INTRODUCTION
Aceh Province in Indonesia experienced the most severe earthquake and tsunami disaster in 2004. After the tsunami infrastructure development occurred rapidly in Banda Aceh City. Banda Aceh City became the administrative center of Aceh Province and was the most tsunami-affected area. Residential settlement development continues to increase yearly in Banda Aceh City (Gadeng et al., 2019). The return of the community to new settlements with all the activities of daily life has created a habitat for Aedes and an explosion of cases of dengue hemorrhagic fever (DHF). Reports from the Ministry of the Health Republic of Indonesia revealed that cases of dengue fever in Aceh continued to increase after the tsunami (Ministry of Health Indonesia, 2007). Cases of dengue fever in Banda Aceh City after the tsunami from 2005 to 2007 experienced a significant increase. The explosion of very high dengue cases occurred in 2010, with 759 cases after the return of the community to new housing in 2009 (Agustina et al., 2021).
This condition is related to the high population of Aedes and other supporting factors that caused the presence of Aedes in the tsunami’s neighborhood area of Banda Aceh City. Aedes is an invasive species that can adapt to and interact with the surrounding environment. The invasive species interactions vary in space and time and depend on local conditions (Cunze et al., 2018).
Mosquitoes exist around humans because of the availability of breeding places, eating, and resting habitats. Therefore, it is necessary to have control efforts oriented to the habitat conditions and the necessities of life for it. The life of Aedes depends on plants. Plants are resting places and sources of food for male and female Aedes aegypti L. and Aedes albopictus Skuse (Agustina et al., 2019). Plants can serve as attractors or repels, and each type has a different attraction. Mosquitoes come to plants because smells or colors attract them to get food (Barredo and DeGennaro, 2020).
The selection of plant species by various mosquitoes is related to the volatile compounds produced by each plant. The olfactory systems of A. aegypti, Aedes mcintoshi Huang, and Anopheles gambiae l. can detect different volatile compounds in plants (Nyasembe et al., 2018) because they have an affinity for certain plant species (host plants). Their olfactory system influences the selection of host plants. Plants that attract mosquitoes or are called attractants are very limited in the study, even though these attractant plants also have the potential to control mosquitoes (Nyasembe et al., 2015). Other studies also revealed that eliminating the mesquite plant [Prosopis juliflora (Sw.) DC.], which is the food source of Anopheles, can reduce the population of malaria vector mosquitoes by 69% (Muller et al., 2017). Plant attractant compounds can serve as an effective biological control strategy. The content of secondary metabolites in plants that attract mosquitoes can as act bait in mosquito surveillance and control programs (Dormon et al., 2021). Parts of the plants have attractions such as flowers, sharp aromas, and high nectar content that can attract mosquitoes.
A repellent is an insecticide that is repelling and nonkilling. Every plant has a composition of chemical compounds called secondary metabolites. Essential oils, flavonoids, alkaloids, and aromatic compounds are metabolites in plants that have the potential to be mosquito repellents (Boate and Abalis, 2020). Essential oils, also called volatile oils, are secondary metabolites of volatile plants. This oil exists in fruits, seeds, leaves, flowers, stems, bark, roots, and rhizomes (Sengül Demirak and Canpolat, 2022). Therefore, plants as a place for mosquito feeding and resting activities also have a favorable opportunity to become one of the mosquito control strategies by utilizing secondary metabolite compounds. Plants around us have the potential insecticides, but it is necessary to identify which bioactive molecules in repellent plants have a higher effect on disease-transmitting mosquitoes (Athuman et al., 2016).
House yard plants not only support the life of mosquitoes but also have the potential to control the mosquito population. Based on the content of plant compounds, these plants can act as repellents or attractants for Aedes. This information is necessary to know an effective and targeted control strategy. This study was to determine the potential of house yard plants as an alternative to dengue vector control in the tsunami area settlements of Banda Aceh City.
MATERIALS AND METHODS
Study area
The study is in Banda Aceh City, as it was severely affected by the earthquake and tsunami disasters in 2004 (Fig. 1). Banda Aceh City is between 5°30? – 05°35? north latitude and 95°30? – 99°16? east longitude, with an average elevation of 0.80 meters above sea level, with an area of 61.36 km2 (BPS, 2019). The city of Banda Aceh consists of nine subdistricts, and the research sites are the Meuraxa Subdistrict and Syiah Kuala Subdistrict. The Meuraxa and Syiah Kuala Subdistricts were chosen as the research sites because of the endemic areas for dengue cases. In addition, these two areas were also the worst affected by the tsunami.
Data collection
This research begins with a preliminary survey using an explorative method to determine the condition of the houses in the Meuraxa Subdistrict and Syiah Kuala Subdistrict, Banda Aceh City. Purposive sampling was used to sample 200 sample houses. The selection was houses suspected of having an Aedes breeding place and plants in the yard. The collection of house yard plants involved the larva monitoring community in each village. To determine secondary metabolites that attract or repel Aedes using a literature study, all plants found in the house yards were collected and documented. The data were then summarized, and the results of the studies arranged in the tabular form of the list of secondary metabolites in plants that can function as repellents or attractants.
Data analysis
The data from this research are presented and analyzed using descriptive statistics.
Statistical analysis
Statistical analysis for calculation of graph and table data was carried out with Microsoft Excel.
RESULTS AND DISCUSSION
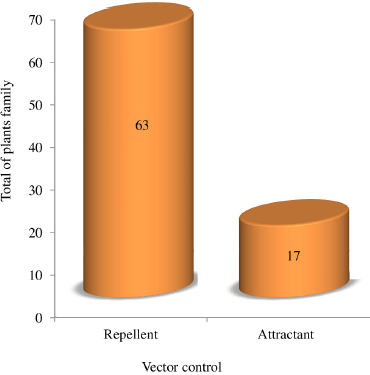
The results of 200 houses in the tsunami area settlement of Banda Aceh City found 150 species and 63 families of house yard plants (Fig. 2). The category of plant habitus found in the study area comprised herbs (44%), shrubs (32%), and trees (24%) (Fig. 3). The tsunami disaster caused the coast to be badly damaged, and almost all the vegetation was destroyed and lost. After the tsunami, much vegetation of the damaged coastal area naturally changed (succession), namely, the emergence of pioneer plant species such as herbs, shrubs, and trees (Suryawan, 2007).
The family’s highest number of species are from the group Araceae (14 species), Euphorbiaceae (8 species), Asparagaceae (7 species), Lamiaceae (6 species), Apocynaceae (5 species), Arecaceae (5 species), Fabaceae (4 species), Myrtaceae (4 species), Portulacaceae (4 species), Solanaceae (4 species), and Zingiberaceae (4 species) (Fig 2). Many species in this family found at the research sites are related to the COVID-19 pandemic. Restrictions on activities outside the home during the COVID-19 pandemic have provided much free time at home and made many people take up new hobbies such as caring for ornamental plants and business opportunities for buying and selling plants. Plants in pots are obtained easily by ordering through online media or direct purchase. Residents in the tsunami area of Banda Aceh City planted the Araceae family such as Aglaonema and other species because they follow trends and other aspects such as the benefits that they can filter air pollution at home and are easy to maintain (Zahara and Win, 2020).
The families Apocynaceae, Arecaceae, Asparagaceae, Euphorbiaceae, Fabaceae, Lamiaceae, Myrtaceae, Portulacaceae, Solanaceae, and Zingiberaceae are species commonly grown by communities in tsunami areas for various needs or uses such as food crops, medicine, ornamental plants, and traditional ceremonies. Home gardens have contributed to increasing food security, social, cultural, health, and economic community (Du Toit et al., 2022 ; Galhena et al., 2013). Table 1 and Figure 4 shows that 63 families have potential as Aedes mosquito repellent plants. 16 families have potential as Aedes attractants. Analysis of the determination of the plant acting as a repellent or attractant based on the content of secondary metabolites obtained information from the literature study sought.
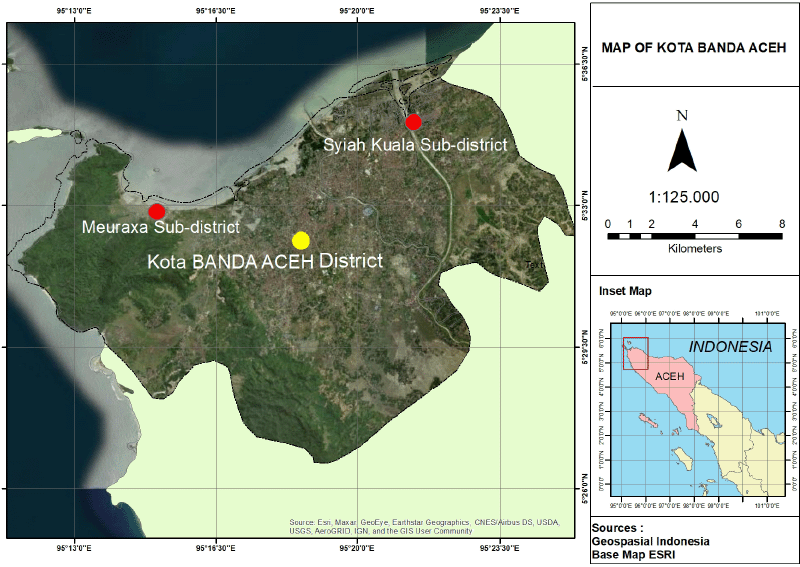 | Figure 1. Location of study area. [Click here to view] |
Potential of house yard plants as repellent of Aedes spp.
The repellants used by the public to prevent mosquito bites are synthetic repellants, one of which contains diethyltoluamide (DEET). These compounds can protect longer than other synthetic and botanical repellents. This synthetic active ingredient has health effects such as contact urticaria, skin eruptions, and encephalopathy. Plants around us have potential as insecticides, but it is necessary to identify bioactive molecules that have the effect of repelling or killing disease-transmitting vectors (Athuman et al., 2016).
Plant parts studied for their repellant content were the roots, stems, leaves, and flowers. Research on protecting from the bites of A. aegypti, Anopheles minimus Theobald, and Culex quinquefasciatus Say using essential oils showed different responses from mosquito species. The group of plant essential oils used was Zingiber cassumunar Roxb. (Zingiberaceae), Ocimum basilicum L. (Lamiaceae), and Cymbopogon nardus L. (Poaceae). These three essential oils are effective as repellents and food inhibitors against A. minimus, C. quinquefasciatus, and A. aegypti. However, the period of protection against A. Aegypti is lower than other mosquito species (Phasomkusolsil and Soonwera, 2010). The Z. cassumunar essential oil consists of sabinene, b-pinene, caryophyllene oxide, and caryophyllene (Bhuiyan et al., 2008). In the basil leaf extract of O. basilicum, the active compounds are flavonoids, saponins, tannins, and essential oils, which are considered toxic to mosquitoes (Ramayanti et al., 2017). The stems and leaves of citronella contain a toxin, and that substance can act as a repellant (Arcani et al., 2017). Essential oil Z. cassumunar was tested at several concentrations showing that the higher the concentration, the higher the activity to repel mosquitoes (Yulianis et al., 2018).
Volatile oils from four plant species Curcuma longa L. (Zingiberaceae), Citrus hystrix DC. (Rutaceae), Cymbopogon winterianus Jowitt, and Ocimum americanum added with 5% the vanillin showed a repellent effect against A. aegypti, Anopheles dirus Peyton & Harrison, and C. quinquefasciatus. The volatile oils of turmeric, lemongrass, and basils were able to repel the three mosquito types for 8 hours, while the kaffir lime oil was effective in repelling mosquitoes for up to 3 hours (Tawatsin et al., 2001). One of the plants that contain biologically active ingredients and can be used as an alternative controller is turmeric. The essential oils of turmeric can be used as natural insecticides to replace chemicals to kill mosquito larvae. In addition, the essential oil is also effective as a mosquito repellent for Aedes (Aseptianova, 2019). The essential oil content in kaffir lime leaves is citronellal, citronellol, linalool, and geraniol compounds (Munawaroh and Astuti, 2010). The largest components produced in citronella oil are citronellal, citronellol, and geraniol (Eden et al., 2018). The components of basil oil (O. americanum) are linalool, neral, citral, β-caryophyllene, α-humulene, and germacrene-d (Hapsari and Feroniasanti, 2019). The compounds contained in all the plants above have the potential and act as mosquito repellents and larvicides.
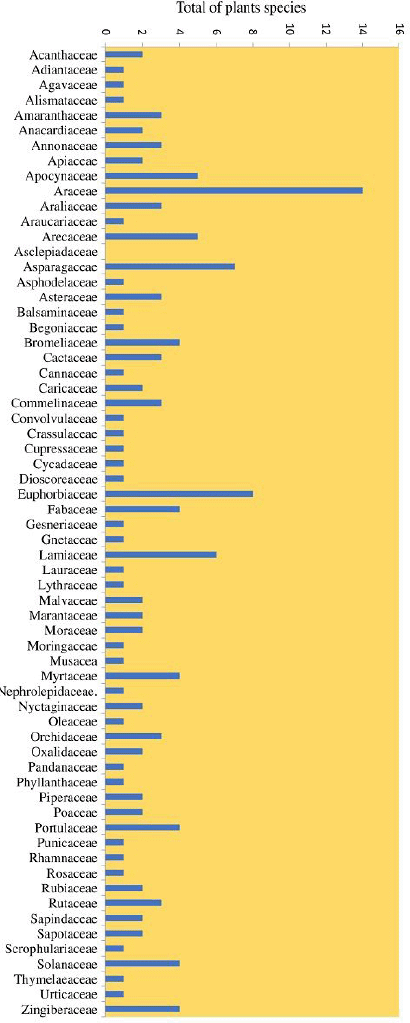 | Figure 2. Plant families found in study area. [Click here to view] |
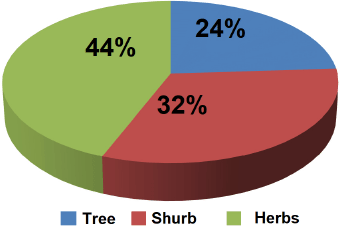 | Figure 3. Category of habitus plants in study are. [Click here to view] |
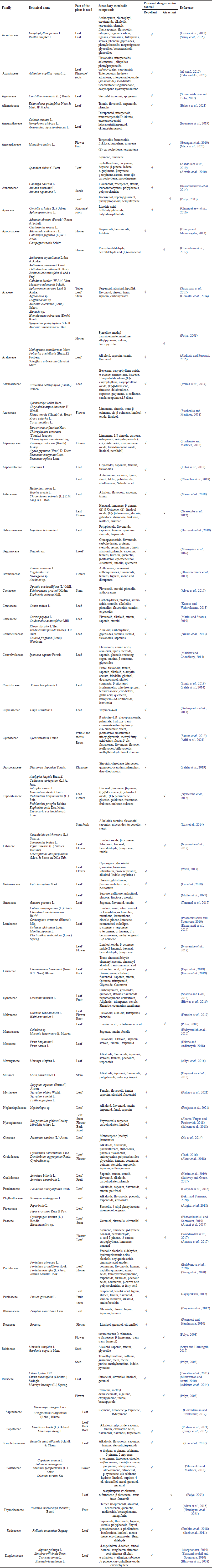 | Table 1. Families of plants that have the potential dengue vector control in the tsunami area settlement of Banda Aceh City. [Click here to view] |
 | Figure 4. Families of plants that have the potential dengue vector control in study area. [Click here to view] |
The community has traditionally used Tagetes minuta L. (Asteraceae) to repel mosquitoes. The essential oil from T. minuta showed the presence of limonene, camphene, and verbenone as the main constituents. The essential oil of T. minuta is effective in repelling mosquitoes (Athuman et al., 2016). Mentha piperita L. (Lamiaceae) oil has potential as a larvicidal and repellant of A. aegypti (Manh and Tuyet, 2020). The M. piperita oil contains pulegone, menthone, menthol, carvone, 1,8-cineole, limonene, and β-caryophyllene (Singh and Pandey, 2018). The study of the potential of O. Americanum and Blumea alata (D.Don) DC. (Asteraceae) extracts as a source of mosquito repellent showed that O. Americanum gave 100% repellency for 1.5 hours, B. alata for 2 hours, and a mixture of O. Americanum and Blumea alata for 2.5 hours. The O. Americanum extract contains linalool, neral, citral, isocaryophyllene, and humulent, while B. alata contains terpinene-4-ol, germacrene-D, sabinene, and terpinene. The compound components contained in both types of plants potentially have mosquito protection power (Kazembe et al., 2012). The addition of the concentration of Evodia suaveolens Scheff (Rutaceae) essential oil increases the protection power as a repellant. The addition of 1.5 ml of E. suaveolens essential oil has 81% protection against A. aegypti. The ingredients in Evodia leaves are linalool, and pinene can repel mosquitoes such as A. aegypti, which causes DHF (Simaremare et al., 2017).
The infusion of the leaves of the fragrant Pandanus amaryllifolius Roxb. (Pandanaceae) has the power to repel the laying of the eggs of the Aedes spp. The optimum concentration effective for repelling mosquito eggs is in the range of 4.5 to 5 ml/l. Pandanus leaves have a fragrant aroma that affects preventing oviposition against Aedes spp. The contents of compounds in Pandanus are alkaloids, saponins, flavonoids, tannins, and polyphenols (Cahyadi et al., 2016). Illicium verum Hook.f. (Illiciaceae) contains an essential oil that can be used as a repellent of A. aegypti. The results showed that the clove flower essential oil at concentrations of 10%, 20%, 30%, 40%, and 50% was able to protect against the bites of A. aegypti for 1–2 hours. The contents of the essential oil of the clove flower are cineole, linalool, and limonene. The clove flower extract contains the linalool compound that has mosquito repellent properties from the distinctive aroma it produces. The linalool compound is a kind of stable alkali. The clover flower oil often is used as a fragrance for soaps and perfumes. Mosquitoes do not like the aroma of the clove flower essential oil and linalool compounds because they cause irritation to the mosquito’s body parts and damage the mosquito’s nervous system (Lestari et al., 2019). The Pogostemon cablin Benth (Lamiaceae) oil has major (patchouli alcohol) and minor (patchoulen, guaien, sychellen, and caryophyllene) components. These minor components can potentially act as repellants or as attractants to insects. The activity of Culex sp. using patchouli oil showed that the repellency activity had better protection than synthetic DEET (Nidianti et al., 2014).
Insect bioassay results showed that the essential oil and extract of Nepeta parnassica Heldr & Sart (Lamiaceae) were highly active against Aedes cretinus Edwards and Culex pipiens L. The protective power of N. parnassica extracts against A. cretinus was for 3 hours, while for C. pipiens the protective power was up to 2 hours after application. Analysis essential oil N. parnassica, dominated by oxygenated monoterpenes, 4aα,7α,7aβ-nepetalactone, 1,8-cineole, dichloromethane-methanol, and 4aα,7β,7aβ-nepetalactone as the main constituents. The content of dichloromethane-methanol and 4aα,7α,7aβ-nepetalactone isolated from N. parnassica showed very high mosquito repellency for at least 2 hours against both types of mosquitoes. This study demonstrated the potential use of essential oil extracts, especially dichloromethane-methanol and 4aα,7α,7aβ-nepetalactone N. parnassica, as control agents for A. cretinus and C. pipiens (Gkinis et al., 2014).
The Angelica sinensis Oliv. (Apiaceae) extract has potential as a repellent against female A. aegypti. The results of the GC-MS analysis revealed that the A. sinensis extract contains at least 21 phytochemical compounds, and the main constituent is 3-N-butylphthalide. The protective power of the A. sinensis extract provides an average protection time of 2.0–6.5 hours against A. aegypti. The combination of A. sinensis extracts with 5% vanillin can increase to 4.0–8.5 hours (Champakaew et al., 2016).
Potential of houses yard plants as attractant of Aedes spp.
One of the effective biological control strategies is necessary to do by finding and identifying attractant compounds produced by plants. Attractive flowers, intense aromas, and nectar content need to find metabolites that attract or repel mosquitoes (Peach and Gries, 2020). If plant-based chemicals can be identified, especially those from plants that are attractive to mosquitoes, these plants can serve as bait in mosquito control and surveillance programs (Nyasembe et al., 2012). Each mosquito species has a particular preference for plant sources of nutrients. Mosquitoes can detect general and plant-specific chemical cues within their ecological range. The ability of mosquitoes to detect chemical compounds in certain plants will find suitable host plants for them. The interaction of mosquitoes with plants provides information on mosquito control strategies that target plant-eating behavior like attractive toxic sugar baits and the resulting odor (Nyasembe et al., 2018).
The volatile compound released by the host plant is attractive to mosquitoes. This compound attracts both male and female mosquitoes. Mosquitoes prefer volatile compounds produced by plants; for example, A. gambiae can detect certain chemical compounds from plants (Pachuwah, 2016). The visual appearance of flowers and the volatile compounds released by them are cues for mosquitoes to distinguish and locate host plants. Some species of mosquitoes, such as A. gambiae, C. Pipiens, and A. aegypti, can detect and respond to certain compounds from plants and detect and respond to volatile compounds from plants. Flower volatile organic compounds are mainly composed of four chemical groups: aromatics, monoterpenes, sesquiterpenes, and fatty acid derivatives (Yu et al., 2015).
Female A. aegypti prefer ovitrap with jenu [Derris elliptica (Wall.) Benth.] leaf extract to lay their eggs compared to other ovitraps. This plant from the Fabaceae family has the potential to be an attractant to A. aegypti in the oviposition process. Methyl eugenol compounds such as sex pheromones are effective at attracting insects and influencing insect behavior, such as searching for a mate, searching for food, and laying eggs. Visual and olfactory integration affects oviposition search media behavior, but the olfactory signal is more influential than visuals. The olfactory organ of the mosquito is the sensilla (hair), and these spread all over its body surface. Sensilla are mostly in many mosquito antennae, and this organ is sensitive to the smell of chemical compounds (Wibowo and Astuti, 2015).
Analysis of the extract of Silene otitis L. (Caryophyllaceae) using gas chromatography-mass spectrometry identified 35 compounds. Most of the extract compounds are monoterpenoids, fatty acid derivatives, and benzene. Phenyl acetaldehyde was the most dominant compound found in S. otites flowers. The test results of a mixture of S. otites flower aroma extract compounds on male and female Cx. pipiens showed different responses. Oxide compounds linalool (furanoids) and linalool showed strong responses in male and female mosquitoes. The compound (Z)-3-hexenyl acetate had positive responses only from female mosquitoes. Male mosquitoes showed moderate responses to compound (Z)-3-hexenyl acetate. Female mosquitoes have a moderate reaction to benzaldehyde and methyl salicylate compounds. Meanwhile, the lilac aldehyde, lilac alcohol, and linalool oxide (pyranoid) compounds had moderate responses from both sexes of mosquitoes (Jhumur et al., 2008).
The extract Asclepias syriaca L. (Asclepiadaceae) showed significant orientation of male and female Cx. pipiens. The mixture compounds of benzaldehyde, phenylacetaldehyde, and (E)-2-nonenal most attracted mosquito responses. Therefore, we recommend further research to examine the potential use of synthetic floral scent mixtures for monitoring or controlling disease-transmitting mosquitoes (Otienoburu et al., 2012). The maize/Zea mays L. (Poaceae) crop contributes to the prevalence of malaria mosquitoes and exacerbates malaria transmission in sub-Saharan Africa. Pollen from corn serves as a food source for Anopheles larvae and imago. Female mosquitoes can detect breeding sites where corn pollen is abundant. The Anopheles mosquito uses olfactory cues to locate, distinguish, and select breeding sites by utilizing volatile compounds to guide it. The pollen is a source of energy and attractant mosquitoes. Pollen contains pinene, limonene, p-cymene, nonanal, and benzaldehyde compounds (Wondwosen et al., 2017).
The selections of the oviposition site strongly influence the reproductive success and population dynamics of Anopheles, a vector for malaria in female mosquitoes. Mosquitoes choose oviposition sites at different spatial scales, starting with selecting the habitat to search. Anopheles arabiensis Patton larvae were the most common species found in various grassy habitats. The highest larva density in habitats was found overgrown by Echinochloa pyramidalis (Lam.) Hitchc. & Chase (Poaceae). This condition caused the volatile compounds of E. pyramidalis grass to be more attractive than Typha (Typhaceae) and Cyperus (Cyperaceae). The preference is shown by Anopheles coluzzii Coetzee & Wilkerson and A. arabiensis prove volatile grass compounds in larval habitat vegetation have an effect in the selection of oviposition sites (Asmare et al., 2017).
CONCLUSION
This study shows that various house yard plants have secondary metabolites that have the potential to control adult Aedes. Plants in the tsunami settlement area of Banda Aceh City contain secondary metabolites that function as repellents and attractants of adult Aedes. However, further testing is necessary in the laboratory to ensure Aedes’ preference for plants in the yard and the secondary metabolite content of each plant. This research information can be an alternative to Aedes control and elimination. Plants in the house yard in the tsunami settlement area of Banda Aceh City have the potential to be used as a strategy for controlling disease-transmitting vectors.
ACKNOWLEDGMENTS
The authors express their deepest gratitude to the volunteer team “Jumantik instar 3 UINAR” and the residents of the Asoe Nanggroe Village, Meuraxa Subdistrict, and Rukoh Village, Syiah Kuala Subdistrict, in the tsunami area of Banda Aceh City.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
This research was supported by the MoRA Scholarship for Islamic Higher Education (MoRA -SIHE).
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abarca-vargas R, Petricevich VL. Bougainvillea genus?: a review on phytochemistry, pharmacology, and toxicology. eCAM, 2018: 1–17. CrossRef
Adrianto H, Yotopranoto S, Hamidah H. Effectivity of kaffir lime (Citrus hystrix), nasnaran Mandarin (Citrus amblycarpa), and pomelo (Citrus maxima) leaf extract against Aedes aegypti larvae. Aspirator J, 2014; 6(1):1–6. CrossRef
Afifi N, Moawad A, Hassan M, Al Amir D, Elwekeel A, Amin E. Phytochemical content and biological activity of the genus Cycas, family Cycadaceae?: a review. Pharm Sci Asia, 2021; 48(4):300–19. CrossRef
Agustina E, Leksono AS, Gama ZP, Yanuwiadi B. Analysis of climatic variability and dengue hemorrhagic fever incidence at the Tsunami Area Banda Aceh City. IOP Conf Ser Earth Environ Sci, 2021; 948(1):012076; doi:10.1088/1755-1315/948/1/012076 CrossRef
Agustina E, Sari W, Ofreza A. The preferred plant by Aedes in houseyard of Kopelma Village Banda Aceh. Aspirator J, 2019; 11(1):59–66. CrossRef
Ahdiyah I, Purwani KI. Pengaruh ekstrak daun mangkokan (Nothopanax scutellarium) sebagai larvasida nyamuk Culex sp. J Sain Seni ITS, 2015; 4(2):32–6.
Akter M, Huda MK, Hoque MM. Investigation of secondary metabolites of nine medicinally important orchids of Bangladesh. JPP, 2018; 7(5):602–6.
Al-Snafi AE. The chemical constituents and pharmacological effects of Adiantum capillus-veneris—a review. Asian J Pharm Sci Technol, 2015; 5(2):106–111.
Alara O, Alara J, Olalere O. Review on Phaleria macrocarpa pharmacological and phytochemical properties. Drug Des, 2016; 5(3):1–5. CrossRef
Alighiri D, Cahyono E, Eden WT, Kusuma E, Supardi KI. Study on the improvement of essential oil quality and its repellent activity of betel leaves oil (Piper betle L .) from Indonesia. Orient J Chem, 2018; 34(6): 2913–2926. CrossRef
Aliyu A, Chukwuma UD, Omoregie EH, Folashade KO. Qualitative phytochemical analysis of the leaf of Moringa oleifera Lam. from three climatic zones of Nigeria. J Chem Pharm Res, 2016; 8(8):93–101.
Alves FAL, Andrade AP, Bruno RLA, Silva MGV, Souza MFV, Santos DC. Seasonal variability of phenolic compounds and antioxidant activity in prickly pear cladodes of Opuntia and Nopalea genres. Food Sci Technol, 2017; 37(4):536–43. CrossRef
Alwala OJ, Wanzala W, Inyambukho RA, Osundwa EM, Ndiege IO. Characterization and evaluation of repellent effect of essential oil of Mangifera indica L. from Kenya. Jeobp, 2010; 13(1):85–96. CrossRef
Arcani NLKS, Sudarmaja IM, Swastika IK. Efektifitas ekstrak etanol serai wangi (Cymbopogon Nardus L.) sebagai larvasida Aedes aegypti. J Med Udayana, 2017; 6(1):1–4.
Asadollahi A, Khoobdel M, Ramazani AZ, Azarmi S, Mosawi SH. Effectiveness of plant ? based repellents against different Anopheles species?: a systematic review. Malar J, 2019; 18(436):1–20. CrossRef
Aseptianova. Pengaruh ekstrak daun kunyit (Curcuma longa Linn.) sebagai insektisida elektrik terhadap mortalitas nyamuk Culex sp. L. Pro-Life, 2019; 6(1):44–54. CrossRef
Asmare Y, Hill SR, Hopkins RJ, Tekie H, Ignell R. The role of grass volatiles on oviposition site selection by Anopheles arabiensis and Anopheles coluzzii. Malar J, 2017; 16(1):1–9. CrossRef
Athuman I, Innocent E, Machumi F, Augustino S. Repellency properties of oils from plants traditionally used as mosquito repellents in Longido district, Tanzania. IJMR, 2016; 3(2): 04–08.
Barredo E, DeGennaro M. Not just from blood: Mosquito nutrient acquisition from nectar sources. Tren in Parasitol J, 2020; 36(5):473–84. CrossRef
Balabanova V, Hristov I, Zheleva-dimitrova D, Sugareva P, Lozanov V, Gevrenova R. Bioinformatic insight into Portulaca oleracea L. (Purslane) of Bulgarian and greek origin. Acta Biol Cracov Ser Bot, 2020; 62(1):1–16.
Behera T, Mounika M, Ratnakar M, Choudhari SS, Madhuri V, Kumar S. Antibacterial activity of Butomopsis latifolia?: a plant of sand dune. In: Devi RS, Mahanti P, Kumar S (eds.). Medico biowealth of India, Vol. IV. Ambika Prasad Research Foundation, Odisha, India, pp 62–9, 2021.
Bhuiyan MNI, Chowdhury JU, Begum J. Volatile constituents of essential oils isolated from leaf and rhizome of Zingiber cassumunar Roxb. Bangladesh J Pharmacol, 2008; 3(2):69–73. CrossRef
Biswas KK, Sharmin N, Rabbi MA. Evaluation of insecticidal activity of Lawsonia inermis Linn. against the red flour beetle, Tribolium castaneum (Herbst). NPAIJ, 2016; 12(1): 8–11.
Boate UR, Abalis OR. Review on the bio-insecticidal properties of some plant secondary metabolites: types, formulations, modes of action, advantages and limitations. AJRIZ, 2020; 3(4):27–60. CrossRef
BPS. Master file wilayah Provinsi Aceh 2019. BPS, Aceh, Indonesia, 2019.
Cahyadi A, Wahdaningsih S, Natalia D. Daya tolak infusa daun pandan wangi (Pandanus amaryllifolius Roxb.) terhadap peletakan telur nyamuk Aedes spp. JFFI, 2016; 1(2):65–71. CrossRef
Champakaew D, Junkum A, Chaithong U, Jitpakdi A, Riyong D, Wannasan A, Intirach J, Muangmoon R, Chansang A, Tuetun B, Pitasawat B. Assessment of Angelica sinensis (Oliv.) diels as a repellent for personal protection against mosquitoes under laboratory and field conditions in northern Thailand. Parasit Vectors, 2016; 9(373):1–14. CrossRef
Choudhri P, Rani M, Sangwan RS, Kumar R, Kumar A, Chhokar V. De novo sequencing, assembly and characterisation of Aloe vera transcriptome and analysis of expression profiles of genes related to saponin and anthraquinone metabolism. BMC Genom, 2018; 19(427):1–21. CrossRef
Cunze S, Kochmann J, Koch LK, Klimpel S. Niche conservatism of Aedes albopictus and Aedes aegypti—two mosquito species with diferent invasion histories. Sci Rep, 2018; 8(7733):1–10. CrossRef
Dhivya R, Manimegalai K. Mosquito repellent activity of Calotropis gigantea (Apocynaceae) flower extracts against the filarial vector Culex quinquefasciatus. Hygeia J, 2013; 5(2):56–62.
Dormon L, Mulatier M, Carassco D, Cohuet A. Mosquito attractants. J Chem Ecol, 2021; 47(4-5): 351–393. CrossRef
Du Toit MJ, Rendón O, Cologna V, Cilliers SS, Dallimer M. Why home gardens fail in enhancing food security and dietary diversity. Front Ecol Evol, 2022; 10(804523):1–13. CrossRef
Eden WT, Alighiri D, Cahyono E, Supardi KI, Wijayati N. Fractionation of Java citronella oil and citronellal purification by batch vacuum fractional distillation. IOP Conf Ser Mater Sci, 2018; 349(12):012067; doi:10.1088/1757-899X/349/1/012067 CrossRef
Ervina M, Han LS, Diva J, Caroline TS, Tewfik I. Optimization of water extract of Cinnamomum burmannii bark to ascertain its in vitro antidiabetic and antioxidant activities. Biocatal Agricul Biotech, 2019; 19(101152):1–7. CrossRef
Fajar A, Ammar GA, Hamzah M, Manurung R, Abduh MY. Effect of tree age on the yield, productivity, and chemical composition of essential oil from Cinnamomum burmannii. CRBB, 2019; 1(1):17–22. CrossRef
Ferreira MDL, Fernandes DA, Nunes FC, Teles YCF, Rolim YM, Silva CM, Albuquerque JBL, Souza MFV. Phytochemical study of Waltheria viscosissima and evaluation of its larvicidal activity against Aedes aegypti. Rev bras farmacogn, 2019; 29(5):582–90. CrossRef
Fikri F, Purnama MTE. Pharmacology and phytochemistry overview on Sauropus androgynous. Sys Rev Pharm, 2020; 11(6):124–8. CrossRef
Gadeng AN, Nandi, Hafizul FM. The Development of Settlement in the Tsunami Red Zone Area of Banda Aceh City. KnE Soc Sci, 2019; 1–13.
Galhena DH, Freed R, Maredi KM. Home gardens?: a promising approach to enhance household food security and wellbeing. Agricul Food Secur, 2013; 2(8):1–13. CrossRef
Giatropoulos A, Pitarokili D, Papaioannou F, Papachristos DP, Koliopoulos G, Emmanouel N, Tzakou O, Michaelakis A. Essential oil composition, adult repellency and larvicidal activity of eight Cupressaceae species from Greece against Aedes albopictus (Diptera?:Culicidae). Parasitol Res, 2013; 112:1113–23. CrossRef
Gkinis G, Michaelakis A, Koliopoulos G, Ioannou E, Tzakou O, Roussis V. Evaluation of the repellent effects of Nepeta parnassica extract, essential oil, and its major nepetalactone metabolite against mosquitoes. Parasitol Res, 2014; 113(3):1127–34. CrossRef
Gomathi R, Indrakumar I, Karpagam S. Larvicidal activity of Monstera adansonii plant extracts against Culex quinequefaciatus. JPP, 2014; 3(1):160–2.
Gouagna LC, Poueme RS, Dabiré KR, Ouédraogo JB, Fontenille D, Simard F. Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera: Culicidae). J Vector Ecol, 2010; 35(2): 267–276. CrossRef
Govindarajan M, Sivakumar R. Repellent properties of Cardiospermum halicacabum Linn. (Family?: Sapindaceae) plant leaf extracts against three important vector mosquitoes. Asian Pac J Trop Biomed, 2012; 2(8): 602–607. CrossRef
Handayani R, Purnamasari W, Mun’im A. Optimization of ionic liquid-microwave assisted extraction method of Mahkota Dewa (Phaleria macrocarpa (Scheff.) Boerl.) fruit pulp. J Appl Pharm Sci, 2021; 11(02):059–65.
Hapsari IP, Feroniasanti YML. Phytochemical screening and in vitro antibacterial activity of sweet basil leaves (Ocimum basilicum L.) essential oil against Cutibacterium acnes ATCC 11827. AIP Conf Proc, 2019; 2099(1):020007; doi:10.1063/1.5098412 CrossRef
Hariyanto, Fajriaty I, Wijaya T, Hafizh, M. The potential ethnomedicine plant of Impatiens balsamina leaves from Pontianak, West Kalimantan, Indonesia for wound healing. Nusantara Biosci, 2018; 10(1):58–64. CrossRef
Hasim, Arifin YY, Andrianto D, Faridah DN. Ethanol extracts of Averrhoa bilimbi leaf demonstrated anti-inflammatory activity. JATP, 2019; 8(3):86–93. CrossRef
Hidayatullah, Anam S, Tandah MR. Chemical compounds profile and antibacterial activity of methanolic extract of bamban (Donax canniformis (G.Forst.) K.Schum.) leaf against Staphylococcus aureus. Galenika, 2015; 1(2):141–8. CrossRef
Hikma SR, Ardiansyah S. Combination of kelor leaf extract (Moringa oleifera Lamk) with tin leaf extract (Ficus carica Linn) as larvasida on Aedes aegypti larva. Medicra, 2018; 1(2):94–102.
Ibrahim M, Rehman K, Razzaq A, Hussain, I, Farooq T, Hussain A, Akash MSH. Investigations of phytochemical constituents and their pharmacological properties isolated from the genus Urtica?: critical review and analysis. CRE, 2018; 28(1):22–66. CrossRef
Idris M, Mudi S, Datti Y. Phytochemical screening and mosquito repellent activity of the stem bark extracts of Euphorbia Balsamifera (Ait ). CSJ, 2014; 5(2):46–51.
Iwuagwu MO, Ogbonna NC, Okechukwu UH. Insecticidal effects of some plant leaf extracts in the control of insect field pests of Amaranthus hybridus L. Int J Plant Sci Hor, 2019; 1:71–9. CrossRef
Jayaprakash A. Punica granatum?: a review on phytochemicals, antioxidant and antimicrobial properties. JAIR, 2017; 5(9):132–8.
Jhumur US, Dötterl S, Jürgens, A. Floral odors of Silene otites: their variability and attractiveness to mosquitoes. J Chem Ecol, 2008; 34(1):14–25. CrossRef
Kanase V, Vishwakarma S. Treatment of various diseases by Canna indica L. a promising herb. Asian J Pharm Clin Res, 2018; 11(12):51–6. CrossRef
Kazembe T, Chaibva M, Nkomo S. Evaluation of mosquito repellence of Ocimum americanum and Blumea alata plants. J Sci Res Pharm, 2012; 1(2):55–7.
Lestari E, Wahyudi BF, Ustiawan A, Dewi DI . Potency of star anise (Illicium verum) essential oil as Aedes aegypti mosquito repellent. Balaba, 2019; 15(1):13–22. CrossRef
Lestari P, Khumaida N, Sartiami D, Mardiningsih TL. Selection criteria of Graptophyllum pictum resistance to Doleschallia bisaltide cramer (Lep?: Nymphalidae ) attack based on insect feeding preference. SABRAO J Breed Genet, 2015; 47(2):172–84.
Liu J, Moyankova D, Dijilianov D, Deng X. Common and specific mechanisms of desiccation tolerance in two Gesneriaceae resurrection plants multiomics evidences. Front Plant Sci, 2019;10 (1067):1–6. CrossRef
Lubis R, Ilyas S, Panggabean M. The effectivity test of Aloe vera leaf extract to larvae Aedes sp. Asian J Pharm Clin Res, 2018; 11(7):262–6. CrossRef
Malakar C, Choudhury PPN. Pharmacological potentiality and medicinal uses of Ipomea aquatica Forsk?: a review. Asian J Pharm Clin Res, 2015; 8(2):60–3.
Manh HD, Tuyet OT. Larvicidal and repellent activity of Mentha arvensis L. essential oil against Aedes aegypti. Insects, 2020; 11(198):1–9. CrossRef
Marini, Ni’mah T, Mahdalena V, Komariah RH, Sitorus H. Repellent potency of Marigold ( Tagetes erecta L .) leaves extract againts Aedes aegypti mosquito. Balaba, 2018; 14(1):53–62. CrossRef
Marini, Sitorus H. Some plants potentially as repellent in Indonesia. Spirakel, 2019; 11(1):24–33.
Meza FC, Roberts JM, Sobhy IS, Okumu FO, Tripet F, Bruce TJA. Behavioural and electrophysiological responses of female Anopheles gambiae mosquitoes to volatiles from a mango bait. J Chem Ecol, 2020; 46:387–96. CrossRef
Ministry of Health Indonesia. Indonesia health profile 2005. Departemen Kesehatan RI, Jakarta, Indonesia, 2007.
Muller GC, Junnila A, Traore MM, Traore SF, Doumbia S, Sissoko F, Dembele SM, Schlein Y, Arheart KL, Revay EE, Kravchenco VD, Beier JC. The invasive shrub Prosopis juliflora enhances the malaria parasite transmission capacity of Anopheles mosquitoes: a habitat manipulation experiment. Malar J, 2017; 16(237):2–9. CrossRef
Muller J, Sprenger N, Bortlik K, Boiler T, Wiemken A. Desiccation increases sucrose levels in Ramonda and Haberlea, two genera of resurrection plants in the Gesneriaceae. Physiol J, 1997; 100:153–8. CrossRef
Munawaroh S, Astuti P. Ekstraksi minyak daun jeruk purut (Citrus hystrix D.C.) dengan pelarut etanol dan N-heksana. JKT UNNES, 2010; 2(1):73–8.
Murugesan S, Irulandi K, Siva V, Mehalingam P. A short review on ethnomedicinal uses, phytochemistry and pharmacology of Begonia malabarica Lam. Int J Bot Stud, 2016; 1(6):16–7. CrossRef
Nidianti E, Utomo EP, Himawan T. Studi interaksi molekul komponen minyak nilam dengan reseptor olfaktori sebagai repellent nyamuk Culex sp. secara in silico dan in vitro. J Ilmu Kimia UB, 2014; 1(2):227–33.
Nikam M, Mundada P, Kadam D, Jadhav S, Aparadh V. Comparative screening of various solvent for phytochemical testing using some commelinaceae members. Int Res J Pharm App Sci, 2013; 3(2):18–20.
Nyasembe V O, Cheseto X, Kaplan F, Foster WA, Teal PEA, Tumlinson JH, Borgemeister C, Torto B. The invasive American weed parthenium hysterophorus can negatively impact malaria control in Africa. PLoS One, 2015; 10(9):1–15. CrossRef
Nyasembe VO, Chouassi DP, Pirk CWW, Sole CL, Torto B. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Negl Trop Dis, 2018; 12(2):1–21. CrossRef
Nyasembe VO, Teal PE, Mukabana WR, Tumlinson JH, Torto B. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit Vectors, 2012; 5(234):1–11. CrossRef
Oliveira-Júnior RG, Ferraz CAA, Souza GR, Guimarães AL, Oliveira AP de, Lima-Saraiva SRG, Rolim LA, Rolim-Neto PJ, Almeida JRGS. Phytochemical analysis and evaluation of antioxidant and photoprotective activities of extracts from flowers of Bromelia laciniosa ( Bromeliaceae ). B & BE, 2017; 31(3):600–5. CrossRef
Onyenekwe PC, Okereke OE, Owolewa SO. Phytochemical screening and effect of Musa paradisiaca stem extrude on rat haematological parameters. Curr Res J Biol Sci, 2013; 5(1):26–9. CrossRef
Otienoburu PE, Ebrahimi B, Phelan PL, Foster WA. Analysis and optimization of a synthetic milkweed floral attractant for mosquitoes. J Chem Ecol, 2012; 38(7):873–81. CrossRef
Pachuwah P. The role of floral and fruit scent compounds as mosquito attractants?: developing new methods for monitoring mosquito populations. Thesis. KwaZulu-Natal University, Pietermaritzburg, South Africa, 2016.
Peach DAH, Gries G. Mosquito phytophagy–sources exploited, ecological function, and evolutionary transition to haematophagy. Entomol Exp Appl, 2020; 168:120–36. CrossRef
Phasomkusolsil S, Soonwera M. Insect repellent activity of medicinal plant oils against Aedes aegypti (Linn), Anopheles minimus (Theobald) and Culex quinquefasciatus say based on protection time and biting rate. Southeast Asian J Trop Med Public Health, 2010; 41(4):831–40.
Polya G. Biochemical targets of plant bioactive compounds?: A pharmacological reference guide to sites of action and biological effects. CRC Press, Boca Raton, FL, 2003. CrossRef
Pratiwi N, Retnosari R, Prabaningtyas S. Preliminary study on antibacterial activity of sawo kecik (Manilkara kauki ( L .) Dubard ) roots extract. J Biodjati, 2021; 6(1):146–52. CrossRef
Priyanka P, Bhatt S, Dhyani DS, Jain A. Phytochemical studies of the secondary metabolites of Ziziphus mauritiana Lam. leaves. Int J Curr Pharm Res, 2012; 4(3):153–5.
Rahayu SE, Dharmawan A, Putri VAL. Potential of Jamlang leaf extract (Syzigium cumini L.) as larvacide for control of Aedes aegypti mosquito larva. Biosaintropis, 2021; 6(2):26–33. CrossRef
Ramayanti I, Layal K, Pratiwi PU. Effectiveness test of basil leaf (Ocimum basilicum) extract as bioinsecticide in mosquito coil to mosquito Aedes aegypti death. AMS, 2017; 3(2):6–10. CrossRef
Ravaomanarivo LHR, Razafindraleva HA, Raharimalala FN, Rasoahantaveloniaina B, Ravelonandro PH, Mavingu P. Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus. Asian Pac J Trop Biomed, 2014; 4(10):798–806. CrossRef
Renjana E, Nikmatullah M, Firdiana ER, Ningrum LW, Angio MH. The Potential of Nephrolepis spp. as medicinal plant, a collection of Purwodadi botanical garden, based on ethnomedicine and phytochemical studies. BPN, 2021; 27(1):1–10. CrossRef
Riaz M, Rasool N, Bukhari IH, Shahid M, Zahoor F, Gilani MA, Zubair M. Antioxidant , antimicrobial and cytotoxicity studies of Russelia equisetiformis. AJMR, 2012; 6(27):5700–7. CrossRef
Rosnaeni MH, Hendranata KF. Reppelent effect of lavender, rose and rosemary oil on Aedes aegypti mosquitoes. Medika Planta, 2010; 1(1):67–74.
Saeb K, Kakouei A, Hajati RJ, Pourshamsian K, Babakhani B. Investigating the effect of height on essential oils of Urtica diocia L. (case study?: Ramsar, Mazandaran, Iran ). Orient J Chem, 2011; 27(4):1345–50.
Saleem H, Htar TT, Naidu R, Zengin G, Ahmad I, Ahemad N. Phytochemical profiling, antioxidant, enzyme inhibition and cytotoxic potential of Bougainvillea glabra flowers. Nat Prod Research, 2019:1–7. CrossRef
Saleh MM, Ghoneim MM, Kottb S, El-Hela AA. Biologically active secondary metabolites from Kalanchoe tometosa. JBPR, 2014; 3(6):136–40.
Salehi B, Sener B, Kilic M, Sharifi-Rad J, Naz R, Yousaf Z, Mudau FN, Fokou PVT, Ezzat SM, Bishbishy MHE, Taheri Y, Lucariello G, Durazzo A, Lucarini M, Suleria HAR, Santini A. Dioscorea plants?: a genus rich in vital nutra- pharmaceuticals—a review. IJPR, 2019; 18 (Special Issue): 68–89.
Samy MN, Sugimoto S, Matsunami K, Otsuka H, Kamel MS. Chemical constituents and biological activities of genus Ruellia. IJP, 2015; 2(6):270–9.
Santos VAN, Agoo EM, Shen C, Ragasa CY. Secondary metabolites from Cycas lacrimans. IJPCR, 2015; 7(5):356–9.
Sengül Demirak MS, Canpolat E. Plant-based bioinsecticides for mosquito control: impact on insecticide resistance and disease transmission. Insects, 2022; 13(162):1–24. CrossRef
Setya AK, Harningsih T. Pathological effect and repellent of Noni (Morinda citrifolia) seed extract toward dengue fever vector. IJMS, 2019; 6(1):1–5.
Sharma RK, Goel A. Identification of Phytoconstituents in Lawsonia inermis Linn. leaves extract by GC-MS and their antibacterial potential. Pharmacogn, 2018; 10(6):1101–8. CrossRef
Simaremare ES, Sinaga DI, Agustini V. Zodia soap as repellent against Aedes aegypti mosquitoes. PJI, 2017; 3(1):11–6. CrossRef
Simmons-boyce JL, Tinto W. Steroidal saponins and sapogenins from the Agavaceae. NPC, 2007; 2(1): 99–114. CrossRef
Singh SK, Patel JR, Dangi A. Physicochemical, qualitative and quantitative determination of secondary metabolites and antioxidant potential of Kalanchoe pinnata (Lam.) Pers. leaf extracts. JDDT, 2019; 9(1):220–4. CrossRef
Singh P, Pandey AK. Prospective of essential oils of the genus Mentha as biopesticides: a review. Front. Plant Sci, 2018; 9(1295):1–14. CrossRef
Singh V, Pandey VN, Shukla K. Quantitative estimation of secondary metabolites from Mimusops elengi L. IJSER, 2015; 5(7):13–5.
Stashenko E, Martinez JR. The expression of biodiversity in the secondary metabolites of aromatic plants and flowers growing in Colombia. In: El-Shemy H (ed.). Potential of essential oils. Intechopen, London, UK, pp 196–59, 2018. CrossRef
Suluvoy JK, Grace VMB. Phytochemical profile and free radical nitric oxide (NO) scavenging activity of Averrhoa bilimbi L. fruit extract. 3 Biotech, 2017; 7(85):1–11. CrossRef
Suparman A, Rupa D, Zulfadli. Identification of secretory structure and histochemical of family Araceae as medicinal plants by Dayak Kenyah Tribe. AST, 2017; 2(1):26–30.
Suryawan F. Study on condition of vegetation and physical condition of coastal area to support conservation effort in Nanggroe Aceh Darussalam. Thesis. IPB, Bogor, Indonesia, 2007.
Taha MA, Ali AAB. The first study for the acaricidal activity of alcoholic extracts of Adiantum capillus-veneris and Funaria hygrometric against Argas persicus. Egypt Acad J Biol Sci, 2020; 12(2):203–17. CrossRef
Tanamal MT, Papilaya PM, Smith A. Kandungan senyawa flavonoid pada daun melinjo (Gnetum gnemon L.) berdasarkan perbedaan tempat tumbuh. Biopendix, 2017; 3(2):142–7. CrossRef
Tawatsin A, Wratten SD, Scott RR, Thavara U, Techadamrongsin Y. Repellency of volatile oils from plants against three mosquito vectors. J Vector Ecol, 2001; 26(1):76–82.
Teoh ES. Secondary metabolites of plants. In: Teoh ES (ed.). Medicinal Orchid of Asia. Springer International Publishing, Cham, Switzerland, pp 559–59, 2016. CrossRef
Verma RS, Padalia RC, Goswani P, Verma SK, Chauhan A, Darokar MP. Chemical composition and anti-bacterial activity of foliage and resin essential oils of Araucaria cunninghamii Aiton ex D.Don and Araucaria heterophylla (Salisb.) Franco from India. Indus Crop Prod, 2014; 61:410–6. CrossRef
Wang Z, Yang R, Li P, Yang Z, Ling R, Shen T, Peng W, Yang Q, Yan J. A homoisoflavonoid and a fatty acid in common purslane (Portulaca oleracea L.) synergistically inhibit growth of Spodoptera litura larvae. Pest Manag Sci, 2020; 76:1513–22. CrossRef
Wibowo SG, Astuti EP. Oviposition preference of Aedes aegypti against various leaf extract as an atractant. Balaba, 2015; 11(01):23–8. CrossRef
Wink M. Evolution of secondary metabolites in legumes (Fabaceae). S Afr J Bot, 2013; 89:164–75. CrossRef
Wondwosen B, Hill SR, Birgersson G, Seyoum E, Tekie H, Ignell R. A(maize)ing attraction: gravid Anopheles arabiensis are attracted and oviposit in response to maize pollen odours. Malar J, 2017; 16(1):1–9. CrossRef
Xu P, Choo YM, Rosa ADL, Leal WS. Mosquito odorant receptor for DEET and methyl jasmonate. PNAS, 2014; 111(46):16592–7. CrossRef
Yu BT, Ding YM, Mo JC. Behavioural response of female Culex pipiens pallens to common host plant volatiles and synthetic blends. Parasit Vectors, 2015; 8(598):1–8. CrossRef
Yulianis, Dachriyanus, Putra AA. Uji aktifitas antinyamuk minyak atsiri sereh dapur dalam bentuk semprot. JIT, 2018; 12(1):78–83. CrossRef
Zahara M, Win CC. Review?: the effect of plant growth regulators on micropropagation of Aglaonema sp. JTHORT, 2020; 3(2):96–100. CrossRef