INTRODUCTION
In the dairy industry, mastitis is a crucial problem that causes economic loss and public health concern. Bovine mastitis is one of the most common diseases in dairy cows worldwide. It typically occurs in the prepartum period and is caused by immunosuppression (Aleri et al., 2016). Therefore, the increased incidence of mastitis problems can occur during early productive life (defined as the first 100 days of the first lactation) (Hertl et al., 2018). The overall prevalence of mastitis was estimated to be 47.0% [95% confidence interval (CI) = 42.0, 52.0] using the meta-analysis of 39 studies. The odds of occurrence of mastitis were also higher at early lactation [odds ratio (OR) = 1.6; 95% CI = 1.4, 1.8] (Getaneh and Gebremedhin, 2017). Currently, the common methods of control and prevention of mastitis in cattle during the dry period are long-acting antibiotic therapy known as dry cow therapy to eliminate current infection and prevent new infection (Niemi et al., 2021). The use of antibiotics, however, might lead to antibiotic-resistance and contamination of the residues in milk. Herein, teat sealant is noted as an alternative method by covering the teat, known as external teat sealant. The capping seal does not contain any antibiotic properties. Internal gel or blocker, on the other hand, is shown and applied in dry cows such as Orbeseal®, an inert nonantibiotic internal teat sealant (Van Houten, 2018). The closeness of the teat canal to the internal teat sealant mimics the physiological mechanisms of plug formation.
Currently, a variety of different types of long-acting drug delivery systems have been introduced as an easy and simplified administration, which enhances the potential of drug stability and prolongs release. Hydrogel or in-situ forming implants using biopolymers have been noted and incorporate various drugs, proteins, molecules, etc. This type of vehicle shows good biocompatibility and biodegradable properties. Interestingly, in-situ-formed vehicles have specific-shaped masses according to the cavity at the injection site (Bode et al., 2018;Yang et al., 2021). Poly(lactic-co-glycolic acid) (PLGA) polymer has been reported as a foundation for many in-situ-forming implants based on the solvent exchange technique. PLGA is also widely accepted for use in drug delivery systems that have garnered the approval of the Food and Drug Administration (Kumar et al., 2022). The mechanism of the formulation is quite simple and is like-dissolve-like or precipitates in the water phase (Ibrahim et al., 2021; Kamali et al. 2021a). In short, PLGA is required to dissolve in an organic solvent together with the delivery molecule before immersing/dropping into the water phase. Herein, drugs or compounds could be precipitated and entrapped in the PLGA vehicles. This vehicle can be called implants, hydrogels or depots depending on the applications. The existing PLGA polymer-based implants for drug delivery on the market are InductOs®S, Sinuva®, Sublocade®, Zilretta®, etc (Ibrahim et al., 2021; Kamali et al., 2021a; Selmin et al., 2020). These compounds are gelled as PLGA vehicles in water, phosphate buffer saline (PBS), and biological solutions. Thus, the PLGA polymer with an organic solvent mixture was able to be used as an in-situ injectable implant for targeted delivery (Kamali et al., 2021a; Toledo et al., 2020).
Gallic acid (GA) is a naturally occurring phenolic acid compound containing trihydroxy. It has been reported that GA can stimulate apoptosis in tumors by downregulating molecular pathways such as PI3K/Akt (Ashrafizadeh et al., 2021). The use of GA also enhances the antibacterial and antioxidant properties (Yadav et al., 2021) and has been reported in several publications such as polyvinylidene fluoride (PVDF)-grafted GA membrane (Li et al., 2021a), chitosan-GA drug delivery (Yi et al., 2021), and GA-hydrogel (acrylic acid, ammonium persulfate, and N,N’-methylenebis acrylamine) (Abebe et al., 2020). Moreover, GA is an antimicrobial agent in dairy industry disinfection, similar to Staphylococcus aureus. This information implies the use of GA as a compound for preventing infection by bacteria.
In this paper, PLGA polymer was selected to formulate an internal hydrogel teat sealant (IHTS) containing GA as an antibacterial compound. The GA-loaded IHTS was characterized for its properties such as hydrogel formation, release profile, surface and cross-section observation, and cytotoxicity against the L929 cell line. This IHTS could be applied as a mastitis control during the dry period by internal injection into the teat as a degradable teat sealant and to prevent bacterial infection.
MATERIALS AND METHODS
Materials
PLGA, molecular ratio of DL-lactide/glycolide 50/50 (Mw 27.2 kDa, PDI 1.51) was purchased from NanoPolyPEG Co., Ltd, (Bangkok, Thailand). GA and 1-methyl-2-pyrrolidinone (NMP) were purchased from Sigma-Aldrich (USA). L929:NCTC clone 929 cell line (IFO50409) was purchased from the Japanese Collection of Research Bioresources Cell Blank (Japan). Dulbecco’s modified eagle medium (DMEM), 0.25% trypsin-EDTA (ethylene diamine tetra-acetic acid) solution, fetal bovine serum (FBS), and antibiotic-antimycotic solution were purchased from Gibco, Thermo Fisher Scientific (NY, USA). Triton X-100, PBS tablets, dimethyl sulfoxide, and 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Amresco Inc. (OH, USA).
Preparation of GA-loaded PLGA IHTS and measurement of in vitro release
GA-loaded poly(D,L-lactide-co-glycolide) IHTSs or GA-loaded PLGA IHTSs were prepared using the solvent exchange principle (Kamali et al., 2021a). Briefly, PLGA (3 g) and GA (0.08 g) were dissolved in 4.6 ml of NMP. The mass ratio of the mixture (PLGA:GA:NMP) was prepared at 39:1:60. The mixture solution was then incubated at 37°C for 24 hours (Li et al., 2021b; Yang et al., 2021). This organic solution was mixed and dropped into 5 ml PBS (pH 7.4) by pipetting (~50 μl). Herein, bare IHTSs were performed without GA.
The released GA from GA-loaded IHTSs was investigated by Folin–Ciocalteu’s reagent (Yadav et al., 2021). GA-loaded IHTSs were prepared in 5 ml of PBS and incubated at 37°C with an 80 rpm orbital shaker. At the determination time points (3, 6, and 12 hours, and on days 1, 2, 3, 5, 7, 10, 14, 21, and 37), PBS was collected and the same volume of fresh PBS was replaced. All collected PBS samples were evaluated for GA quantities. Briefly, 20 μl of the collected sample was mixed with 80 μl of deionized water and 100 μl of 2N Folin–Ciocalteu’s reagent. The reaction was incubated at room temperature for 5 minutes. Then, 100 μl of 5% (w/v) Na2CO3 was added and incubated for another 1 hour. The absorbance was measured with a microplate reader spectrophotometer (HiPo, MPP-96, Biosan, Latvia) at a wavelength of 700 nm.
Stability testing
The mixture solutions (as mentioned in the previous section) were investigated for their stability using accelerated conditions and cycling temperature procedures. For accelerated temperature, the mixture solutions (PLGA, GA, and NMP) were stored at 4°C, 25°C, and 45°C for 4 weeks, whereas cycling temperature was performed at 4°C and 45°C (1 day per temperature) for 5 and 10 rounds. The color, viscosity, and hydrogel gelation of each condition were determined and investigated.
Surface characterization
After 1 and 7 days of formation in PBS, all IHTSs were removed from the vial containing PBS. The samples were washed with deionized water and freeze-dried. The surface and cross-section of the IHTSs were observed by scanning electron microscopy (SEM): JEOL JCM-7000 NeoScope™ Benchtop SEM (JEOL, Japan). Samples were deposited on the stub and coated with gold. For the cross-section study, IHTSs were frozen and divided by a blade before the coating process. All samples were observed under SEM using a 10 kV electron beam voltage.
In-vitro cytotoxicity
The cytotoxicity of NMP (organic solvent), bare IHTS, and GA-loaded IHTS was evaluated in vitro against a fibroblast cell line (L929). L929 cells were maintained in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic. For NMP cytotoxicity, NMP solvent was diluted with serum-free DMEM to various concentrations ranging from 0.01% to 20% (v/v). Cells at 10,000 cells per well were plated at least 1 day before being exposed to NMP and DMEM. Cell viability was measured by MTT assay using a microplate reader (HiPo, MPP-96, Biosan, Latvia) at a wavelength of 568 nm.
Bare and GA-loaded IHTSs were formed in sterilized vials containing 5 ml of serum-free DMEM and incubated at 37°C for 1, 7, 14, and 28 days (Díaz et al., 2017). The DMEM was collected and used to treat L929 cells (10,000 cells per well) at 37°C for another 1 day in 100%, 50%, and 10% collected DMEM. Cell viability was evaluated by MTT assay as mentioned previously. All conditions were normalized to the control (fresh culture medium) and positive control (1% Triton X-100).
Statistical analysis
All experiments were conducted at least in triplicate. Data are expressed as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance. A p-value less than 0.05 was considered to indicate statistical significance.
RESULTS AND DISCUSSION
GA-loaded IHTSs in a ratio of PLGA:GA:NMP (39:1:60) were formed and their properties were evaluated. The IHTSs were self-solidified and formed as hydrogels after dropping the mixture solution into PBS. During gelation, the NMP organic solvent diffused out of the PLGA matrix into the PBS phase. The PLGA (27.2 kDa) matrix and GA were mixed and precipitated, forming a gel/hydrogel (Fig. 1a). The bare IHTSs (without GA) and GA-loaded IHTSs weights were varied at 71.1 ± 2.3 and 75.9 ± 7.4 mg. The loading GA was calculated to be 0.73 ± 0.08 mg, which was a very high encapsulation efficiency (over 98%). This mixture was also drawn into a 1 ml syringe and mimics an injection into the artificial teat made from silicon. The formulation of the hydrogel was observed to seal the leakage of water from the in vitro teat as an inserter plug for up to 3 weeks (Fig. 1b).
The in-vitro release profile of GA from GA-loaded IHTSs was studied over 30 days as shown in Figure 1c. GA release from the IHTS was very fast as the initial burst release on the first day due to the property of GA which is a water-soluble compound. Approximately 48.9% cumulative GA release was calculated on day 7 of the study. It was approximately 352.1 μg of GA. The residual GA (unreleased GA) was still encapsulated inside the core of the IHTS, which provided a sustained release of GA after 7 days. At this point, we studied higher GA loading in the IHTS. PLGA:GA:NMP at 38:2:60 was performed, and the release profile was studied. IHTSs containing more GA showed a greater cumulative release of approximately 58.8% on day 7 of the study compared with PLGA:GA:NMP at 39:1:60. The GA concentration for the release study between 1 and 2 mass ratios did not significantly increase the release of GA within the first 7 days. Herein, the weight and size of the IHTSs were controlled by the pipetting volume. The accumulation of GA might be released upon the degradation of PLGA over time. However, the recommendation of GA of inhibiting common pathogens of bovine mastitis, such as Escherichia coli (Gram negative) and Streptococcus mutant (Gram positive), was noted as 8 mg/ml on biofilm formation (Mohammed et al., 2022). Liu et al. (2017) reported the use of 2 mg/ml GA could inhibit S. aureus (commonly found and isolated from dairy products) in suspension. Thus, the use of the IHTSs (PLGA:GA:NMP at 39:1:60) against the formation of biofilm could be approximately 10-fold higher than this in vitro study (~500 μl of the mixture). Based on the number of daily doses (nDDay) of antibiotics in use for the treatment of mastitis, cows, cows were treated for approximately 3.7 days (± 0.36 SEM, 0.7–8.7 nDDay) with a success rate in the range of 18%–59% (assessed by individual somatic cell count) (Doehring and Sundrum, 2019). A single insertion of the IHTS might reduce the daily doses with a benefit for financial antibiotic consumption and human power and improve animal health and welfare.
The stability testing of PLGA:GA:NMP mixture solutions was investigated under accelerated conditions and cycling temperatures. Long-term preservation (4 weeks) at 4°C confirmed the formulation of the hydrogel in PBS, whereas the mixture at 25°C and 45°C preservation could not be formed as the hydrogel (data not shown). Although all conditions, whether with or without GA, did not make any change in color but slightly decreased the viscosity, the overall observation of the mixture was the same as the initial time (Table 1). Herein, the mixtures after 5 and 10 cycles temperature between at 4°C and 45°C (1 day per temperature) were able to form hydrogels (Fig. 2). Long-term preservation might cause the degradation of PLGA which is the main matrix of IHTS, via humidity. As mentioned previously, the degradation rate of PLGA (25–30 kDa) started on day 10–15 (Bode et al., 2019). A preservation process of less than 10 days might not affect the gelation process. This information recalls the stability process of the mixture.
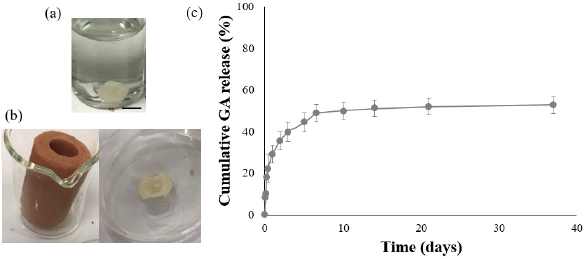 | Figure 1. Photograph of (a) GA-loaded IHTS (PLGA:GA:NMP = 39:1:60) after formulation for 6 hours. (b) Gelation of PLGA:GA:NMP after injection into the silicon-based vehicle. The hydrogel was observed up to 3 weeks after injection. (c) Release profile of GA from the GA-loaded IHTSs. The scale bar is 5 mm. [Click here to view] |
IHTSs with and without GA were analyzed by SEM (Fig. 3). Overall, the surfaces of the IHTSs were not significantly changed within 7 days, whereas the cross section of GA-loaded IHTSs showed high porosity because those surfaces were exposed to water earlier. The acidic condition (GA and degradation product) could build up the blank cavity close to the surface that was not present on the surface. The burst release of GA could help to generate larger clusters of pores (Gu et al., 2016). Thus, a dense matrix of PLGA was observed on the surface under both IHTS conditions. After 10 days of incubation, those IHTSs for SEM could not be prepared due to polymer degradation (Bode et al., 2019).
When assayed independently, NMP reduced the viability of L929 cells to the extent of a 50% inhibitory concentration (IC50 value) at 1.17% ± 0.25% (v/v) NMP in serum-free culture media. Moshikur et al. (2020) reported that the IC50 of NMP solvent was 58.1 mM. Moreover, the viability of L929 cells was approximately 80% after a 1-day incubation period with levothyroxine in NMP which was applied as a drug delivery system (Kamali et al., 2021a). These results showed the potential of NMP as a biocompatible liquid for drug delivery. IHTSs (with and without GA) were carefully formed in serum-free DMEM. The extracted medium was prepared as described in the Methods section. The evaluation of IHTS followed an adaptation of ISO10993 Part 5 (biocompatibility test). Then, the extracted medium was harvested (days 1, 7, 14, and 28) and replaced in a culture plate at 100%, 50%, and 10%, which was seeded with L929 cells for 1 day prior to the experiments. The viability of L929 cells was observed to be 80% after the cells were treated with the extracted DMEM (Fig. 4a). Generally, the extracted media from bare IHTSs did not show cytotoxicity as low as 80% cell viability upon treatment of L929 cells with 50% extracted DMEM. The 100% extracted medium showed an approximately 20%–30% decrease in cell viability according to the MTT assay in the presence of NMP. Each condition of IHTS formation would contain NMP of approximately 0.3%–0.6% (v/v). However, the GA-loaded IHTSs significantly decreased the percentage of cells to around 60%. This evidence could be due to the released GA in the culture media. A high concentration of GA could provide an acidic environment that could not be recovered by sodium bicarbonate in DMEM. Overall, the cellular observation confirmed that the extracted medium prepared from PLGA IHTS did not significantly change the cell morphology under light microscopy (Fig. 4b).
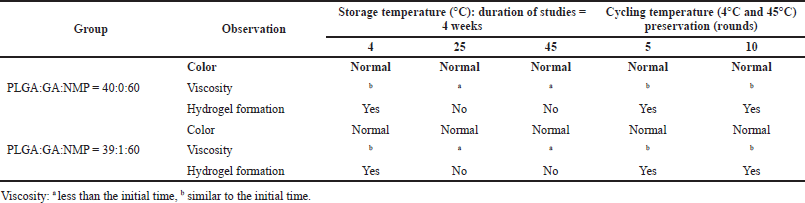 | Table 1. Physical observation of the mixture of PLGA, GA, and NMP before IHTS preparation. [Click here to view] |
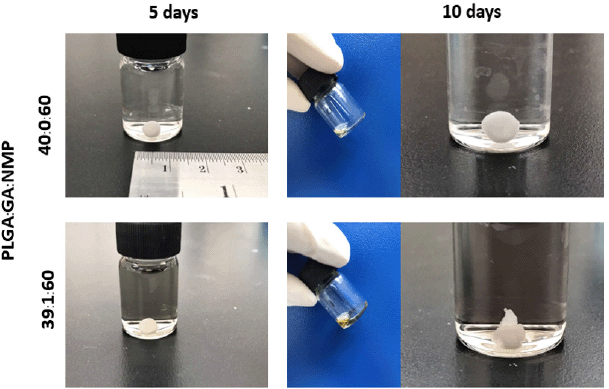 | Figure 2. Gelation of the IHTS after cycling at 4°C and 45°C for 5 and 10 days (1 day per temperature). [Click here to view] |
This study demonstrates the preparation of a GA-loaded IHTS and its main properties. The formulation would be used in the dairy industry to prevent mastitis in cows during the dry period. Released GA from the teat sealant hydrogel showed slight toxicity because it provided an acidic environment, whereas the bare internal teat sealant exhibited little cytotoxicity from the NMP organic solvent. However, the amount of GA released from IHTS would be able to inhibit microorganisms. More studies will be carried out to examine the antibacterial efficacy of the released GA, in-vivo irritation of the IHTS, in-vivo efficacy of the IHTS, etc.
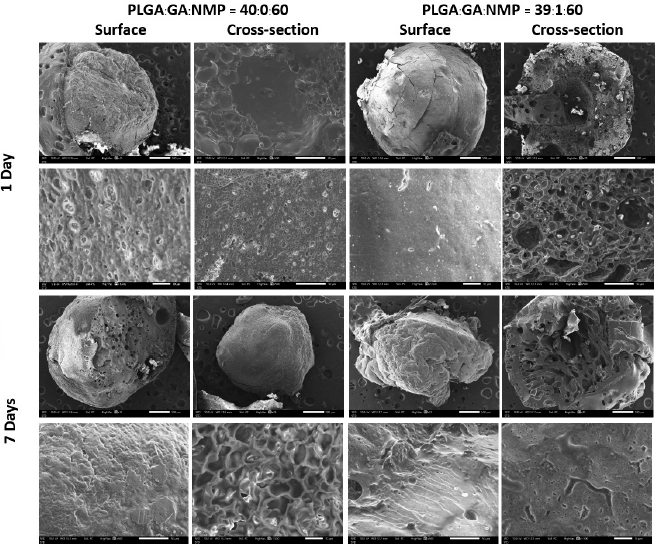 | Figure 3. Series of SEM images of bare IHTS and GA-loaded IHTS (PLGA:GA:NMP = 39:1:60) at different times in PBS (pH 7.4, 37°C). The images were captured at low magnification to observe the overall morphology (rows 1 and 3) and high magnification for the pores and microstructure of the hydrogel (rows 2 and 4). The scale bar of the image (rows 1 and 3) is 500 μm and the high magnification image (rows 2 and 4) is 10 μm. [Click here to view] |
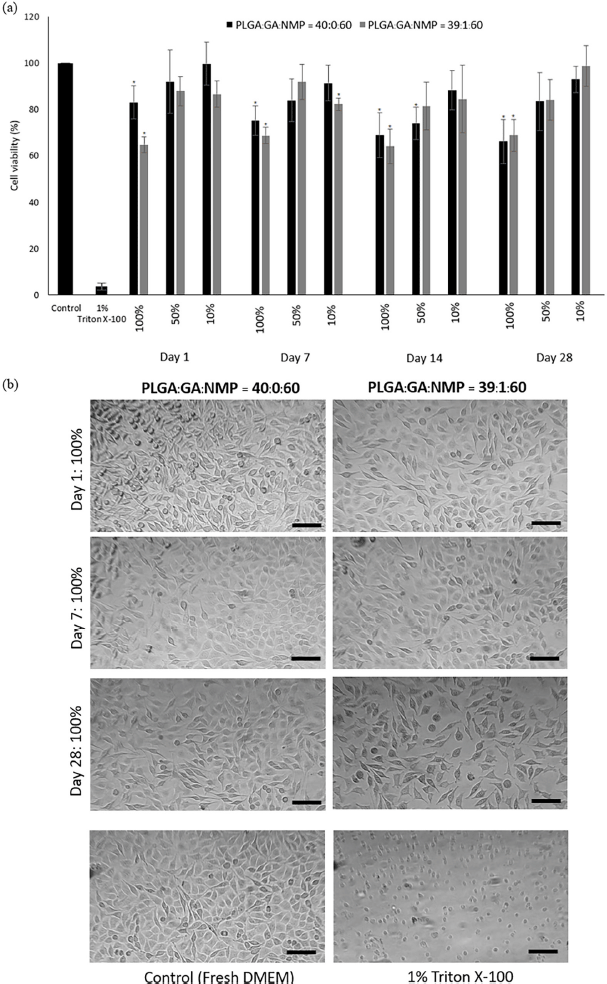 | Figure 4. In-vitro cytotoxicity of (a) cell viability of L929 cells against extracted medium from bare IHTS and GA-loaded IHTS by the medium extraction method at different incubation times (1, 7, 14, and 28 days) and (b) morphological changes in L929 cells treated with 100% extracted medium from 1, 7, and 28 days). The bottom row shows control L929 cells. The scale bar is 0.2 mm. (*p <0.05). [Click here to view] |
CONCLUSION
GA-loaded IHTSs were successfully prepared and formed in salt buffer or aqueous environments with a high percentage of sustained release of GA. GA could be controllably released by the PLGA matrix. The PLGA vehicle showed a low cytotoxic effect against L929 cells at different incubation times. These results were confirmed as noncytotoxic carriers that could be used as internal teat sealants. However, further examination of the antibacterial efficacy is needed.
FUNDING
This research was supported by the Faculty of Veterinary Medicine, Chiang Mai University, Thailand (CMU-MIS R000025318).
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
AUTHORS’ CONTRIBUTION
PB contributed to experimental design, data acquisition, material characterization, statistical analysis, data interpretation, manuscript drafting, and SEM images. RM contributed to study conceptualization, experimental design, data acquisition, and data interpretation. CM contributed to experimental design, data acquisition, statistical analysis, data interpretation, and manuscript drafting. All authors critically revised the manuscript and gave final approval for publication. All authors read and approved the final manuscript.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abebe-Medhen W, Appiah-Ntiamoah R, Kim H. Gallic acid modified alginate self-adhesive hydrogel for strain responsive transdermal delivery. Int J Biol Macromol, 2020; 163:147–55. CrossRef
Aleri JW, Hine BC, Pyman MF, Mansell PD, Wales WJ, Mallard B, Fisher AD. Periparturient immunosuppression and strategies to improve dairy cow health during the periparturient period. Res Vet Sci, 2016; 108: 8–17. CrossRef
Ashrafizadeh M, Zarrabi A, Mirzaei S, Hashemi F, Samarghandian S, Zabolian A, Hushmandi K, Ang HL, Sethi G, Kumar AP, Ahn KS. Gallic acid for cancer therapy: molecular mechanisms and boosting efficacy by nanoscopical delivery. Food Chem Toxicol, 2021; 157:112576. CrossRef
Bode C, Kranz H, Fivez A, Siepmann F, Siepmann J. Often neglected: PLGA/PLA swelling orchestrates drug release: HME implants. J Control Release, 2019; 306:97–107. CrossRef
Bode C, Kranz H, Siepmann F, Siepmann J. In-situ forming PLGA implants for intraocular dexamethasone delivery. Int J Pharm, 2018; 548(1):337–48. CrossRef
Díaz E, Puerto I, Ribeiro S, Lanceros-Mendez S, Barandiarán JM. The influence of copolymer composition on PLGA/nHA scaffolds’ cytotoxicity and in vitro degradation. Nanomaterials, 2017; 7(7):173. CrossRef
Doehring C, Sundrum A. The informative value of an overview on antibiotic consumption, treatment efficacy and cost of clinical mastitis at farm level. Prevent Vet Med, 2019; 165:63–70. CrossRef
Getaneh AM, Gebremedhin EZ. Meta-analysis of the prevalence of mastitis and associated risk factors in dairy cattle in Ethiopia. Trop Anim Health Prod, 2017; 49(4):697–705. CrossRef
Gu B, Sun X, Papadimitrakopoulos F, Burgess DJ. Seeing is believing, PLGA microsphere degradation revealed in PLGA microsphere/PVA hydrogel composites. J Control Release, 2016; 228:170–8. CrossRef
Hertl JA, Schukken YH, Tauer LW, Welcome FL, Gröhn YT. Does clinical mastitis in the first 100 days of lactation 1 predict increased mastitis occurrence and shorter herd life in dairy cows? J Dairy Sci, 2018; 101(3):2309–23. CrossRef
Ibrahim TM, El-Megrab NA, El-Nahas HM. An overview of PLGA in-situ forming implants based on solvent exchange technique: effect of formulation components and characterization. Pharm Dev Technol, 2021; 26(7):709–28. CrossRef
Kamali H, Khodaverdi E, Kaffash E, Saffari AS, Shiadeh SN, Nokhodchi A, Hadizadeh F. Optimization and in vitro evaluation of injectable sustained-release of levothyroxine using PLGA-PEG-PLGA. J Pharm Innov, 2021a; 16:688–98. CrossRef
Kamali H, Khodaverdi E, Mohammadpour F, Kakavand A, Shiadeh SN, Oroojalian F, Hadizadeh F. The impacts of PLGA/PEG triblock copolymers with variable molecular weights on the sustained release of buprenorphine. Curr Drug Deliv, 2021b; 19:357–68. CrossRef
Kumar SP, Asokan Y, Balamurugan K, Harsha B. A review of wound dressing materials and its fabrication methods: emphasis on three-dimensional printed dressings. J Med Eng Technol, 2022; 46(4):318–34. CrossRef
Li C, Chen X, Luo J, Wang F, Liu G, Zhu H, Guo Y. PVDF grafted gallic acid to enhance the hydrophilicity and antibacterial properties of PVDF composite membrane. Sep Purif Technol, 2021a; 259:118127. CrossRef
Li Z, Mu H, Larsen SW, Jensen H, Østergaard J. An in vitro gel-based system for characterizing and predicting the long-term performance of PLGA in situ forming implants. Int J Pharm, 2021b; 609:121183. CrossRef
Liu M, Wu X, Li J, Liu L, Zhang R, Shao D, Du X. The specific anti-biofilm effect of gallic acid on Staphylococcus aureus by regulating the expression of the ica operon. Food Control, 2017; 73:613–8. CrossRef
Mohammed AN, Radi AM, Khaled R, Abo El-Ela FI, Kotp AA. Exploitation of new approach to control of environmental pathogenic bacteria causing bovine clinical mastitis using novel anti-biofilm nanocomposite. Environ Sci Pollut Res, 2020; 27(34):42791–805. CrossRef
Moshikur RM, Chowdhury MR, Wakabayashi R, Tahara Y, Kamiya N, Moniruzzaman M, Goto M. Ionic liquids with N-methyl-2-pyrrolidonium cation as an enhancer for topical drug delivery: synthesis, characterization, and skin-penetration evaluation. J Mol Liq, 2020; 299:112166. CrossRef
Niemi RE, Hovinen M, Vilar MJ, Simojoki H, Rajala-Schultz PJ. Dry cow therapy and early lactation udder health problems-associations and risk factors. Prev Vet Med, 2021; 188:105268. CrossRef
Selmin F, Musazzi UM, Magri G, Rocco P, Cilurzo F, Minghetti P. Regulatory aspects and quality controls of polymer-based parenteral long-acting drug products: the challenge of approving copies. Drug Discov Today, 2020; 25(2):321–9. CrossRef
Toledo CR, Pereira VV, Andrade GF, Silva-Cunha A. PLGA-corosolic acid implants for potential application in ocular neovascularization diseases. Braz J Pharm Sci, 2020; 56:e18484.
Van Houten B. The future of mastitis control. Dairy Mail, 2018; 25(12):83–7. CrossRef
Yadav S, Mehrotra GK, Dutta PK. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem, 2021; 334:127605. CrossRef
Yang S, Hu M, Liu W, Hou N, Yin K, Shen C, Shang Q. Fabrication of PLGA in situ forming implants and study on their correlation of in vitro release profiles with in vivo performances. J Biomater Sci Polym Ed, 2021; 32(8):994–1008. CrossRef
Yi J, Huang H, Wen Z, Fan Y. Fabrication of chitosan-gallic acid conjugate for improvement of physicochemical stability of β-carotene nanoemulsion: impact of Mw of chitosan. Food Chem, 2021; 362:130218. CrossRef