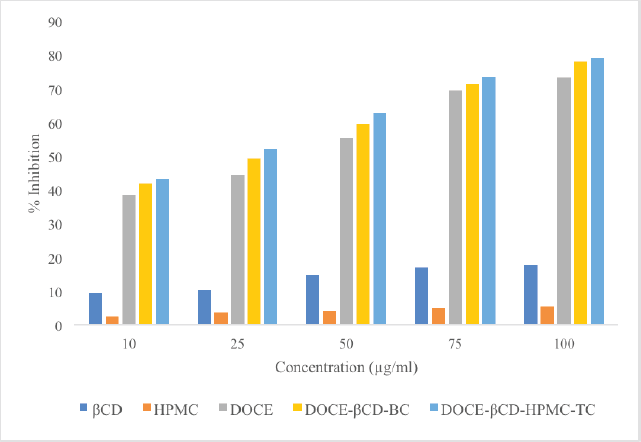 | Figure 10. Percentage inhibition values of β-CD, HPMC, DOCE, DOCE-β-CD-BC, and DOCE-β-CD-HPMC-TC with respect to concentration. [Click here to view] |
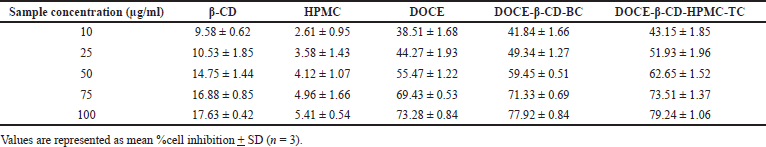 | Table 2. %Cell inhibition of docetaxel, carriers, and the prepared formulations [Click here to view] |
CONCLUSION
Binary and ternary inclusion complexes of docetaxel, DOCE-β-CD-BC, and DOCE-β-CD-HPMC-TC were prepared in the present study by the freeze-drying technique. HPMC E5 was chosen as a ternary co-complexing agent from assessed hydrophilic polymers based on phase solubility study data. A noticeable rise in drug dissolution was obtained, which is attributed to increased aqueous solubility of the drug due to complexation. The complex formation was confirmed by different solid-state characterization techniques like DSC, PXRD, FTIR, and SEM. Shifts of exothermic events associated with change in enthalpy of complexes as compared to pure drug during DSC studies suggests complex formation of docetaxel with β-CD and HPMC E5. The data were also supported by the results of PXRD studies, which showcased the attainment of the amorphous form of complexes since the partial crystallanity of β-CD had completely disappeared. The morphological evaluation of inclusion complexes carried out using SEM revealed the attainment of fine, microscopic-sized, irregular, fluffy particles, which additionally support the enhanced release tendency of docetaxel in cyclodextrin carrier. FTIR analysis revealed the interaction of C=C and C-O group of docetaxel with β-CD in the binary complex, and the interaction of all the major groups of docetaxel that are -OH, -NH, C=O, C-O, C=C, and -CH with β-CD in the ternary complex due to presence of HPMC E5. Thus, it can be stated that this hydrophilic polymer significantly enhanced the interaction of docetaxel with β-CD. In-vitro anticancer activity studies carried out using the MCF-7 cell lines also showcased improved cell growth inhibition with both binary and ternary inclusion complexes as compared to pure docetaxel. Thus, all the tested parameters in this study have given promising results due to the achievement of increased aqueous solubility of docetaxel with β-CD and HPMC E5.
ABBREVIATIONS
DOCE: Docetaxel; DSC: Differential scanning calorimetry; HPMC E5: Hydroxylpropyl methylcellulose E5; PEG 4000: Polyethylene glycol 4000; PVP K30: Polyvinylpyrrolidone K30; PXRD: Powder X-ray diffraction; SEM: Scanning electron microscopy; β-CD: β-cyclodextrin MCF-7 cell line: Michigan Cancer Foundation-7 cell line; DMEM :Dulbecco’s Modified Eagle Medium
ACKNOWLEDGMENTS
The authors are very thankful to Mr. Dhiraj Khattar and Dr. Sandip Zode, Fresenius Kabi Oncology Ltd., Gurgaon, India, for providing a free gift sample of the valuable docetaxel. They also thank Dr. S.S. Chitlange, DYPIPSR, Pune, India, for allowing to use the lyophilizer for freeze drying the formulations. The authors also express their sincere thanks to Dr. Sandip Patil, Director, Biocyte Laboratories, for supporting the in-vitro anticancer activity studies in this research.
AUTHORS’ CONTRIBUTIONS
In the conceptualization, writing, data collection, rewriting, and editing, all authors contributed substantially. The final manuscript was read and approved by all authors. Also, all the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest. The authors alone are responsible for the accuracy and integrity of the paper content.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All generated and analyzed data are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alshamrani M, Ayon NJ, Alsalhi A, Akinjole O. Self-assembled nanomicellar formulation of docetaxel as a potential breast cancer chemotherapeutic system. Life, 2022; 12:485; doi: 10.3390/life12040485 CrossRef
Alshehri S, Imam SS, Hussain A, Altamimi MA. Formulation of piperine ternary inclusion complex using βCD and HPMC: physicochemical characterization, molecular docking and antimicrobial testing. Processes, 2020; 8:1450; doi: 10.3390/pr8111450 CrossRef
Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ. Advances on oral drug delivery. Front Pharmacol, 2021; 12:618411.
Batrisyia RN, Janakiraman AK, Ming LC, Helal Uddin ABM, Sarker ZI, Kai Bin L. A review on the solubility enhancement technique for pharmaceutical formulations. Nat Volatiles Essent Oils, 2021; 8(4):3976–89.
Campani V, Salaroglio IC, Nele V, Kopecka J, Bernkop-Schnürch A, Riganti C, De Ros G. Targeted self-emulsifying drug delivery systems to restore docetaxel sensitivity in resistant tumors. Pharmaceutics, 2022; 14(2):292; doi: 10.3390/pharmaceutics14020292 CrossRef
Chen XU, Yang HW, Chi SM, Yue LL, Ruan Q, Lei Z, Zhub HY, Zhao Y. Solubility and biological activity enhancement of docetaxel via formation of inclusion complexes with three alkylenediamine-modified β-cyclodextrins. RSC Adv, 2021; 11:6292–302.
Giri BR, Leea J, Limb DY, Kim DW. Docetaxel/dimethyl-β-cyclodextrin inclusion complexes: preparation, in vitro evaluation and physicochemical characterization. Drug Develop Indus Pharm, 2021; 47(2):319–28.
Jansook, P, Kurkov SV, Loftsson T. Cyclodextrins as solubilizers: Formation of complex aggregates. J Pharm Sci, 2010; 99:719–29.
Kicuntod J, Sangpheak K, Mueller M, Wolschann P, Viernstein H, Yanaka S, Kato K, Chavasiri W, Pongsawasdi P, Kungwan N, Rungrotmongkol T. Theoretical and experimental studies on inclusion complexes of pinostrobin and β-cyclodextrins. Sci Pharm, 2018; 86:5; doi:10.3390/scipharm86010005
Li X, Wang D, Zhang J, Pan W. Preparation and pharmacokinetics of docetaxel based on nanostructured lipid carriers. J Pharm Pharmacol, 2009; 61:1485–92.
Lim SM, Pang ZW, Tan HY, Shaikh M, Adinarayana G, Garg S. Enhancement of docetaxel solubility using binary and ternary solid dispersion systems. Drug Dev Ind Pharm, 2015; doi: 10.3109/03639045.2015.1014818. CrossRef
Miranda JC, Martins TEA, Veiga F, Ferraz HG. Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drug. Braz J Pharml Sci, 2011; 47(4):665–81.
Messner M, Kurkov SV, Jansook P, Loftsson T. Self-assembled cyclodextrin aggregates and nanoparticles. Int J Pharm, 2010; 387:199–208.
Muhammad FS, Rehman M, Sarwar HS, Naveed S, Salman O, Bukhari NI, Hussain I, Webster TJ, Shahnaz G. Advancements in the oral delivery of docetaxel: challenges, current state-of-the-art and future trends. Int J Nanomed, 2018; 13:3145–61.
Ren L, Yang X, Guo W, Wang J, Chen G. Inclusion complex of docetaxel with sulfobutyl ether β-cyclodextrin: preparation, in vitro cytotoxicity and in vivo safety. Polym, 2020; 12, 2336; doi: 10.3390/polym12102336 CrossRef
Sadaquat H, Akhtar M. Comparative effects of β?cyclodextrin, HP?β?cyclodextrin and SBE7?β?cyclodextrin on the solubility and dissolution of docetaxel via inclusion complexation. J Incl Phenom Macro Chem, 2020; doi: 10.1007/s10847-020-00977-0 CrossRef
Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules, 2018; 23:1161; doi:10.3390/molecules23051161
Savolainen J, Jarvinen K, Taipale H, Jarho P, Loftsson T, Järvinen T. Coadministration of a water-soluble polymer increases the usefulness of cyclodextrins in solid oral dosage forms. In Proceedings of the Ninth International Symposium on Cyclodextrins, Dordrecht, The Netherlands, pp 261–4, 31 May–3 June 1998.
Taupitz T, Dressman JB, Buchanan CM, Klein S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: Itraconazole. Eur J Pharm Biopharm, 2013; 83:378–87.
Vakili-Ghartavol R, Rezayat SM, Faridi-Majidi R, Sadri K, Jaafari MR. Optimization of docetaxel loading conditions in liposomes: proposing potential products for metastatic breast carcinoma chemotherapy. Sci Rep, 2020; 10:5569; doi: 10.1038/s41598-020-62501-1 CrossRef
Zawilska P, Machowska M, Wisniewski K, Grynkiewicz G, Rafal Hrynyk, Rzepecki R, Gubernator J. Novel pegylated liposomal formulation of docetaxel with 3-n-pentadecylphenol derivative for cancer therapy. Eur J Pharm Sci, 2021; 163:105838; doi: 10.1016/j.ejps.2021.105838 CrossRef