INTRODUCTION
Mangrove plants grow under stressful habitat conditions where marine and freshwater systems meet. They are able to adapt morphologically and survive steep temperature gradients and extreme conditions of salinity at the interface of sea and land. To survive in these complex conditions, these plants are endowed with unique and diverse classes of phytochemicals. The use of these plants in the treatment of various ailments may be attributed to the presence of bioactive phytochemicals and secondary metabolites such as phenolics, flavonoids, tannins, alkaloids, and saponins (Das et al., 2020; Gurudeeban et al., 2013; Sobolewska et al., 2020). Several mangrove plants are used in traditional medicine or insecticides and pharmaceuticals (Gajula et al., 2020; Premanathan et al., 1999; Ravikumar et al., 2011). Mangrove plants have been reported by a number of authors as potential sources of natural antioxidant and antimicrobial agents that could be used in medicines for the treatment of bacterial infections and cancer (Arulkumar et al., 2020; Eswaraiah et al., 2020a, 2020b). Therefore, the identification of the phytochemical constituents of bioactive components of these plants is necessary to predict the biological activities that may be exhibited by them (Vasanthi et al., 2014).
Several phytochemical surveys of mangrove plants have been published. For example, the major phytochemical substances of interest in these reports are polyphenols and tannins (Das et al., 2020). Other naturally occurring significant level substances, like alkaloid and saponin groups, have also been reported (Aberoumand, 2012; Samatha et al., 2012). In medicine, the antioxidant, anticancer, and anti-inflammatory activities of saponins are used to treat hypercholesterolemia (Haslam et al., 1989) as well as cardiovascular diseases (Samatha et al., 2012), and saponins have also been used to facilitate antibody access to intracellular proteins (Gowri and Vasantha, 2010). Although many studies on bioactivity have been performed in the last few decades (Ahmad et al., 2013; Arulkumar et al., 2020; Eswaraiah et al., 2020b; Gajula et al., 2020; Hong et al., 2011; Loo et al., 2007; Premanathan et al., 1999; Rahim et al., 2008; Saad et al., 2011; Sulaiman et al., 2011), relatively fewer reports have been published on the antibacterial potential of mangrove plant in inhibiting the growth of aquatic pathogenic bacteria.
Rhizophora mucronata and Rhizophora apiculata are mangrove trees belonging to the family Rhizophoraceae. They generally grow in tropical and subtropical areas. When applied against various animal, plant, and human pathogens, the extracts of different parts of both plants exhibited antiviral (Vijayavel et al., 2006), antibacterial (Ahmad et al., 2013), and antioxidant (Loo et al., 2007; 2008; Rahim et al., 2008) activities. These activities were derived from phytochemical or bioactive substances, including polyphenols (Hong et al., 2011; Sulaiman et al., 2011). Present in several parts of the plants, these substances combine with proteins to form stable complexes and antioxidants characterized by free radical scavenging activity (Szyd?owska-Czerniak et al., 2008). Besides polyphenols, other phytochemicals presented various properties already mentioned above. However, the antibacterial activities of these two plants with various parts against aquatic pathogens have not been investigated.
Due to the lack of research in this area, aquatic pathogens are susceptible to diseases caused by many different bacteria such as Streptococcus agalactiae, Aeromonas hydrophila, Vibrio harveyi, and Vibrio parahemolyticus. As a result of this point, synthetic chemicals or synthesized antibiotics are generally used to combat pathogenic bacteria. Thus, finding bioactive substances in plants could provide essential data for the development of novel bioactive agents in order to use them as an alternative replacement for synthetic chemicals against bacterial pathogens in aquaculture and future application in the pharmaceutical industry. This work aims to study phytochemical analyses and antioxidant activities in the pod, leaf, twig, and bark of R. mucronata and R. apiculata and to evaluate the correlation between the plants and plant parts and three bioactive components (saponin, phenolic, and flavonoid) against aquatic pathogens.
MATERIALS AND METHODS
Plant materials
The fresh pods, leaves, twigs, and bark of R. mucronata and R. apiculata were collected in November 2015 from Trang Province, Thailand (7°52?56.2″N 99°32?75.8″E for R. mucronata and 7°52?29.5″N 99°31?07.7″E for R. apiculata), as shown in Figure 1. The specimen voucher (BKF 194808 for R. mucronata and BKF.194836 for R. apiculata) was deposited at Bangkok Forest Herbarium, Department of National Parks, Wildlife and Plant Conservation, Thailand.
Extraction procedure
Each part collected from R. mucronata and R. apiculata was prepared by drying for 10 d and then reduced mechanically. The dried sample of 1,000 g pod, leaf, twig, and bark was soaked in a sealed container for 5 days with methanol in the ratio of 1:4 (w/v) (Vittaya et al., 2020b). The resulting extracts were filtered and concentrated using a rotary evaporator at 45oC. All extracts were refrigerated until used for analysis, and the qualitative and quantitative analyses of the major contents of phytochemical compounds and antibacterial activity were studied.
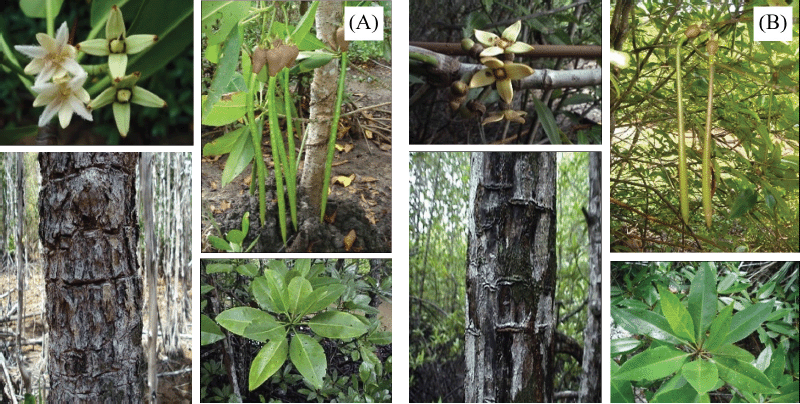 | Figure 1. Each part of R. mucronata (A) and R. apiculata (B). [Click here to view] |
Phytochemical analysis
Qualitative analysis
The major components of the secondary metabolite were searched in the crude extract (CE) using qualitative analysis based on precipitation and coloring reactions. All procedures were examined using the standard method described by Vittaya et al. (2020a). Briefly, saponin was determined by the formation of a stable form (fourth test). The appearance of the dark green extract after the addition of ferric chloride indicated the existence of phenolic composition. Flavonoid was indicated by the reaction of metal and the appearance of yellow. Borntrager’s test was used to determine anthraquinone, the rose-pink color that occurred in the ammonia layer. Terpenoid and alkaloid were tested by using Salkowski’s test and Dragendroff’s solution, respectively. The reddish-brown ring occurred at the junction of two layers, indicating the presence of terpenoid. The appearance of orange-yellow precipitates confirmed the presence of alkaloid.
Quantitative analysis
Determination of saponin content
The saponin content (SC) of each R. mucronata and R. apiculata extract was determined according to the method described by Senguttuvan et al. (2014), with a modification to the procedure that reduced the volume of the extract solution. Briefly, 0.2 mL of extract was mixed with 0.5 ml of a 0.8% (w/v) vanillin solution, 5 ml of 72% (v/v) sulphuric acid was added, and the mixture was allowed to stand for a 1 min. After standing, the mixture was incubated at 70°C for 10 minutes and rapidly cooled with ice water to room temperature. The absorbance of the mixture was measured at 560 nm using UV-vis spectroscopy (U-1800 Spectrophotometer, Hitachi High-Tech Science Corp., Tokyo, Japan). Methanol and escin were used as a control and standard, respectively. SC was expressed as milligram of EE/g CE through the calculation curve of escin (y = 0.0006x + 0.0284). The experiment was performed in triplicate, and the obtained data were expressed as the means ± standard deviation with linearity R2 equal to 0.9931.
Determination of phenolic content
The total phenolic content was determined using the Folin–Ciocalteu reagent by the method described by Vittaya et al. (2019). Briefly, a volume of 0.2 ml of eight methanolic extracts or gallic acid was added to a centrifuge tube, followed by 2.5 ml of distilled water. After 0.2 ml of the Folin–Ciocalteu reagent was added, 2.0 ml of a 7% sodium carbonate solution was added. Immediately after that, each sample was vigorously shaken by a vortex mixer. It was kept away from light for 60 minutes. The absorbance of the standard and all extracts was measured at 765 nm against the blank. Total phenolic content (TPC) was expressed as milligram of gallic acid equivalent per gram CE through the calibration curve of gallic acid (y = 0.004x + 0.0028) with linearity R2 equal to 0.9995.
Determination of flavonoid content
The total flavonoid content of all extracts of R. mucronata and R. apiculata was measured by the colorimetric method of aluminum chloride, based on the method described previously by Vittaya et al. (2019). Briefly, a volume of 0.2 ml of each sample or rutin was added to a centrifuge tube. After 0.5 ml of a 5% sodium nitrite solution was added, the reaction mixture was left to stand at room temperature for 6 minutes. Two hundred microliters of aluminum chloride (10%) was then added, followed by 0.5 ml of 1 M sodium hydroxide. The total volume was made up to 1.5 ml with distilled water. The reaction mixture was mixed well again, and the absorbance was recorded against a blank at 510 nm. Total flavonoid content (TFC) was expressed as milligram of rutin equivalent per gram of CE through the calibration curve of gallic acid (y = 0.001x − 0.004) with linearity R2 equal to 0.9990.
Antioxidant activity
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical free radical scavenging
The DPPH method was conducted using spectroscopy to determine activity based on the method described by Vittaya et al. (2020b). Briefly, the stock solution of extracts or standard 1 mg/mL was prepared, and 0.5 ml was then mixed with 0.5 ml of a 0.15 M DPPH solution. The reaction mixture was incubated to stand at room temperature away from light for 30 minutes. Their absorbance was measured at 517 nm against the blank. The percentage of free radical scavenging activity of the sample was calculated as follows:
DPPH radical scavenging (%) = [1– (Asample–Asample blank)/Acontrol] ×100, (1)
where Asample is the absorbance of the test sample with DPPH solution, Asample blank is the absorbance of the test sample only, and Abscontrol is the absorbance of the DPPH solution.
2,2/-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical free radical scavenging
The ABTS assay was examined according to the method of Vittaya et al. (2020(b)). Briefly, the dilution of the ABTS+ working solution that gave an absorbance of 0.700 ± 0.025 units at 734 nm was prepared. The 0.1 ml of standard or sample extract was added to a centrifuge tube, and then 0.9 ml of the diluted ABTS+ working solution was added to each test tube. The reaction mixture was incubated for 6 min at room temperature. After incubation time, the absorbance at 734 nm was measured. The percentage of free radical scavenging activity of the sample tested was calculated as follows:
ABTS radical scavenging (%) = [(Acontrol –Asample)/Acontrol] ×100, (2)
where Acontrol is the absorbance of the extract without ABTS+ solution and Asample is the absorbance of the extract with ABTS+ solution.
Antibacterial investigation
The antibacterial activities of the pod, leaf, twig, and bark extracts (n = 4) of R. mucronata and R. apiculata were tested against the Gram-positive strain S. agalactiae SAAQ001 (derived from Kasetsart University) and the Gram-negative strains A. hydrophila AHAQ001 and V. harveyi VHAQ001 (derived from Kasetsart University) and V. parahemolyticus (derived from Songkhla Aquatic Animal Health Center, Thailand). The hole-plate diffusion method was conducted according to the method of Brantner et al. (1994), with slight modification. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the extracts were determined using the standard method (Eloff, 1998; National Committee for Clinical Laboratory Standard, 2000) as outlined by Vittaya et al. (2020b).
Statistical analysis
The results were expressed as mean ± standard deviation in triplicate for chemical analysis and quadruplet for antibacterial activity. Statistical analysis was performed by one-way analysis of variance, followed by Duncan’s comparison. A two-way analysis of variance was used to evaluate the interaction between the two plants (R. mucronata and R. apiculata) with four parts (pod, leaf, twig, and bark). The correlation between the phytochemical composition and free radical scavenging activity was carried out using Pearson’s analysis.
RESULTS AND DISCUSSION
Yield and phytochemical analysis
Yield and phytochemical screening
The yields of the pod, leaf, twig, and bark of R. mucronata were obtained as 14.42% (216.25 g), 29.95% (299.53 g), 15.37% (153.73 g), and 19.15% (191.52 g), respectively. For R. apiculata, the yields of the pod, leaf, twig, and bark were 12.57% (125.74 g), 16.81% (168.06 g), 9.81% (98.14 g), and 17.24% (172.39 g), respectively. Phytochemical screening of the pod, leaf, twig, and bark of R. mucronata and R. apiculata was carried out by using a qualitative characterization reaction. These reactions were based on the appearance of color or precipitation by specific reagents. The results of this phytochemical screening are reported in Table 1. All parts of these plants have revealed the presence of saponin, phenolic, flavonoid, anthraquinone, terpenoid, and alkaloid contents differently. For R. mucronata, all phytochemical components were found in the leaf and bark, whereas anthraquinone and terpenoid were not detected in the pod, and anthraquinone and alkaloid were not found in the twig. In contrast to R. apiculata, no parts of this plant contain all kinds of phytochemicals. However, the bark extract of R. apiculata showed the most phytochemicals except terpenoid. Anthraquinone was not detected in the pod and leaf. Terpenoid was not found in the twig and pod, and alkaloid was not observed in the leaf and twig. Interestingly, the high levels of three chemical compositions, like saponin, phenolic, and flavonoid, were observed in both plants, especially in R. mucronata. The part of the plant may be related to the phytochemical composition. The current research in this area has focused on the content of phenolic compounds because they are known to provide therapeutic properties (Adebayo and Ishola, 2009; Gajula et al., 2020). Studies also showed that mangrove plants contain significant levels of saponins, which have anti-inflammatory, antioxidant, and anticancer properties (Sobolewska et al., 2020; Verma et al., 2013). Since they were also found to exercise antimicrobial activity against a wide range of microorganisms in vitro (Saad et al., 2011), the SCs, as well as phenolic and flavonoid levels, may contribute to explain their antioxidant and antibacterial activities.
Determination of the saponin, phenolic and flavonoid contents
In the present study, the SCs of the extracts of both plants were calculated from the equation: y = 0.0006x + 0.0284 (R2 = 0.9931), as escin equivalents [escin equivalent (EE)/g CE], and the data are presented in Table 2. The calculations produced values from 0.46 to 8.05 mg EE/g CE. All extracts of the R. mucronata part used had higher contents of saponins (2.94 and 8.05 mg EE/ g CE) than those of R. apiculata (0.46 and 4.57 mg EE/ g CE). Two-way analysis of variance clearly indicated that the main effects (species and plant parts) had a significant influence on the content of saponin with p < 0.001. Additionally, the phenolic contents of the extracts of both plants were calculated from the following equation: y = 0.0040x + 0.0028 (R2 = 0.9995), as gallic acid equivalents [gallic acid equivalent (GAE)/g CE], and the data are presented in Table 2. The calculations produced values from 0.22 to 2.21 mg GAE/g CE. All extracts of the R. mucronata part used had higher phenolic contents (1.05 and 2.21 mg GAE/g CE) than those of R. apiculata (0.22 and 1.56 mg EE/g CE). Two-way analysis of variance clearly indicated that the main effects (species and plant parts) had a significant influence on the phenolic content with p < 0.001. Moreover, the flavonoid contents of the extracts of both plants were calculated from the equation y = 0.0010x − 0.0040 (R2 = 0.9990), as rutin equivalents (rutin equivalent (RU)/g of CE), and the data are presented in Table 2. The calculations produced values from 1.10 to 6.73 mg RU/g CE. All extracts of the R. mucronata part used had higher contents of flavonoid (4.30 and 6.73 mg RU/g CE) than those of R. apiculata (1.10 and 3.71 mg RU/g CE). Two-way analysis of variance revealed that the main effects (species and plant parts) had significant effect on the content of saponin with p < 0.001. Based on a two-way analysis of variance, all phytochemical compositions (saponin, phenolic, and flavonoid) were affected by species (R. mucronata and R. apiculata) and plant parts (pod, leaf, twig, and bark) (p < 0.01). Interestingly, species × plant parts had a highly significant effect, as shown in the interaction in Figure 2. The findings from the current study showed that the pod of R. mucronata had the highest saponin and flavonoid, while the bark showed phenolic content at its highest. In addition, the SC was significantly correlated with the phenolic and flavonoid contents at r = 0.893 and r = 0.828 for R. mucronata and r = 0.775 and r = 0.975 for R. apiculata (p < 0.01, Fig. 3). In the same way, the phenolic and flavonoid contents were correlated significantly with r = 0.961 and r = 0.798 for R. mucronata and R. apiculata (p < 0.01), respectively. From the above, rich sources of phytochemicals (saponin, phenolic, and flavonoid) from both plants might be related directly to their biological activities, especially antioxidant and antibacterial activities further studied in the next section.
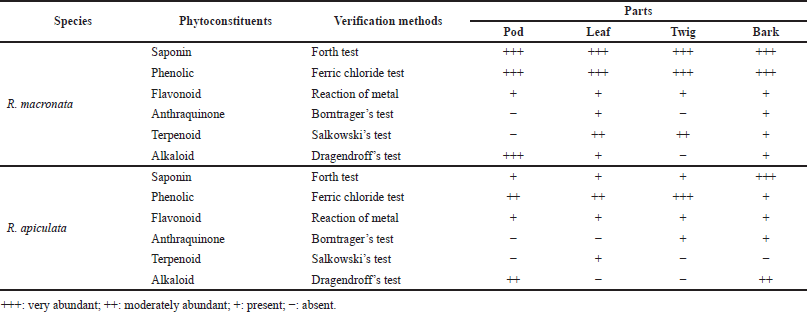 | Table 1. Phytochemical screening performed on aerial parts of R. mucronata and R. apiculata. [Click here to view] |
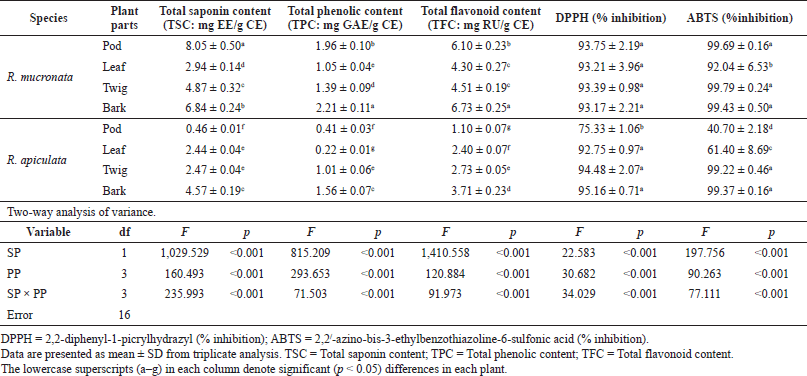 | Table 2. Chemical compositions and free radical scavenging activity in four parts of R. mucronata and R. apiculata. Two-way analysis of variance of effectors SP and PP. [Click here to view] |
Antioxidant activity
The free radical scavenging activity was measured in vitro using two methods, DPPH and ABTS, for each of the eight extracts, and the data are shown in Table 2. The percentage of free radical scavenging of DPPH was determined by spectroscopic analysis at 517 nm of quenched DPPH after reaction with samples. Free radical scavenging by R. mucronata extracts ranged between 93.17% and 93.75% and between 75.33% and 95.16% for R. apiculata extract. All the extracts of R. mucronata and R. apiculata showed very high levels of inhibitory activity (>90%( except the pod of the latter. The ABTS method is a good tool for determining free radical scavenging, and ABTS+ is a synthetic compound widely used to evaluate the antioxidant activity. This radical is produced by the oxidation of ABTS with potassium persulfate. This radical converts to a nonradical form, when exposed to antioxidants. The percentage of free radical scavenging of ABTS ranged between 92.69% and 99.79% for R. mucronata and between 40.70% and 99.37% for R. apiculata (Table 2). Interestingly, the percentage of free radical scavenging activity of the R. apiculata leaf has the trend to decrease slightly. It may be due to the least total phenolic content of the R. apiculata leaf, which affected the trend of free radical scavenging activity. Two-way analysis of variance revealed that the main effect of species and plant parts had an influence on antioxidant capacity as shown in Figure 3. Additionally, species × plant parts have a highly significant effect (F = 34.029, p < 0.001) as shown in the interaction in Figure 4. Furthermore, there was good agreement between the results of DPPH and ABTS in our study with the correlation coefficient (r = 0.834; p < 0.01 for only R. apiculata), presented in Figure 3. It is promising that a significant linear correlation was found between the free radical scavenging activity determined and phytochemical composition at a significant level of 0.01 in only R. apiculata. However, R. mucronata had only SC correlated with ABTS (r = 0.623; p < 0.05). It is possible that saponin has more effect on free radical scavenging activity than the phenolic and flavonoid contents in this plant. Moreover, it was observed in this study that other phytochemical compounds such as anthraquinone and alkaloid exist in R. mucronata and they are assumed to have a role in free radical scavenging activity, which is observed in previous research. According to the report of Gurudeeban et al. (2013), the alkaloid-rich extracts from Rhizophora mucronate displayed a potential application in antibacterials and antioxidants. Also, Kremer et al. (2012) reported that most derivatives of anthraquinone extracts from Frangula rupestris and Frangula alnus bark had high antioxidant and antimicrobial activities. The determination of the bioactive constituents in R. mucronata would be recommended for further study.
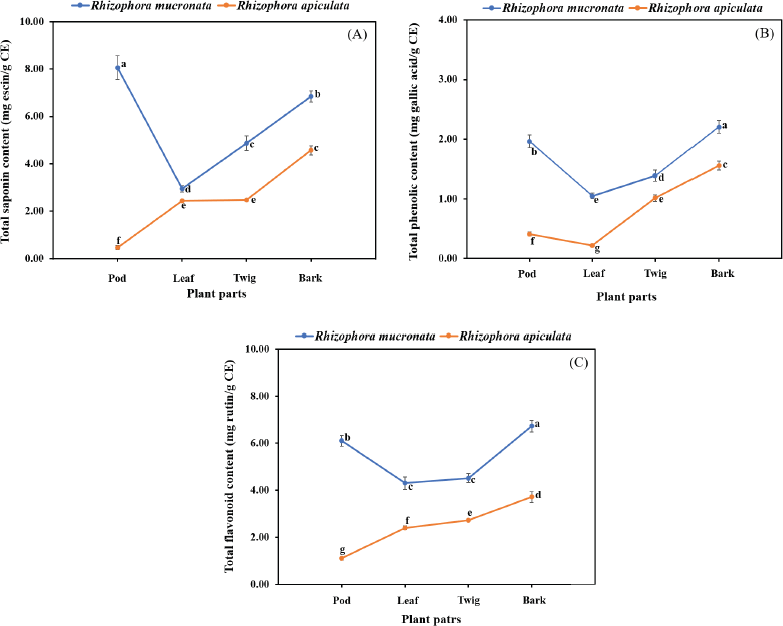 | Figure 2. Interaction effect of species and plant parts on quantity of total saponin content (A), total phenolic content (B), and total flavonoid content (C) in R. macronata and R. apiculata extracts, where different lowercase letters indicate significant (p < 0.05) difference among mean and error bars represent ± SD. [Click here to view] |
Antibacterial screening
The antibacterial activities of the pod, leaf, twig, and bark extracts of R. mucronata and R. apiculata were determined with four bacteria strains, S. agalactiae, A. hydrophila, V. harveyi, and V. parahemolyticus. Antibacterial potency was evaluated by measuring the diameter of the zone of inhibition in millimeters (mm) and determining MIC and MBC values. The results of antibacterial activity by the hole-plate diffusion method are reported in Table 3. The antibacterial activities of various part extracts of R. mucronata and R. apiculata were compared with the activity of the antibiotic agent oxolinic acid. The area of inhibition produced by each extract against the four pathogenic bacterial strains has a wide spectrum of the zone of inhibition, which varies depending on the strain tested, species, and the part extracted. According to Table 3, all extracts were active against all tested strains of the diameters of the inhibition zones ranging from 6.00 to 13.31 mm. It is tempting to explore or explain the effect of species (SP) and plant parts (PP) to inhibit pathogenic bacteria. Therefore, a two-way analysis of variance was used to measure the antibacterial activity of the two plants (R. mucronata and R. apiculata) with various parts (pod, leaf, twig, and bark), to inhibit pathogenic bacteria (Table 3). The results showed that the species of the plant and parts used influenced antibacterial activity (p < 0.001), except for V. harveyi. The observed antibacterial activity resulted from active compounds like phenolics, flavonoids, and saponins, which were more significantly found in R. mucronata than R. apiculata in all parts, as shown inFigure 2. For the above reason, more antibacterial activity was observed in R. mucronata than in R. apiculata. Interestingly, all parts of R. mucronata have more antibacterial activity than in R. apiculata as shown in Figure 5. Some of these phytochemical compounds have already been reported to have antibacterial properties (Krishnavignesh et al., 2012). In agreement with Santhi and Sengottuve (2016), they found that the potential of the extracts such as flavonoids, phenol, and saponin was an important source of useful drugs.
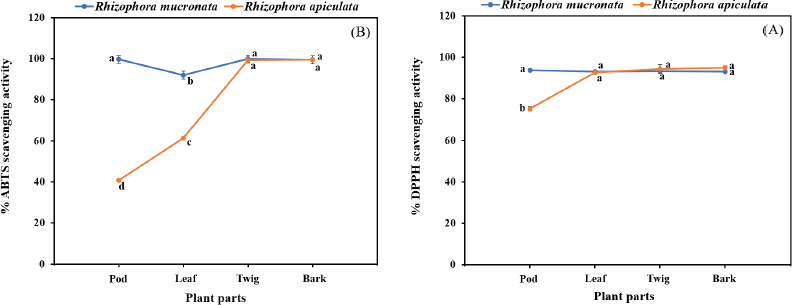 | Figure 3. Interaction effect of species and plant parts on quantity of DPPH assay (A) and ABTS assay (B) in R. macronata and R. apiculata extracts, where different lowercase letters indicate significant (p < 0.05) difference among mean and error bars represent ± SD. [Click here to view] |
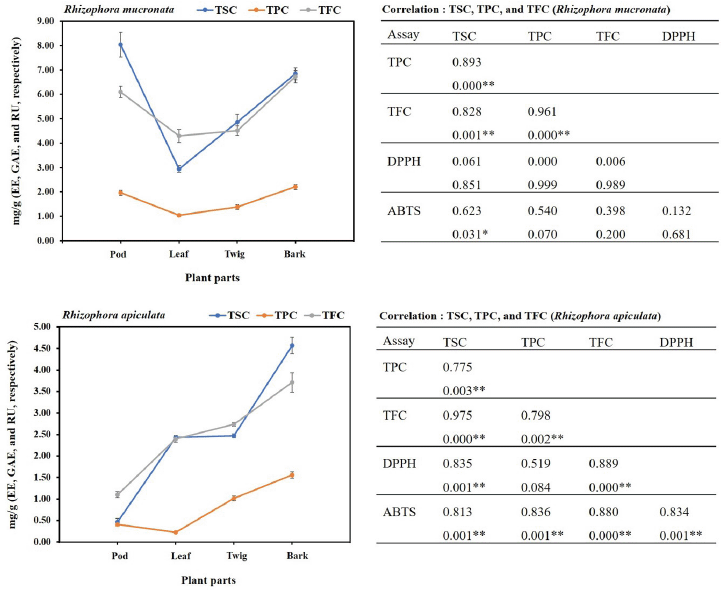 | Figure 4. TSC, TPC, and TFC are positively correlated with antioxidant activity [2,2-diphenyl-1-picrylhydrazyl (% inhibition): DPPH and 2,2'-azinobis- 3-ethylbenzothiazoline-6-sulfonic acid (% inhibition): ABTS] in pod, leaf, twig, and bark of R. mucronata and R. apiculata. Data shown are mean ± SD. Pearson’s correlation coefficient (r) between each pair of plants (R. macronata and R. apiculata) and chemical compositions (n = 12). * p < 0.05, ** p < 0.01. [Click here to view] |
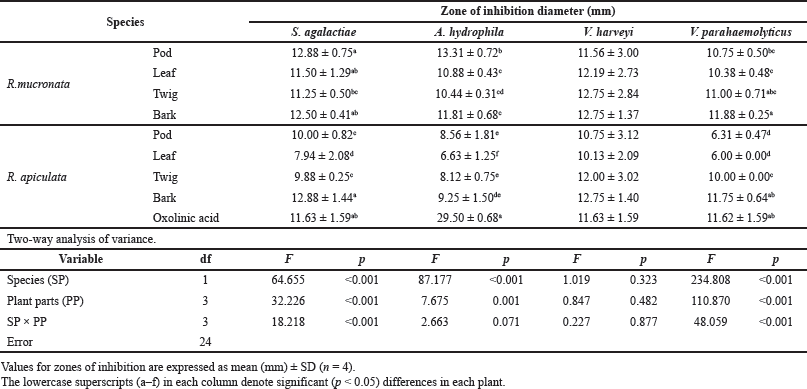 | Table 3. Zones of inhibition from methanolic extracts of R. mucronata and R. apiculata against four pathogenic bacteria (S. agalactiae, A. hydrophila, V. harveyi, and V.parahaemolyticus) that cause infectious diseases in aquatic conditions. Two-way analysis of variance of effectors plant species (SP) and plant parts (PP). [Click here to view] |
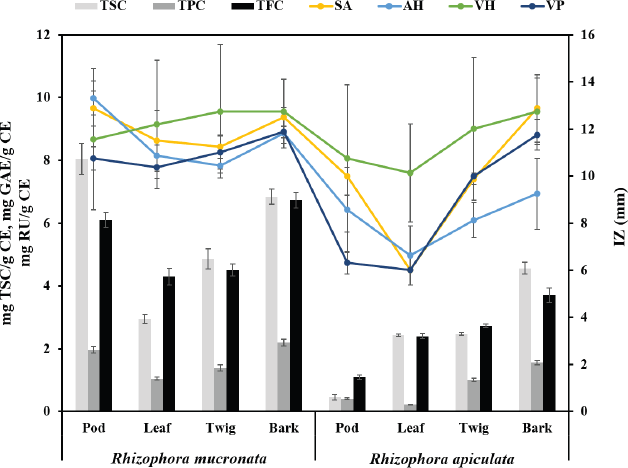 | Figure 5. The relative between quantitative contents with antibacterial activity of species and plant parts in R. macronata and R. apiculata extracts (TSC: Total saponin content, TPC: Total phenolic content, TFC: Total flavonoid content; IZ: Zone of inhibition; SA: Streptococcus agalactiae, AH: Aeromonas hydrophila, VH: Vibrio harveyi, and VP: Vibrio parahemolyticus). [Click here to view] |
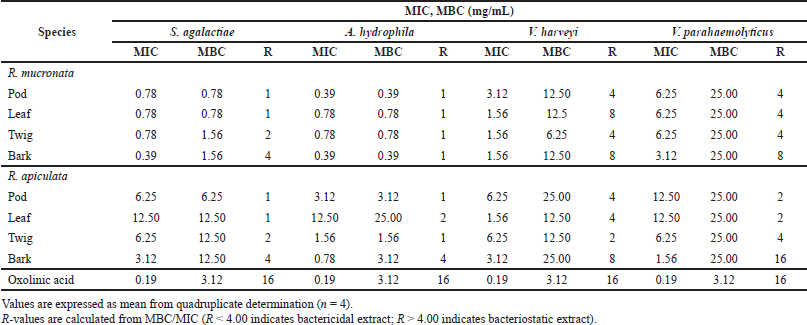 | Table 4. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of extracts of R. mucronata and R. apiculata against four pathogenic bacteria that cause infectious diseases in aquatic conditions. [Click here to view] |
MIC and MBC were used to determine the lowest concentration of antibacterial agent to inhibit the growth of the microorganism and the minimum bactericidal concentration of extracts. Table 4 presents the results obtained from the minimum inhibitory and bactericidal concentrations of the extracts of R. mucronata and R. apiculata. All tested extracts of antibacterial activity gave MIC values between 0.39 and 25.00 mg/ml. It was found that the pod and bark of R. mucronata extracts were the most active with an MIC value of 0.39 mg/mL against A. hydrophila and S. agalactiae. MIC values of 0.78 mg/mL were found in the leaf and twig extracts, which performed less well against A. hydrophila and S. agalactiae. On the contrary, only the bark extract of R. apiculata showed a MIC of 0.78 mg/ml and worked against A. hydrophila. These results showed that the extracts of R. mucronata were more active against the tested pathogens than the extracts of R. apiculata, especially against S. agalactiae and A. hydrophila. Therefore, the bark extract of R. mucronata strongly inhibited both strains with a MIC of 0.39 mg/ml while the pod extract of R. mucronata showed strong activity against only A. hydrophila with a MIC of 0.39 mg/ml. Data from the quantitative analysis of the phytochemical compositions (Table 2) show that the R. mucronata extracts contained higher amounts of saponins, phenolic, and flavonoid contents than the equivalent extracts of R. apiculata almost part of which is supported by Figure 4. This difference may play an important role in the antibacterial activity of the two plants.
The MBC/MIC values examined the nature of the antibacterial effect of the extracts tested. The extract is considered a bactericidal extract when this ratio is lower than 4, and when it is higher than 4, it is considered a bacteriostatic extract (Boulfia et al., 2021). In Table 4, it can be seen that almost all extracts tested have a bactericidal effect on S. agalactiae and A. hydrophila. In contrast to harveyi, it is found that the pod and twig of R. mucronata and the pod, leaf, and twig of R. apiculata have a bactericidal effect. For V. parahemolyticus, only the bark of R. mucronata and R. apiculata has a bacteriostatic effect and the other extracts have a bactericidal effect.
Phytochemical constituents were effective in inhibiting the growth of these pathogenic strains, even in the face of this barrier (Ravikumar et al., 2011). Consisting of a hydrophobic triterpene or steroid skeleton and hydrophilic carbohydrate molecules, the structure of saponins gave rise to amphipathic properties (Them et al., 2019). It is possible that both properties enable the active substance to pass through the cell membrane to destroy the cell, which is in excellent agreement with the previous theoretical reports (Sam and Anne, 2012). Additionally, the phenolic and flavonoid compounds confirmed by the phytochemical analysis of the extracts could inhibit the cell protein synthesis of the bacteria (Ravikumar and Kathiresan, 1993; Scalbert, 1991). Based on the results, it is possible to conclude that the methanolic extract of R. mucronata is a potential source of antibacterial agents against pathogenic aquatic bacteria.
CONCLUSION
From the present study, it was concluded that both plants Rhizophora mucronata and Rhizophora apiculata have the potential to act as a source of useful drugs because of their antibacterial activity against especially S. agalactiae and A. hydrophila. The determination of biologically active compounds, such as predominant saponin, phenolic, and flavonoid contents in the extracts, provided evidence of the presence in these plants of antimicrobial phytochemicals. These species could provide natural bioactive agents to replace the synthetic compounds that are currently used to treat the diseases caused by aquatic pathogens.
ACKNOWLEDGMENTS
This research was supported by the Faculty of Science and Fisheries Technology, Rajamangala University of Technology Srivijaya, Trang Campus. The authors would like to thank the Department of National Park, Wildlife and Plant Conservation, Thailand, for the specimen vouchers of R. mucronata and R. apiculata and also thank Mr. Thomus Duncan Coyne for editing a draft of this manuscript.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
This work was supported by Thailand Government Budget and Rajamangala University of Technology Srivijaya.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
REFERENCES
Aberoumand A. Screening of phytochemical compounds and toxic proteinaceous protease inhibitor in some lesser-known food based plants and their effects and potential applications in food. Int J Food Sci Nutr Eng, 2012; 2(3):16–20. CrossRef
Adebayo E, Ishola,O. Phytochemical and antimicrobial screening of crude extracts from the root, stem bark, and leaves of Terminalia glaucescens. Afr J Pharmacy Pharmacol, 2009; 3(5):217–21.
Ahmad AS, Musa N, Assaw S, Mohtar N, Yunus KB, Wahid ME, John A. Antibacterial properties of selected mangrove plants against Vibrio species and its cytotoxicity against Artemia salina. World Appl Sci J, 2013; 25(2):333–40.
Arulkumar A, Kumar K, Sampath P. Antibacterial and invitro antioxidant potential of Indian mangroves. Biocatal Agric Biotechnol, 2020; 23:101491. CrossRef
Boulfia M, Lamchouri F, Toufik H. Mineral analysis, in Vitro evaluation of alpha-amylase, alpha-glucosidase, and beta-galactosidase inhibition, and antibacterial activities of Juglans regia L. bark extracts. Biomed Res Int, 2021; Article ID 1585692:14. CrossRef
Brantner A, Pfeiffer KP, Brantner H. Applicability of diffusion methods required by the pharmacopoeias for testing antibacterial activity of natural compounds. Pharmazie, 1994; 49(7):512–6.
Das AK, Islam MN, Faruk MO, Ashaduzzaman M, Dungani R. Review on tannins: Extraction processes, applications and possibilities. S Afr J Bot, 2020; 135:58–70. CrossRef
Eloff JN. A Sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med, 1998; 64(8):711–3. CrossRef
Eswaraiah G, Peele KA, Krupanidhi S, Kumar RB, Venkateswarula TC. Identification of bioactive compounds in leaf extract of Avicennia albaby GC-MS analysis and evaluation of its in-vitro anticancer potential against MCF7 and HeLa cell lines. J King Saud Univ Sci, 2020a; 32:740–4. CrossRef
Eswaraiah G, Peele KA, Krupanidhi S, Kumar RB, Venkateswarula TC. Studies on phytochemical, antioxidant, antimicrobial analysis and separation of bioactive leads of leaf extract from the selected mangroves. J King Saud Univ Sci, 2020b; 32:842–7. CrossRef
Gajula H, Kumar V, Vijendra PD, Rajashekar J, Sannabommaji T, Basappa G. Bioprospecting potential of mangrove resources. Biotechnological Utilization of Mangrove Resources. Elsevier Science & Technology, San Diego, CA, 2020.
Gowri S, Vasantha K. Phytochemical screening and antibacterial activity of Syzygium cumini (L.) (Myrtaceae) leaves extracts. Int J Pharmtech Res, 2010; 2(2):1569–73.
Gurudeeban S, Ramanathan T, Satyavani K. Antimicrobial and radical scavenging effects of alkaloid extracts from Rhizophora mucronata. Pharm Chem J, 2015; 49(1):34–7.
Haslam E, Lilley TH, Cai Y, Martin R, Magnolato D. Traditional herbal medicines-The role of polyphenols. Planta Med, 1989; 55:1–8. CrossRef
Hong, L.S., Ibrahim, D, Kassim, J., Sulaiman, S. Gallic acid: An anticandidal compound in hydrolysable tannin extracted from the barks of Rhizophora apiculata Blume. J Appl Pharm Sci, 2011; 1(6):75–9.
Kremer D, Kosalec I, Locatelli M, Epifano F, Genovese S, Carlucci G, Zovko Kon?i? M. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark. Food Chem, 2012; 131(4):1174–80.
Krishnavignesh L, Mahalakshmipriya,A, Ramesh M. Phytochemical Screening and In Vitro Antimicrobial activity of Vitex negundo L. var. purpurascens Sivar. and Mold. against pathogenic microorganisms. Drug Invent Today, 2012; 4(12):667–70. CrossRef
Loo AY, Jain K, Darah I. Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculata. Food Chem, 2007; 104(1):300–7. CrossRef
Loo AY, Jain K, Darah I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem, 2008; 107(3):1151–60. CrossRef
National Committee for Clinical Laboratory Standard (NCCLS). Method for dilution antimicrobial susceptibility tests for bacteria that growth aerobically: approved Standard M7–A5. Clinical Laboratory Standards Institute, Wayne, PA, 2000.
Premanathan M, Arakaki R, Izumi H, Kathiresan K, Nakano M, Yamamoto N, Nakashima H. Antiviral properties of a mangrove plant, Rhizophora apiculata Blume, against human immunodeficiency virus. Antiviral Res, 1999; 44(2):113–22. CrossRef
Rahim AA, Rocca E. Steinmetz J, Kassim MJ, Ibrahim MS, Osman H. Antioxidant activities of mangrove Rhizophora apiculata bark extracts. Food Chem, 2008; 107:200–7. CrossRef
Ravikumar S, Kathiresan K. Influence of tannins, amino acids and sugar on fungi of marine halophytes. Mahasagar, 1993; 26(1):21–5.
Ravikumar S, Syed M, Ferosekhan, A.R.M. Antibacterial activity of chosen mangrove plants against bacterial specified pathogens. World Appl Sci J, 2011; 14(8):1198–202.
Saad S, Taher M, Susanti D, Qaralleh H, Binti NA, Rahim A. Antimicrobial activity of mangrove plant (Lumnitzera littorea). Asian Pac J Trop Med, 2011; 4(7):523–5. CrossRef
Sam TM, Anne O. 2012. Saponin synthesis and function. In: Bach TJ, Rohmer M (ed.). Isoprenoid synthesis in plants and microorganisms, Springer, New York.
Samatha T, Srinivas P, Shyamsundarachary, R., Marka, R., Nanna, R.S. Phytochemical analysis of seeds, stem bark and root of an endangered medicinal forest tree Oroxylum indicum(L) kurz. Int J Pharma Bio Sci, 2012; 3(3):B1063–75.
Santhi KS, Sengottuvel R. Qualitative and quantitative phytochemical analysis of Moringa concanensis Nimmo. Int J Curr Microbiol Appl Sci, 2016; 5(1):633–40. CrossRef
Scalbert A. Antimicrobial properties oftannins. Phytochemistry, 1991; 30(12):3875–83. CrossRef
Senguttuvan J, Paulsamy S, Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac J Trop Biomed, 2014; 4(1):S359–67. CrossRef
Sobolewska D, Galanty S, Agnieszka GK, Makowska-W?s J, Wróbel-Biedrawa D, Podolak I. Saponins as cytotoxic agents: an update (2010–2018). Part I—steroidal saponins. Phytochem Rev, 2020; 19:139–89. CrossRef
Sulaiman S, Ibrahim D, Kassim J, Sheh-Hong L. Antimicrobial and antioxidant activities of condensed tannin from Rhizophora apiculata barks. J Chem Pharm Res, 2011; 3(4):436–44.
Szyd?owska-Czerniak A, Dianoczki C, Recseg K, Karlovits G, Sz?yk E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophoto metric methods. Talanta, 2008; 76:899–905. CrossRef
Them LT, Dung PTN, Trinh PTN, Hung QT, Vi, LNT, Lam TD, Nguyen VT, Dung LT. Saponin, polyphenol, flavonoid content and α-glucosidase inhibitory activity, Antioxidant potential of Launaea sarmentosa leaves grown in Ben Tre province, Vietnam. IOP Conf Ser.: Mater Sci Eng, 2019; 542:012036. CrossRef
Vasanthi P, Ganapathy M, Evanjelene VK, Ayyavuv N, Angamuthu J. Phytochemical screening and antioxidant activity of extracts of the leaf and bark of Albizzia lebbeck (Benth). Acad J Med Plants, 2014; 2(2):026–31.
Verma S, Mohanta T, Revathy T, Suthindhiran K, Jayasri MA. Phytochemical and pharmacological evaluation of selected plants. Am J Biochem Biotechnol, 2013; 9(3):291–9. CrossRef
Vijayavel K, Anbuselvam C, Balasubramanian MP. Free radical scavenging activity of the marine mangrove Rhizophora apiculata bark extract with reference to naphthalene induced mitochondrial dysfunction. Chem Biol Interact, 2006; 163(1–2):170–5. CrossRef
Vittaya L, Khongsai S, Ui-eng J, Chalad C, Leesakul N. Effect of total phenolic and flavonoid contents of Ampelocissus martini on radical scavenging and antibacterial activities. Agric Nat Resour, 2019; 53:154–60.
Vittaya L, Charoeadat U, Junyong S, Leesakul N. Phytochemical screening of Bruguiera cylindrica extracts and pathogenic antibacterial activities. RMUTSV R J, 2020a; 12(2):196–207. Available via https://li01.tci-thaijo.org/index.php/rmutsvrj/article/view/245397
Vittaya L, Na Ranong S, Charoeadat U, Junyong S, Leesakul, N. Bio-activity investigation of extracts of different parts of Lumnitzera littorea Voigt. Trop J Nat Prod Res, 2020(b); 4(8):365–71. CrossRef