INTRODUCTION
Tuberculous meningitis (TBM) infection affects the central nervous system and is considered the most fatal form of tuberculosis (TB), particularly among those infected with HIV and young children. The disease most frequently manifests as a pulmonary disease; however, it also affects other parts of the body, resulting in extrapulmonary TB. After inhalation, infectious droplet nuclei containing M. tuberculosis begin to spread hematogenously throughout the body or by seeding oxygen-rich tissues, including the brain and other parts of the central nervous system (CNS). The proportion of TBM among TB cases varies across studies, depending on the prevalence of TB in the respective settings (between 1% and 10%). Experts have estimated that 100,000 people get TBM each year. The global mortality rate from tuberculosis (TB) is higher, ranking second on a global scale after COVID-19. The advent of multidrug-resistant M. tuberculosis strains over the last couple of years has placed devastating effects on the socioeconomic state of our country. According to the World Health Organization’s global TB report for 2020, about 10 million people contracted TB in 2019. Of these, 56% of men, 32% of women, and 12% of children (aged >15 years) contracted the disease. Almost two-thirds of these cases occurred in eight countries—India (26%), Indonesia (8.5%), China (8.4%), the Philippines (6.0%), Pakistan (5.7%), Nigeria (4.4%), Bangladesh (3.6%), and South Africa (3.6%). Even after adequate treatment, the survivors of TBM often suffer residual neurological sequelae, for which the estimated rate is more than 28% of cases. Mortality tends to be higher in early childhood because young children are at a higher risk of developing severe forms of the disease.
The current treatment is optimized for pulmonary tuberculosis but not for TBM (Horwitz et al., 2014; Nieuwenhuizen and Kaufmann 2018). As it is well known, CNS has multiple compartments [e.g., brain parenchyma and cerebrospinal fluid (CSF)] that are separated from systemic circulation by the blood–brain barrier (BBB) and blood–CSF barrier, and key antimicrobials, including rifampin (Cresswell et al., 2019), do not penetrate into the brain adequately. Aside from being difficult to diagnose and treat, it also causes significant morbidity and mortality even when treated with an appropriate and prolonged (at least 12 months) multidrug regimen of higher doses of antibiotics. The increasing number of cases of multidrug-resistant and extensively drug-resistant M. tuberculosis (MTB) strains complicates TB control and may lead to the Ineffectiveness of most of the current anti-TB drugs (Faksri et al., 2011; Ghajavand et al., 2019; Singh et al., 2019; Soria et al., 2019). In a study, antitubercular peptides were identified, and chimeric vaccine was prepared in silico to target multidrug-resistant MTB. The studies are being carried out to find the potent cure for tuberculosis (TB) (Batta et al., 2022). Thus, there arises the need for safe, cost-effective, and less toxic antitubercular compounds that can easily pass through the BBB and cope with disease resistance. There are several studies on the bioactive phytocompounds to have ethanomedicinal uses against M. tuberculosis. Araujo et al., (2021) studied the in-vitro antitubercular activity of plants from Rio, Brazil, from which the dichloromethane extracts of Eremanthus crotonoides and Kielmeyera membranacea showed to have lowest MIC50 against M. tuberculosis H37Rv (15.28 ± 1.21 and 4.38 ± 1.19 μg/ml, respectively) and Mycobacterium bovis BCG (2.17 ± 1.11 and 0.95 ± 1.08 μg/ml, respectively).
Trigonella foenum-graecum, a perennial plant belonging to the family Fabaceae, is used by people in Asia, Africa, and the Mediterranean as an ingredient in daily diets. It has been used in numerous fields such as medicine, nutrition, beverages, fragrances, and cosmetics, as well as other industrial uses (Gu et al., 2017; Munshi et al., 2020). Fenugreek is known to have several pharmacological effects including hypoglycemia, hypocholesterolemia, gastroprotective, chemopreventive, antioxidant, anti-inflammatory, antipyretic, and appetite stimulating effects (Gu et al., 2017; Norziah et al., 2015). The neuroprotective effects of fenugreek seeds were revealed in a study in which hypercholesterolemia and lipid peroxidation were controlled as bioactive antioxidant compounds which passed through the BBB (Im et al., 2015).
Phytochemical analysis has revealed that fenugreek contains many active ingredients, including alkaloids (such as trigonelline, gentianine, and choline), flavonoids (such as luteolin, apigenin, quercetin, and vitexin), steroids (such as cholesterol and sitosterol), saponins (such as diosgenin, gitogenin, and tigogenin) (Akbari et al., 2019; Assam et al., 2020; Norziah et al., 2015; Sethi et al., 2018; Wang et al., 2019), proteins, volatile oils, polysaccharides, triterpenoids, and nicotinic acid, which are proven to have therapeutic value. These phytochemicals can be extracted using different polar and nonpolar solvents. The quality and quantity of pharmaceutically active phytocompound extracts are determined by several factors, such as solvent type, temperature, pH, the number of extraction steps, liquid-to-solid ratios, as well as particle size of the solute, which affect extraction efficiency. Response surface methodology (RSM) is a useful extraction optimization method for evaluating the effects of multiple factors and their interactions on one or more response variables and is used instead of classical optimization experiments where only one factor is variable at a time, which is a tedious, time-consuming, expensive approach that fails to elaborate on interaction effects between variables (Bogdanovic et al., 2016; Jumeri and Kim 2011; Ong, 2011; Wang et al., 2019).
The main purpose of this research was to determine the factors that can enhance the yield of antitubercular compounds from crude extract of fenugreek [T. foenum-graecum (Methi)] seeds. Various extraction factors, such as temperature, time of exposure, solid-to-solvent ratios, etc., were studied using RSM and the Plackett–Burman design (PBD) approach.
MATERIALS AND METHODS
Plant extract preparation
Trigonella foenum-graecum (Methi) seeds were collected from local Aminabad market (26.8449°N, 80.9249°E). They were washed and shade-dried. The seed sample was then crushed to form powder. Three solvents (ethyl acetate, chloroform, and methanol) were used for the extracts’ preparation. Phytochemical analysis using different methods was carried out to know about active constituents in seed extract (Table 1).
Mycobacterium tuberculosis sample collection
Clinical isolates of tuberculosis meningitis (TBM, extrapulmonary TB) and pulmonary TB were collected from the Department of Microbiology at SGPGIMS, Lucknow, UP, India. Acid-fast (AFB) microscopy was also carried out to test their growth (Table 2).
 | Table 1. Phytochemical Analysis of Plant Extract Samples in solvents of different polarity. [Click here to view] |
Screening of process variables using PBD
Fenugreek [T. foenum-graecum (Methi)] seeds’ crude extract was analyzed using the PBD (Ong, 2011) to optimize the relative factors that affect the yield of antitubercular compounds. In this study, five determinative variables (solvent, compound concentration, temperature, shaking rate, and time) and two dummy variables (seed particle size and light) were tested in eight experimental designs. Table 3 shows the PBD of the factors studied with high and low levels. A series of duplicate experiments were carried out, and the yield of the anti-TB compound was taken as a response. Resazurin microtiter assay (REMA) was used to determine the in vitro antitubercular activity of the crude extract on M. tuberculosis (Helal et al., 2019; Katawera et al., 2014).
Antitubercular activity REMA
REMA was used to screen all the extracts of experimental design for antitubercular activity (Coban et al., 2014; Helal et al., 2019; Katawera et al., 2014; Tuyiringire et al., 2022). Briefly, in 96-well plate inoculums were dispensed in 100 μl Middlebrook 7H9 broth medium, single Mycobacterium samples per plate. Furthermore, 100 μl of plant extract was added in duplicate in the wells. Isoniazid and rifampicin were used as positive controls, while Mycobacterium culture was used as a negative control, as shown in Figure 1. The plate was then incubated for 24 hours at 37°C after loading. Following the incubation period, 30 μl of resazurin (0.02% w/v) was added to each well and reincubated for 24 hours. The optical density of each well was recorded using an enzyme-linked immunosorbent assay reader at 450 and 620 nm.
Optimization of process variables by response surface methodology
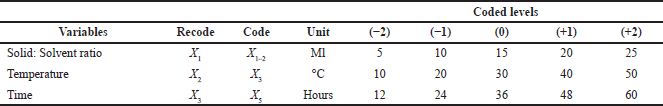
The optimization of variables for extraction of antitubercular compounds from fenugreek was carried out by using two statistical models, i.e., the PBD and the central composite design (CCD) of response surface methodology (Akbari et al., 2019). The CCD model was used in this study. Table 6 shows the variables for crude fenugreek extract at five coded levels (–2, –1, 0, +1, and +2). Various combinations of the independent variables within the central composite design matrix and the observed response are presented in Tables 4, 5, and 7. The experiments were repeated twice, and the average results of the duplicates were taken as the response (yield of antitubercular compound of fenugreek).
Cytotoxicity of fenugreek to Hek293T cells
For screening of compounds, cell-based assays are used to determine the cytotoxic effects of the test compound that can lead to cell death. In this colorimetric assay, reduction of yellow 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Mitra et al., 2016) by mitochondrial succinate dehydrogenase is measured. Hek293T cells (human embryonic kidney 293 cells containing the SV40 T-antigen) were cultured for 24 hours till they reached confluency. The cells were then seeded on a 96-well plate with a volume of 100 μg/well. Different concentrations of the extract (10, 50, 100, and 200 μg/ml) were dissolved in Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum (FBS) and added to each well in triplicate. The plate was incubated for 24 hours. Positive control (1% SDS and 1% Trition X-100) and negative control (Hek293T cells in DMEM) were used. After incubation, 0.5 mg/ml MTT in phenol red free DMEM without FBS was added in each well and the plate was incubated for 4 hours. After incubation, the media was discarded and 100 μg/well DMSO was added. The absorbance at 570 nm was determined using an automated microplate reader (Bio-Rad). The MTT data represent the mean + SD of the experiment carried out in triplicate.
Statistical analysis
Statistical significance was assessed by the two-sample Student’s t-test using GraphPad Prism 7 (Mitteer et al., 2018); p-values <0.05 were considered statistically significant. Furthermore, Design Expert Version 8.0 (Elsayed, 2018) and TIBCO Statistica® v13.0 (Tibco Software 2018) were used to develop the experimental design and analyze the data. One-way analysis of variance (ANOVA) was carried out for the fitness of the model.
 | Table 2. Number of Mycobacterial samples (n). [Click here to view] |
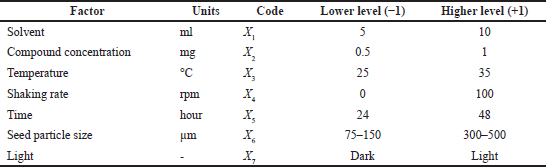 | Table 3. Plackett–Burman design. [Click here to view] |
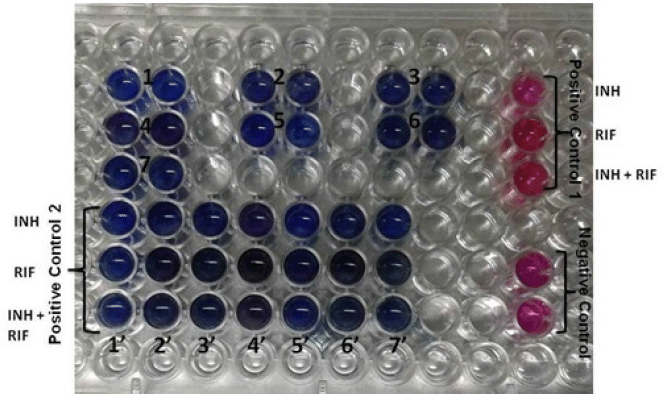 | Figure 1. In 96-well plate, well 1 and 1’ contains blank dye in media; from 2 to 7 were different solvent extracts of fenugreek in duplicate. Negative control contains mycobacteria in media only. Positive control 1 contains first line drugs (INH: Isonizid; RIF: Rifampicin; & Combination), in Positive control 2 from 2’ to 7’ contain extracts, drugs and mycobacteria. REMA assay was then performed. [Click here to view] |
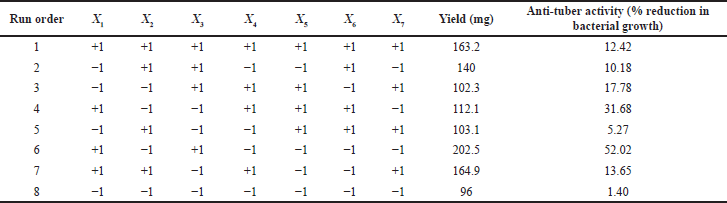 | Table 4. Screening design study. [Click here to view] |
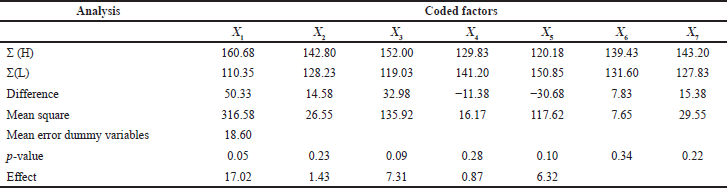 | Table 5. Screening design study effect and p-values. [Click here to view] |
 | Table 6. RSM. [Click here to view] |
RESULTS AND DISCUSSION
Phytochemical analysis
As evident from various studies, fenugreek seed crude extract in different solvents contained many therapeutic phytochemical compounds that were analyzed in this study using different methods (Adhikari and Rai 2021; Aylanc et al., 2020; Hasan Khan et al., 2019). Table 1 shows that out of three extracts, those in methanol gave positive results for flavonoids, phenols, and terpinoids in phytochemical testing. These phytochemicals are known to have antitubercular activity. In a study, Shabbir et al., (2020) utilized Maytenus royleanus of the Celastraceae family methanolic leaf extract to reduce liver injury caused by anti-TB drugs. The study was designed by inducing liver injury in mice by Myrin®-p Forte. It was seen that the antioxidants of M. royleanus methanolic leaf extract exerted an hepatoprotective effect in liver injured mice (Shabbir et al., 2020). Thus, methanolic extracts were selected for further studies and in-vitro tested on MTB using REMA. Table 2 presents the number of clinical isolates of MTB culture acquired from pulmonary sputum (n = 100) and extrapulmonary CSF (40). The cultures were of three categories: first, drug-sensitive where the tubercular bacteria is not resistant against the anti-TB drugs; second, drug-resistant where the tubercular bacteria is resistant against the anti-TB drugs; and third were control samples, which are of other disease than TB. For pulmonary isolates, 69 were drug-sensitive, 14 were drug-resistant, and 17 were control samples. Similarly, the number of CSF isolates was 21, 7, and 12, respectively. Figure 1 shows the REMA where the calorimetric assay was performed using Resazurin dye. Mycobacterium tuberculosis sample used here is resistant to the first line of antituberculosis therapy (ATT) drugs, i.e., isoniazid (INH) and rifampicin (RIF) as shown as pink color in positive control 1 (drug + M. tuberculosis). The wells were compared to negative control (media + M. tuberculosis) and to blank dye in well 1 and 1?. In wells 2–7 are plant extracts and M. tuberculosis. In wells 2?–7? are positive control 2 (drug + plant extract + M. tuberculosis).
Screening of process variables using the PBD
Using the PBD, various factors influencing the extraction yield of antitubercular therapeutic compounds from T. foenum-graecum seeds were identified. Extraction yield values are shown in Table 4 for the high (+1) and low (+1) levels of the eight run screen experiments. The study results in Table 5 indicate that the significant factor effect (Sf) and critical difference (CD) were computed at 17.02 and 316.58, respectively. X1–X5 were independent variables and X6 and X7 were dummy variables. According to the p-values and effect calculated by the PBD (Table 5 and Fig. 2), factors solvent, compound concentration, temperature, and time showed significant values of 0.05, 0.23, 0.09, 0.010 and 17.02, 1.43, 7.31, 6.32, respectively. In a recent study, Rizi et al., (2021) utilized the PDB and response surface methodology approach for the optimization of DNA biosensor which is PCR-free and can detect MTB in as low as 0.141 nm concentration (Rizi et al., 2021). Hence, three variables of the present study solid-to-solvent ratio, extraction temperature, and extraction time were statistically significant at a 90% confidence level and were further optimized using response surface methodology.
Optimization of the selected process variables by response surface methodology
The selected variables were recoded and further optimized using five levels of screening as shown in Table 6 where solid-to-solvent ratio, temperature, and time were studied using 20 combinational experiments. The crude fenugreek methanolic extract yield was tested in vitro on M. tuberculosis (MTB) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was further carried out to test the toxicity of the extract on human embryonic kidney 293 cells (Hek293T cells) (Fig. 6). The extract was nontoxic to normal cells even to the 100 μg/ml concentration. The experimental combination factors were carried out in 20 runs as shown in Table 7. ANOVA for the response surface mathematical model is shown in Table 8. Figure 3 shows the normal probability plot of randomized observed and expected yield values. A below regression equation was developed using the RSM analysis which shows an empirical relationship between the logarithmic values of extracted metabolites in terms of yield and the coded units of the process variables.
Y = +169.73 +15.87*X1 +0.62*X2 − 5.63*X3 + 0.75*X1*X2 + 23.25*X1*X3 − 12.25*X2*X3 − 16.14*X12 − 10.39*X22 − 8.64*X32
Here is a quadratic polynomial equation where Y is the yield and X1, X2, and X3 are variables with values for the model. With a model F-value of 11.36 and a probability value of 0.0004, it is evident that the model is highly significant. The value of R2 (0.9109) indicates that the sample variation of 91% for the extraction yield is caused by the independent parameters and indicates that the model equation is significant (Singh et al., 2008). There is a close correlation between the experimental values and the predicted values, as the multiple correlation coefficient R2 (0.8307) is near 1. The signal-to-noise ratio for the analysis gives a precision of 11.15 (ratios >4 are desirable) and shows the polynomial quadratic model to be adequate. The contour and response surface plots from Figure 4 show that the variables solid-to-solvent ratio, temperature, and time coordinate with the mathematical model and the interaction between them shows synergy in enhancing the yield (extraction of antituberculosis compound). Figure 5 (in supplementary files) shows that by using this model it is possible to achieve more than 90% of anti-TB compound yield (169.727 mg) from fenugreek seed crude extract, which could be produced when solid-to-solvent ratio, temperature, and time were set at 15 ml, 30°C, and 36 hours, respectively.
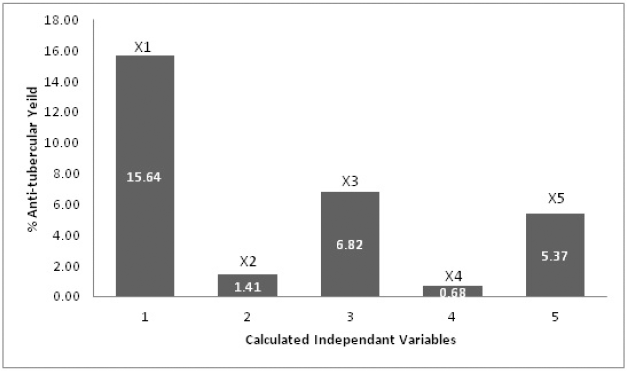 | Figure 2. Plackett–Burman design calculated effects for variables. X1= Solvent, X2= Compound Concentration, X3= Temperature, X4= Shaking rate, X5= Time. [Click here to view] |
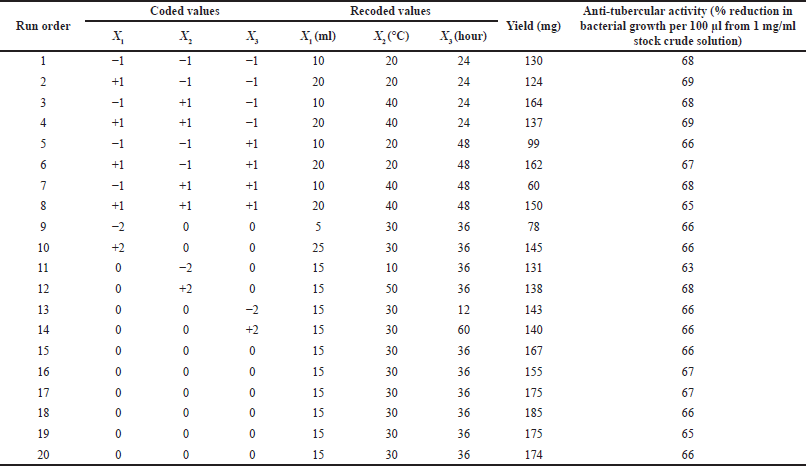 | Table 7. Optimization study. [Click here to view] |
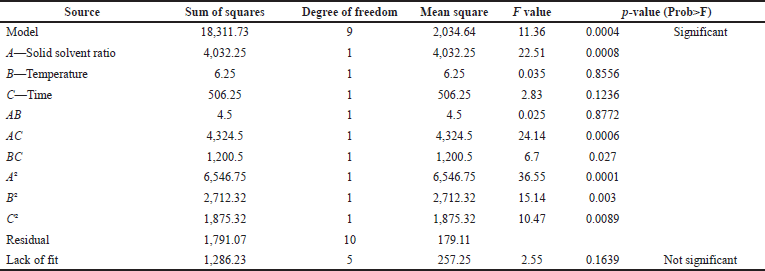 | Table 8. ANOVA. [Click here to view] |
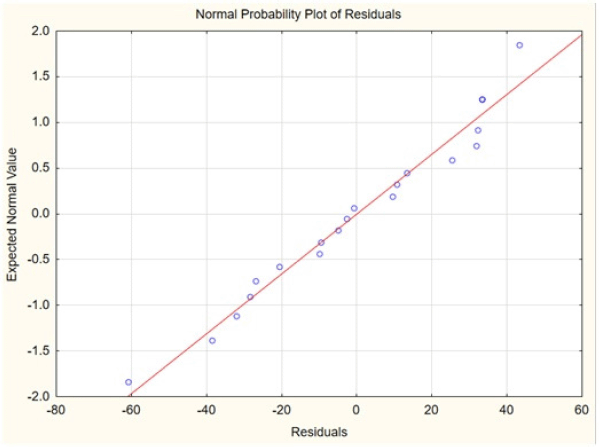 | Figure 3. Normal probability plot. [Click here to view] |
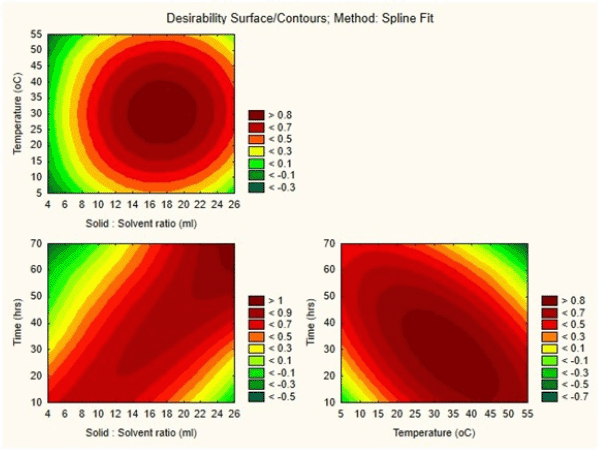 | Figure 4. Contour response surface plot a: Solid-solvent ratio to Temperature; b: Solid-solvent ratio to Time; c: Temperature to Time all with respect to anti- tubercular compound yield from Fenugreek. [Click here to view] |
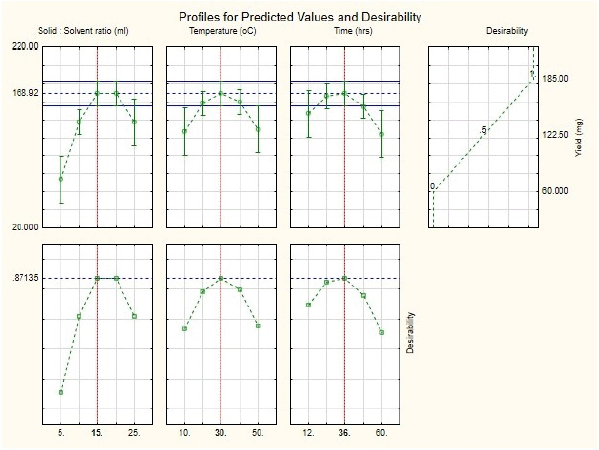 | Figure 5. Model Desirability plots a: Solid-solvent ratio; b: Temperature; c: Time all with respect to anti- tubercular compound yield. [Click here to view] |
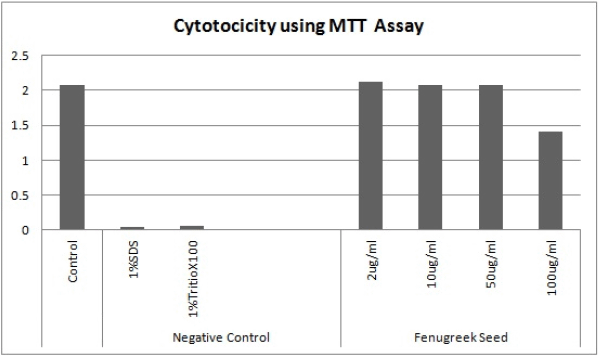 | Figure 6. Cytotoxicity test of crude fenugreek extract was done on human embryonic kidney 293 cells (Hek293T cells) using MTT assay. [Click here to view] |
CONCLUSION
From this study, it is evident that the PBD can be successfully used for enhancing the yield of antituberculosis therapeutic compounds from T. foenum-graecum seeds. The response surface and the mathematical relationship between the screened factors were used to refine the best combination of solid-to-solvent ratio, temperature, and extraction time. Face-centered central composite design provided fairly accurate predictions for a wide area around the center point of the model. From MTT assay, it is evident that this study complements previous studies and the studied compounds are nontoxic and easy to use to combat tuberculosis infection. In the future, the prospective compounds can be isolated and studied in vivo in animal models.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adhikari B, Rai S. Processing impact on nutritional, antinutritional, and phytochemical of fenugreek seeds (Trigonella foenum-graecum L.). Himalayan J Sci Technol, 2021; 5(01):36–44; doi:10.3126/hijost.v5i01.42131 CrossRef
Akbari S, Abdurahman NH, Yunus RM. Optimization of saponins, phenolics, and antioxidants extracted from fenugreek seeds using microwave-assisted extraction and response surface methodology as an optimizing tool. Comptes Rendus Chimie 2019; 22(11–12):714–27; doi:10.1016/j.crci.2019.07.007 CrossRef
Assam JPA, Tcham MFY, Moni NEDF, Betote DPH, Fossi TC, Penlap BV. Phytochemical screening, antimycobacterial activity of three medicinal cameroonians plants and acute toxicity of hydroethanolic extract of vitellaria paradoxa. J Drug Deliv Ther, 2020; 10(1-s):96–104; doi:10.22270/jddt.v10i1-s.3848 CrossRef
Aylanc V, Eskin B, Zengin G, Dursun M, Cakmak YS. In vitro studies on different extracts of fenugreek (Trigonella spruneriana boiss.): phytochemical profile, antioxidant activity, and enzyme inhibition potential. J Food Biochem, 2020; 44(11):1–10; doi:10.1111/jfbc.13463 CrossRef
Batta A, Singh V, Mishra BN, Dhole TN, Seth PK. Chimeric vaccine against multi-drug resistant mycobacterium tuberculosis using in silico reverse vaccinology approach. J Appl Pharm Sci, 2022; 12(06):86–114; doi:10.7324/japs.2022.120609 CrossRef
Bogdanovic A, Tadic V, Ristic M, Petrovic S, Skala D. Optimization of supercritical CO2 extraction of fenugreek seed (Trigonella foenum-graecum L.) and calculating of extracts solubility. J Supercrit Fluids, 2016; 117:297–307; doi:10.1016/j.supflu.2016.07.010 CrossRef
Coban AY, Deveci A, Sunter AT, Palomino JC, Martin A. Resazurin microtiter assay for isoniazid, rifampicin, ethambutol and streptomycin resistance detection in mycobacterium tuberculosis: updated meta-analysis. Int J Mycobacteriol, 2014; 3(4):230–41; doi:10.1016/j.ijmyco.2014.09.002 CrossRef
Cresswell FV, Brake LT, Atherton R, Ruslami R, Dooley KE, Aarnoutse R, Crevel RV. Intensified antibiotic treatment of tuberculosis meningitis. Expert Rev Clin Pharmacol, 2019; 12(3):267–88; doi:10.1080/17512433.2019.1552831 CrossRef
de Araujo MH, Simao TLBV, Konno TUP, Guimarães DO, Leal ICR, Lasunskaia EB, Muzitano MF. Anti-mycobacterial and anti-inflammatory activity of restinga plants: a dual approach in searching for new drugs to treat severe tuberculosis abstract tuberculosis (TB) still constitutes a threat to public health in various regions of the world. Rodriguésia, 2021; 1–13. Available via https://www.scielo.br/j/rod/a/8knTQJYHb84XvhkppXzJ5cq/ CrossRef
Elsayed I. Design of pharmaceutical experiments using design expert® software. J Pharm Care Health Syst, 2018; 05:4172; doi:10.4172/2376-0419-c4-035 CrossRef
Faksri K, Drobniewski F, Nikolayevskyy V, Brown T, Prammananan T, Palittapongarnpim P, Prayoonwiwat N, Chaiprasert A. Epidemiological trends and clinical comparisons of mycobacterium tuberculosis lineages in Thai TB meningitis. Tuberculosis, 2011; 91(6):594–600; doi:10.1016/j.tube.2011.08.005 CrossRef
Ghajavand H, Kamakoli MK, Khanipour S, Dizaji SP, Masoumi M, Jamnani FR, Fateh A, Yaseri M, Siadat SD, Vaziri F. Scrutinizing the drug resistance mechanism of multi- and extensively-drug resistant Mycobacterium tuberculosis: mutations versus efflux pumps. Antimicro Resist Infect Control, 2019; 8(1):70; doi:10.1186/s13756-019-0516-4 CrossRef
Gu LB, Liu XN, Liu HM, Pang HL, Qin GY. Extraction of fenugreek (Trigonella foenum-graceum L.) seed oil using subcritical butane: characterization and process optimization. Molecules, 2017; 22(2):228; doi:10.3390/molecules22020228 CrossRef
Hwa CY, Perveen N, Paliwal N, Khan NH. Phytochemical screening, antimicrobial and antioxidant activity determination of Trigonella foenum-graecum seeds. Pharm Pharmacol Int J, 2019; 7(4):175–86; doi:10.15406/ppij.2019.07.00249 CrossRef
Helal ZH, Menofy GE, Ibrahim ZA, Ashour MSE, Abdulall K. Comparative evaluation of Anyplex II MTB/MDR/XDR and resazurin microtiter assay for detection of drug resistant Mycobacterium tuberculosis. J Microbiol Biotechnol Food Sci, 2019; 8(5):1150;doi:10.15414/jmbfs.2019.8.5.1150-1155 CrossRef
Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun, 2003; 71:1672–9; doi:10.1128/IAI.71.4.1672-1679Im K, Maliakel A, Gopakumar G. Improved blood – brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. J Funct Foods, 2015; 14:215–25; doi:10.1016/j.jff.2015.01.049 CrossRef
Jumeri, Kim SM. Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava). Food Sci Biotechnol, 2011; 20(4):1075–85; doi:10.1007/s10068-011-0146-y CrossRef
Katawera V, Siedner M, Ii YB. Evaluation of the modified colorimetric resazurin microtiter plate-based antibacterial assay for rapid and reliable tuberculosis drug susceptibility testing. BMC Microbiol, 2014; 14:259. CrossRef
Mitra I, Mukherjee S, Venkata PBR, Dasgupta S, Bose CKJ, Mukherjee S, Linert W, Moi SC. Benzimidazole based Pt(II) complexes with better normal cell viability than cisplatin: synthesis, substitution behavior, cytotoxicity, DNA binding and DFT study. RSC Adv, 2016; 6(80):76600–613; doi:10.1039/c6ra17788c CrossRef
Mitteer DR, Greer BD, Fisher WW, Cohrs VL. Teaching behavior technicians to create publication-quality, single-case design graphs in Graphpad Prism 7. J Appl Behav Anal, 2018; 51(4):998–1010; doi:10.1002/jaba.483 CrossRef
Munshi M, Arya P, Kumar P. Physico-chemical analysis and fatty acid profiling of fenugreek (Trigonella Foenum-Graecum) seed oil using different solvents. J Oleo Sci, 2020; 69(11):1349–58; doi:10.5650/jos.ess20137 CrossRef
Nieuwenhuizen NE, Kaufmann SHE. Next-generation vaccines based on bacille calmette-guérin. Front Immunol, 2018; 9:121; doi:10.3389/fimmu.2018.00121 CrossRef
Norziah MH, Fezea FA, Bhat R, Ahmad M. Effect of extraction solvents on antioxidant and antimicrobial properties of fenugreek seeds (Trigonella Foenum-Graecum L.). Int Food Res J, 2015; 22(3):1261–71.
Ong ST. Plackett–Burman design and response surface methodological approach to optimize basic dyes removal using sugarcane bagasse. Civil and Environmental Engineering Faculty Publications, 2011. Available via https://engagedscholarship.csuohio.edu/encee_facpub/98
Rizi KS, Hatamluyi B, Rezayi M, Meshkat Z, Sankian M, Ghazvini K, Farsiani H, Aryan E. Response surface methodology optimized electrochemical DNA biosensor based on HAPNPTs/PPY/MWCNTs nanocomposite for detecting Mycobacterium tuberculosis. Talanta, 2021; 226(January):122099; doi:10.1016/j.talanta.2021.122099 CrossRef
Sethi G, Shanmugam MK, Warrier S, Merarchi M, Arfuso F, Kumar AP, Bishayee A. Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review. Nutrients, 2018; 10(5); doi:10.3390/nu10050645 CrossRef
Shabbir M, Afsar T, Razak S, Almajwal A, Khan MR. Phytochemical analysis and evaluation of hepatoprotective effect of Maytenus royleanus leaves extract against anti-tuberculosis drug induced liver injury in mice. Lipids Health Dis, 2020; 19(1):1–15; doi:10.1186/s12944-020-01231-9. CrossRef
Singh R, Dwivedi SP, Gaharwar US, Meena R, Rajamani P, Prasad T. Recent updates on drug resistance in Mycobacterium tuberculosis. J Appl Microbiol, 2020; 128(6):1547–67; https://doi.org/10.1111/jam.14478. CrossRef
Singh V, Tripathi CKM. Production and statistical optimization of a novel olivanic acid by Streptomyces olivaceus MTCC 6820. Process Biochem, 2008; 43(11):1313–17; doi:10.1016/J.PROCBIO.2008.07.015 CrossRef
Soria J, Metcalf T, Mori N, Newby RE, Montano SM, Huaroto L, Ticona E, Zunt JR. Mortality in hospitalized patients with Tuberculous meningitis. BMC Infect Dis, 2019; 19(1); doi:10.1186/s12879-018-3633-4 CrossRef
Tibco Software. TIBCO Statistica TM Quick Reference. TIBCO Software Inc, no. 11, 2018.
Tuyiringire N, Mugisha IT, Tusubira D, Munyampundu JP, Muvunyi CM, Heyden YV. In vitro antimycobacterial activity of medicinal plants Lantana camara, Cryptolepis sanguinolenta, and Zanthoxylum leprieurii. J Clin Tuberc Other Mycobact Dis, 2022; 27:100307; doi:10.1016/j.jctube.2022.100307 CrossRef
Wang N, He F, Li W, Fang X, Li H. Purification of the total steroidal saponins from fenugreek seeds (Trigonella Foenum-Graecum L.) using aqueous two-phase system and determination of diosgenin content using micellar electrokinetic chromatography method. Nat Prod Res, 2019; 33(3):453–56; doi:10.1080/14786419.2018.1455047 CrossRef