INTRODUCTION
Alzheimer’s disease (AD) is an enervating condition that predominantly impacts neuron cells and causes progressive degeneration of the brain and ce?ntral nervous system, disrupting cognitive and motor functioning and potentially leading to death. Currently, the development of effective therapeutic techniques to treat AD is challenging. The protective action of the blood–brain barrier (BBB) is demonstrated by preventing the uptake of chemicals that can cause neuronal damage. The obstructive impact of the BBB is due to brain capillary endothelial cells, which inhibit transcellular transport and form tight junctions between cells by hindering paracellular transport. In the last decade, nanoparticles, which are designed materials in the range of nanoscale diameter with unique physiochemical properties, have been actively investigated as innovative therapeutic modalities to reduce the progression of Alzheimer’s disease. Although considering patient compliance and formulation aspects, the oral route may have certain drawbacks for unstable drugs in GI fluids and poorly soluble drug molecules. Thus, to overcome the disadvantages mentioned above, we have come up with a new nanoplatform that will avoid gastric degradation of the therapeutic moiety and enhance the solubility and bioavailability of the therapeutic moiety. Nanoparticles and their delivery are relatively new concepts and are a rapidly evolving science in the health sector. Various nanoformulations are frequently used in the drug delivery research to improve bioavailability, increase solubility, and target delivery with fewer side effects (Patra et al., 2018).
Liposomes are the most popular and well-researched nanovesicular systems for improved drug delivery. They can successfully incorporate both hydrophilic and lipophilic drugs. The main characteristics of liposomes include spherical vesicles consisting of an inner core hydrophilic partition enclosed by several compact lipidic layers. At the same time, conventional lipid carriers have several advantages. They have significant problems, too, such as stability issues, reproducibility, sterilization methods, poor drug entrapment, particle size control, etc. Instability in the form of drug leakage or separation of the phospholipid layer during the formulation and storage conditions could be possible drawbacks. Conventional liposomes are not advisable for oral administration because of bile salts, highly acidic gastric pH, and gastric enzyme activity in the Gastro-Intestinal Tract (GIT) (Laouini et al., 2012).
Stability is an essential parameter to know the quality of the dosage form, efficacy, and product safety. To further improve the stability and integrity of the conventional liposomes, they can be modulated by several methods, such as stabilization: (i) modulation of lipid composition, (ii) surface coating of the liposome, and (iii) interior thickening of the liposomal layer. Chitosan-coated liposomes are one such modification primarily intended for nonparenteral drug delivery (Malam et al., 2009).
Chitosan is one of the abundant and low-cost biopolymers globally, having multiple properties in drug delivery. Chitosan is formed by residues of glucosamine and acetyl-glucosamine linked with a 1–4 linkage. As a result of the electrostatic interaction between the negatively charged phospholipid group and the positively charged amino group of chitosan, a stable biopolymer coat is formed around the liposome, increasing its stability and preventing drug leakage. The positive charge at the surface of the chitosan is responsible for its mucoadhesive property, leads to prolong drug contact at the intestinal absorption site, and helps release the drug in a sustainable pattern (Le-Vinh et al., 2019). Maintaining stable colloidal liposomal suspension is a task that needs optimum repulsive interaction to prevent drug aggregation. The polyethylene glycol (PEG) coat on liposomal layers accomplishes steric stabilization, which helps in reducing aggregation with improved stability. Excellent drug distribution and bioavailability will be observed in steric liposomes due to the reduction of uptake by the reticuloendothelial system.
Asiatic acid (AA) is a potent pentacyclic triterpene compound obtained from Centella asiatica, also known as Brahmi, a well-established herb for memory enhancement and neuroprotective agent. The bioactive compound AA effectively modulates the enzymatic pathways, such as Beta-site amyloid precursor protein cleaving enzyme (BACE) 1, which is responsible for forming an Aβ protein. AA is sparingly soluble in aqueous media, and its poor dissolution and absorption result in low bioavailability, limiting its clinical use. By adapting it to a nanovesicular system, the effectiveness of its use in terms of bioavailability can be increased (Borhan et al., 2013). As a result, this research aims to create surface-modified AA liposomes and determine their in-vitro evaluation results to ensure the enhanced stability of the liposomal suspension.
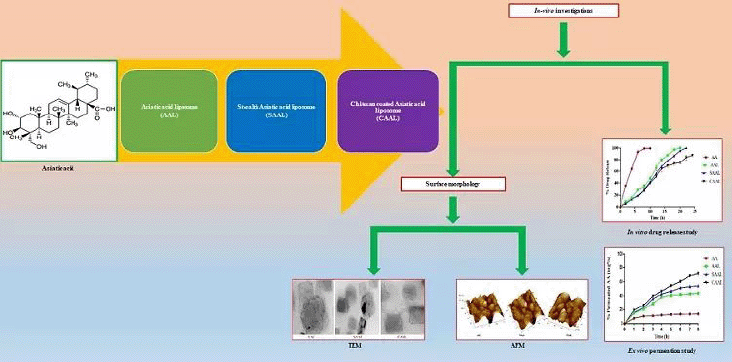
In the present investigation, we developed and optimized three types of liposomes of AA: AA liposome (AAL), chitosan-coated AA liposome (CAAL), and stealth AA liposome (SAAL), respectively. The prepared optimized formulations were then evaluated for compatibility studies by Fourier transform infrared spectroscopy (FTIR), liposomal vesicle size, drug entrapment and drug content studies, polydispersibility index (PDI), surface morphology evaluation by transmission electron microscopy (TEM), and atomic force microscopy (AFM), the percentage of in-vitro drug release, drug permeation studies, and stability evaluation.
MATERIALS AND METHODS
Materials
AA (97%w/w) was procured from Sigma-Aldrich, USA. Chitosan (50 kDa, 75%–85% deacetylated) was bought from Loba Chemie, Mumbai, India. DSPE-m PEG from Nice Chemicals Pvt. Ltd., Kochi, India. Soybean phosphatidylcholine (Lipoid® S 100, Lipoid® S 75) was a gift sample from Lipoid® GmbH, Germany. Cholesterol, potassium dihydrogen phosphate, dimethyl sulfoxide (DMSO), methanol, ethanol, chloroform, acetone, and sodium hydroxide were procured from Himedia Laboratories Pvt. Ltd., Mumbai, India. The remaining analytical grade chemicals were purchased from local distributors in Mangalore, India.
Preformulation studies
Descriptive terminology was used to describe the color, odor, taste, melting point, and solubility of AA. 10 mg of AA was weighed and dissolved in 10 ml of methanol to get 1,000 μg/ml. From this stock solution, it was diluted to achieve 10, 20, 30, 40, and 50 μg/ml. The λ-max and absorbance were measured using a UV spectrophotometer (version UV2600i, Shimadzu Japan) (Raval et al., 2015).
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR analysis was used to investigate the chemical compatibility between drugs and excipients and predict the possible physicochemical interactive nature of components. The IR spectra of drugs AA and soy phosphatidylcholine (SPC), cholesterol, chitosan, 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol) (PEG-DSPE), physical mixture, and liposomal formulation were carried out by FTIR spectroscopy (Alpha Bruker, Japan). A known amount of sample was mounted underneath the FTIR spectrophotometer probe as per the ATR process. The analysis was carried out at a wavelength of 4,000–500 cm−1. The comparison between the pure sample wavenumber and the physical mixture and formulation was determined and interpreted (Wang et al., 2017).
Formulation of conventional AA liposome (AAL)
The thin-film hydration using the solvent evaporation method was adopted to prepare the liposomes according to the previously reported survey with minor modifications. Several trials with different ratios of AA, SPC, and cholesterol were weighed and dispersed in the solvent dichloromethane (10 ml). The mixture was made and evaporated through a rotary evaporator with a rotation of 60 rpm at 55°C. The hydration of dry, thin lipid films was carried out using 6.5 pH phosphate buffer, followed by the handshake method to obtain multilaminar vesicles.
Optimization using the software: design of experiments
Significant factors influencing the AAL were identified according to previously reported articles. Preliminary screening was carried out. The optimization of liposomes was carried out through a central composite design to calculate the effect of the concentration of the phospholipid and cholesterol on dependent variables, such as entrapment efficiency (EE%) and vesicle size (nm), of the liposomes. The experiments were optimized by Design-Expert software (Version 11.0.3.0, 64-bit, Stat-Ease, Inc., MN).
Independent phospholipid and cholesterol concentrations were specified at three different factorial randomized quadratic design levels with 11 independent experimental trials. The implied model describes a quadratic equation with coefficient effects, interactions, and polynomial terms.
Y = b0 + b1X1 + b2X2 + b12X1X2 + b11X12 + b22X22 (1)
where Y is the total response related to each factor level; b0 is an intercept; b1–b22 are the regression coefficients computed from the observed experimental values of Y; and X1 and X2 are the coded levels of independent variables (Sailor et al., 2015), as shown in Table 1.
Formulation of CAAL
Initially, AAL was formulated by thin-film hydration using the thin hydration method. Later, the chitosan coat was rendered using an electrostatic interaction. At room temperature, the experimental structure and concrete values of the independent variables’ molecular weight chitosan were treated with 0.5% w/v acetic acid to solubilize and kept overnight. The optimized liposomal suspension was treated with a drop-wise chitosan solution at room temperature under continuous magnetic stirring for 1 hour. The formulation was then kept uninterrupted for 3–4 h before being treated with an ultraprobe sonicator (CV-18, Sonics and Materials Inc., USA) for 35 minutes to achieve the desired size (Li et al., 2009).
Formulation of electrosteric SAAL
A mixture of SPC, Tween 80, AA, and 1,2-distearoyl-sn-glycerol-3-phosphoethanolamine with conjugated methoxyl poly(ethylene glycol) (DSPE-mPEG) were dissolved in 10 ml of chloroform. The mixture was subjected to a rotary flash evaporator. The chloroform evaporated at 90 rpm to get the film. 10 ml of 7.4 pH phosphate buffer was used to hydrate the formed film layer. The hydrated mixture was kept under the ultraprobe sonicator for 18 minutes at 3 intervals to obtain the desired size range. The formulated samples were stored at a cool temperature for further use (Fatouh et al., 2021).
In-vitro evaluation of formulated AAL, CAAL, and SAAL
The liposomal vesicle size, polydispersity index, and zeta (ζ) potential
The mean hydrodynamic vesicle size (in nm) was determined by the dynamic light scattering method, measuring the width of the size dispersion as the PDI. The Malvern Zetasizer measured the electrokinetic potential of the colloidal liposomal suspensions (Malvern Instruments, Malvern, UK). At the beginning of the analysis, the formulations were sufficiently diluted with deionized water in a ratio of 1:10. Measurements were made in triplicate at a constant temperature of 25°C (Ghule and Bhoyar, 2018).
%Drug EE
The centrifugation method (direct method) was used to evaluate the percentage of drug entrapment of the formulated lipid vesicles. A liposomal sample aliquot (1 ml) was centrifuged at 5,000 rpm for 35 minutes at 4°C in a cold laboratory centrifuge (Remi Elektrotechnik Ltd., Vasai, India). The clear supernatant was carefully removed to isolate the unentrapped drug, and the diluted sample absorbance was taken at 209 nm (Hebbar et al., 2019). The %EE of the formulations was calculated with the following formula:
Drug content analysis
The liposomal formulations were evaluated to determine the concentration of AA. 1 ml of the liposomal formulation was treated with 10 ml of the solvent methanol, filtered through a membrane filter, and scanned under UV spectroscopy at an absorbance maximum of 209 nm with methanol as a blank (Buboltz and Feigenson, 1999).
Surface morphology
Transmission electron microscopical studies
The surface morphology of the formulated liposomes was analyzed using TEM. The samples were diluted, and a drop of this diluted solution was inserted separately onto a copper grid coated with carbon to form a film. The film was negatively stained by incorporating one drop of ammonium molybdate (2% w/w) into 2% w/v ammonium acetate buffer (pH 6.8). The negatively stained film was evaluated by CM 120 Bio Twin TEM Philips Electron optics BV, The Netherland (Sikarwar et al., 2008).
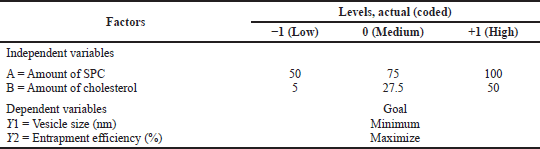 | Table 1. Variables for the 32 level of factorial design. [Click here to view] |
Atomic force microscopical studies
To visualize and compare the 3D surface morphology of the liposomes, AFM (Innova SPM, Bruker, Santa Barbara, CA) was used. The samples were smeared into the mica disk and visualized in contact mode with AFM tips at an image speed of 1.2 Hz and a 267–328 kHz resonance frequency (Aisha et al., 2014; Fadel et al., 2011).
Investigation of drug release
An in vitro AA release investigation was conducted through the Franz diffusion cell. 1 ml sample (100 mg/ml) was added and placed in the beaker containing the dissolution medium in the form of phosphate buffer saline with pH 7.4. The beaker contained a dissolution medium of 37 ± 0.5°C. The dissolution setup was kept on a 50 rpm magnetic stirrer. Buffers were made for similar intestinal environmental conditions and cerebrospinal fluid (pH 7.4). 5 ml of dissolution medium was withdrawn at different time intervals while the sink condition was maintained. The samples were filtered and measured at 209 nm through UV spectroscopy. The experiments were conducted in triplicates and are shown as the mean with standard deviation (Maiti et al., 2007; Preety et al., 2010).
Investigation of drug permeation
The ex-vivo permeation investigation of optimized AAL, SAAL, and CAAL using the intestinal using a noneverted gut sac method was performed. After sacrifice, the rat’s small intestine was isolated and washed using oxygenated saline (0.9% w/v). The rat’s small intestine sac with an 8 ± 0.5 cm length and a 3 ± 0.5 mm diameter was filled with optimized AAL, SAAL, and CAAL (~100 mg AA). The sac ends were bound with the thread and kept in Ringer’s solution (10 ml). The temperature of the solution was kept constant at 37°C, and the pH was made up to 9.0 in a shaking water bath at 100 rpm. The system was exposed to aeration with 5% CO2. The samples were withdrawn, filtered, and analyzed at the predetermined time intervals, i.e., every 20 minutes, up to 8 hours (Maiti et al., 2007).
Stability studies
Stability is the major problem in liposomal formulation. The stability test for formulated liposomes was conducted to comply with the International Council for Harmonisation (ICH) guidelines. The sonicated formulations were studied for stability at the refrigeration temperature (5 ± 2°C), controlled room temperature (23 ± 2°C) and (37 ± 2°C) at 60 ± 2°C % RH in a stability chamber (Thermolab, Scientific Equipment). Sampling was carried out at a definite time interval for 3 months, and suitable dilutions were made; UV absorbance was determined for drug content, %entrapment efficiency, and vesicle size analysis (Khan et al., 2010).
Stability of liposomal formulations in simulated gastric fluid (SGF)
The gastric stability of CAAL was determined by measuring turbidity. CAAL, AAL, and SAAL were added to SGF individually. SGF was prepared by mixing 0.32% (w/v) pepsin, 0.7% (v/v) hydrochloric acid, and 0.25% (w/v) sodium chloride to get a solution of pH 1.2. The turbidity of the formulation represents the chitosan concentration that influences the formulation; the higher the turbidity, the greater the risk of chitosan erosion from the vesicles. The Digital Nephelo Turbidity Meter (Systronics India Ltd, Ahmedabad, India) was used to calculate the turbidity of the liposomes, and the values were expressed as Nephelometric Turbidity Unit (NTU) (Vasanth et al., 2020).
RESULTS
Preformulation studies of AA
FTIR study
The probable physicochemical interaction between AA and other excipients of the liposomes was identified by FTIR spectroscopy. The chemical interaction of AA molecules with the phospholipids was observed and compared with the pure compound in terms of changes in a specific region. In formulations, evident stretch frequency changes were observed in the phenolic O-H of AA from 3,430.41 to 3,416.95 cm−1. Due to the molecular interaction during the formulation of liposomes, the absorption peak of phospholipids was altered at 1,226.66 and 1,061.61 cm−1. Peak retention was observed in the physical mixture of excipients and formulations. That indicates that the drug and other ingredients were physiochemically well suited to each other.
Formulation of AAL
The solvent evaporation process was adopted to formulate the conventional liposome of AA. The preliminary solubility test was conducted with organic solvents. The solvent dichloromethane was selected, considering its lower boiling point (39.6°C) and less toxicity (LD50 value of 1.5 g/kg in rats). As per the preliminary survey, the drug, SPC, and cholesterol ratio were selected and subjected to DoE. The role of cholesterol was to increase the stiffness and stability of liposomes. The mixtures were dissolved in dichloromethane and evaporated. The resulting film was dried at 45°C for 45 minutes to remove the solvent residue before being hydrated with phosphate buffers at pH 7.2. Sonication processes were carried out using an ultraprobe sonicator to achieve the desired size range (Tatode et al., 2018).
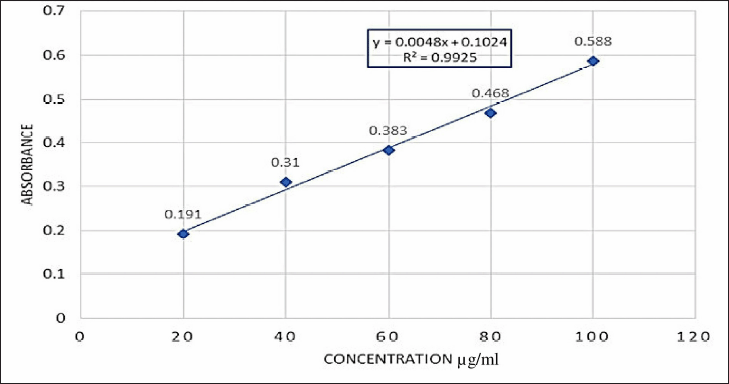 | Figure 1. Calibration curve of AA. [Click here to view] |
Optimization of liposomes
Design of experiments (DoE) by three factorial designs (32)
The optimization of liposomes based on all the responses with minimum particle size and maximum entrapment efficiency was carried out, as shown in Table 2. As suggested by the central composite design, all response variables were observed experimentally for 11 runs and matched to the run design chart. Following the withdrawal of nonimportant coefficients, the appropriate correlations for response variables in terms of coded factors were obtained.
Vesicle size:
Y1 = +271.53 − 15.14A − 6.82B --- (2)
Percentage entrapment efficiency:
Y2 = +70.41 − 3.93A − 1.77B -- (3)
where A indicates SPC concentration and B is cholesterol concentration.
Numerical optimization
The obtained equations were statistically significant (p = 0.5). The independent variables’ influences are shown in Figure 2. The color red represents the maximum value and the color blue represents the minimum value. In Figure 2, the 3D surface response curve shows that the vesicle size decreases when there is an increase in the concentration of both SPC and cholesterol. The drug entrapment rate increased with the drug–SPC ratio increase. The percentage error was calculated and found to be less than 5%. The vesicle size of the optimized formulated liposomes was 274.25 nm, with a 72.98% drug entrapment efficiency (Table 3). The correlation between observed experimental values and predicted median values clearly shows no notable difference between predicted and experimental values, and both are within the suggested model’s range.
Formulation of CAAL and SAAL
Chitosan was coated on their surfaces to improve colloidal stability and control release by increasing mucoadhesion to the negatively charged cell membrane. The negatively charged surface of the liposome tightly coated by the positively charged chitosan is because of the ionic interaction between SPC and the chitosan’s positive charged amino group. The chitosan coating also prevents drugs from leaking out of the vesicular system (Mengoni et al., 2017). SAAL was formulated by coating PEG-DSPE onto the conventional lipid layers to reduce the uptake of macrophages during systemic circulation. So, the surface modulation will lead SAAL to circulate for a prolonged time as a therapeutic reservoir.
In -vitro evaluation of formulated AAL, CAAL, and SAAL
Vesical size, PDI, and zeta (ζ) potential
The physical stability of liposomes in a medium is determined by vesicle size, PDI, and ζ potential. The chitosan coating increases the thickness of the vesicles compared to conventional liposomes. The average vesicle size of sonicated optimized CAAL was obtained at 246.3 ± 0.4 nm. The appropriate chitosan coat and vesicle sizes help in the maximum absorption efficiency of the drug AA, and a sustained release pattern of AA was observed. At the same time, AAL and SAAL were 212.4 ± 0.73 nm and 221.56 ± 0.32 nm, respectively. The PDI values of AAL, CAAL, and SAAL were 0.054, 0.315, and 0.478, respectively, indicating a slight series of vesicle size dispersal. A practical method for evaluating the surface electrical charge of a particle, which shows the potential stability of a vesicular system, is ζ potential measurement. A distinctive quality of the ζ potential was reported to be a surface charge of −20 to +20 mV, followed by flocculation exceeding repulsive forces. The optimized formulations of AAL, CAAL, and SAAL showed ζ potentials of −15.35 mV, 22.8 mV, and −14.8 mV. This ζ potential resulted in a high level of physical stability. The positive charge of CAAL favors cohesion to the cell membrane and facilitates penetration into the mucous membrane (Bannunah et al., 2014).
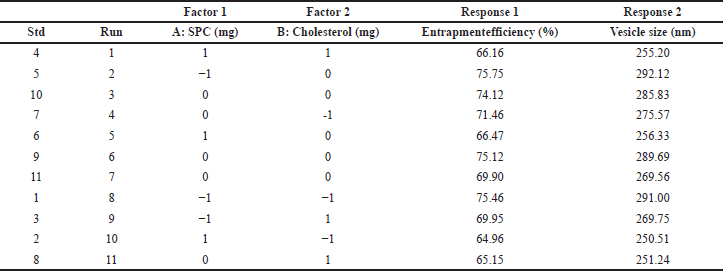 | Table 2. 32 level factorial design experimental trial batches with obtained vesicle size (nm) and entrapment efficiency (%). [Click here to view] |
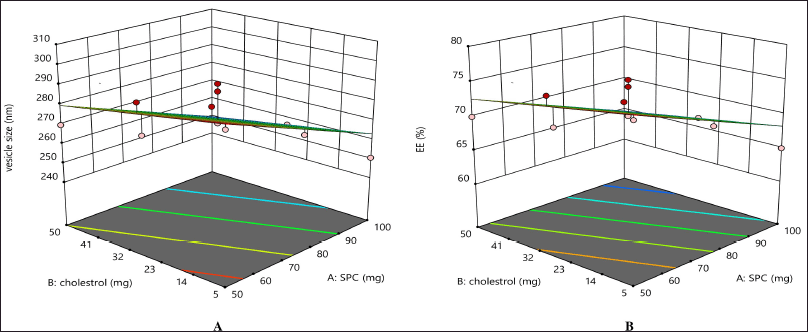 | Figure 2. Independent variables’ influence on the formulation. [Click here to view] |
 | Table 3. Comparison of experimental results with predicted responses of optimized formulation. [Click here to view] |
Percentage entrapment eficiency (%EE)
The drug AA entrapped in CAAL was 74.2 ± 0.1% due to the stable chitosan coat and larger vesicle size. On the contrary, AAL and SAAL were found to be 64.3 ± 0.4% and 69.78 ± 0.2% (n = 3), respectively. The optimized formulation of CAAL had a significantly higher percentage of entrapment of the drug due to its stable vesicular form. The drug is insoluble in the external solvent phase but shows higher affinity toward phospholipid membranes, which results in enhanced entrapment efficiency of the liposome. The free drugs were kept untouched in the formulation to prevent drug loss (Hooresfand et al., 2015; Vijayakumar et al., 2017).
Surface morphology
TEM
The morphological evaluation, such as fusion and aggregation of the colloidal suspension or thickness of the bilayer in multilaminar vesicles, was determined using TEM images. TEM images of optimized AAL, CAAL, and SAAL (Fig. 3) showed well-formed, separate vesicles free from aggregation. When optimized liposomes were swirled in distilled water, they revealed a nanovesicle structure with no aggregation or degradation (Jiang et al., 2016; Ravi et al., 2018).
AFM
The high-resolution three-dimensional image of AFM detects the repulsive or attractive surface forces between the vesicles that could scan and visualize the sample’s surface texture, size, and morphology. The AFM image of the formulations (Fig. 4) revealed well-formed, discrete vesicles with no sign of accumulation or decomposition. The formation indicates an improved dissolution profile than the pure drug and shows better stability (Hossain et al., 2015).
Investigation of drug release
An in -vitro drug release study was conducted with a modified Franz diffusion cell to compare the percentage of drugs released from the formulations. The obtained data can be correlated with the in -vitro and in -vivo drug release values. The drug release profile of AAL, CAAL, and SAAL in pH 7.4 phosphate buffer for 24 hours was determined and compared with the pure AA suspension shown in Figure 5. This investigation demonstrated that AA rapidly releases 99.60 ± 0.43% in about 8 hours. However, compared to AA, AAL and SAAL demonstrated sustained release and improved drug release rates, with 97.00 ± 0.56% and 86.42 ± 0.38% release in 18 hours, respectively. However, the drug release of CAAL was found to be 87.42 ± 0.43% in 24 hours, which was a better-sustained drug release compared to all other forms of the liposome. The physicochemical alteration and complexation between AA and SPC and chitosan are responsible for the drug release from the optimized CAAL. The mechanism of drug discharge involves layer swelling and dispersion, and erosion of the polymer layer due to the hydrophilic nature of chitosan. Compared to the uncoated liposome of pure drugs, it possibly enhanced the solubility and wettability of the complex (Dash et al., 2010; Jain et al., 2012).
">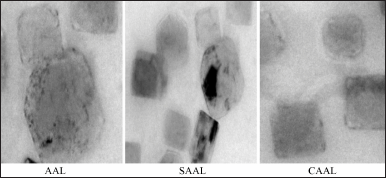 | Figure 3. TEM images of optimized AAL, CAAL, and SAAL formulations. [Click here to view] |
The organoleptic characteristics of AA were compared to those of the Merck index’s standard document. The AA powder was crystalline in nature, mildly pungent in taste, odorless, and white in color. The melting point of AA was found to be 325 ± 1°C. The solubility of the AA powder in different solvents, such as distilled water, 7.4 pH phosphate buffer, methanol, dichloromethane, ethanol, chloroform, acetone, and methylene chloride, about 20 mg/ml in DMF and DMSO, was examined visually and qualitatively. It was found that methanol, dichloromethane, and other organic solvents showed a clear AA solution (approximately 10 mg/ml). According to the Biopharmaceutics ClassificationSystem (BCS) classification,AA belongs to class IV, with low aqueous solubility and low permeability (Mengoni et al., 2017; The Merck Index Online—chemicals, drugs and biologicals, n.d.). The calibration of AA was determined with an absorbance of 209 nm. It exhibited a linear graph with a regression coefficient Rs) of 0.992523 (Kapp, 2006) (Fig. 1).
FTIR study
The probable physicochemical interaction between AA and other excipients of the liposomes was identified by FTIR spectroscopy. The chemical interaction of AA molecules with the phospholipids was observed and compared with the pure compound in terms of changes in a specific region. In formulations, evident stretch frequency changes were observed in the phenolic O-H of AA from 3,430.41 to 3,416.95 cm−1. Due to the molecular interaction during the formulation of liposomes, the absorption peak of phospholipids was altered at 1,226.66 and 1,061.61 cm−1. Peak retention was observed in the physical mixture of excipients and formulations. That indicates that the drug and other ingredients were physiochemically well suited to each other.
Formulation of AAL
The solvent evaporation process was adopted to formulate the conventional liposome of AA. The preliminary solubility test was conducted with organic solvents. The solvent dichloromethane was selected, considering its lower boiling point (39.6°C) and less toxicity (LD50 value of 1.5 g/kg in rats). As per the preliminary survey, the drug, SPC, and cholesterol ratio were selected and subjected to DoE. The role of cholesterol was to increase the stiffness and stability of liposomes. The mixtures were dissolved in dichloromethane and evaporated. The resulting film was dried at 45°C for 45 minutes to remove the solvent residue before being hydrated with phosphate buffers at pH 7.2. Sonication processes were carried out using an ultraprobe sonicator to achieve the desired size range (Tatode et al., 2018).
Optimization of liposomes
Design of experiments (DoE) by three factorial designs (32)
The optimization of liposomes based on all the responses with minimum particle size and maximum entrapment efficiency was carried out, as shown in Table 2. As suggested by the central composite design, all response variables were observed experimentally for 11 runs and matched to the run design chart. Following the withdrawal of nonimportant coefficients, the appropriate correlations for response variables in terms of coded factors were obtained.
Vesicle size:
Y1 = +271.53 − 15.14A − 6.82B --- (2)
Percentage entrapment efficiency:
Y2 = +70.41 − 3.93A − 1.77B -- (3)
where A indicates SPC concentration and B is cholesterol concentration.
Numerical optimization
The obtained equations were statistically significant (p = 0.5). The independent variables’ influences are shown in Figure 2. The color red represents the maximum value and the color blue represents the minimum value. In Figure 2, the 3D surface response curve shows that the vesicle size decreases when there is an increase in the concentration of both SPC and cholesterol. The drug entrapment rate increased with the drug–SPC ratio increase. The percentage error was calculated and found to be less than 5%. The vesicle size of the optimized formulated liposomes was 274.25 nm, with a 72.98% drug entrapment efficiency (Table 3). The correlation between observed experimental values and predicted median values clearly shows no notable difference between predicted and experimental values, and both are within the suggested model’s range.
Formulation of CAAL and SAAL
Chitosan was coated on their surfaces to improve colloidal stability and control release by increasing mucoadhesion to the negatively charged cell membrane. The negatively charged surface of the liposome tightly coated by the positively charged chitosan is because of the ionic interaction between SPC and the chitosan’s positive charged amino group. The chitosan coating also prevents drugs from leaking out of the vesicular system (Mengoni et al., 2017). SAAL was formulated by coating PEG-DSPE onto the conventional lipid layers to reduce the uptake of macrophages during systemic circulation. So, the surface modulation will lead SAAL to circulate for a prolonged time as a therapeutic reservoir.
In -vitro evaluation of formulated AAL, CAAL, and SAAL
Vesical size, PDI, and zeta (ζ) potential
The physical stability of liposomes in a medium is determined by vesicle size, PDI, and ζ potential. The chitosan coating increases the thickness of the vesicles compared to conventional liposomes. The average vesicle size of sonicated optimized CAAL was obtained at 246.3 ± 0.4 nm. The appropriate chitosan coat and vesicle sizes help in the maximum absorption efficiency of the drug AA, and a sustained release pattern of AA was observed. At the same time, AAL and SAAL were 212.4 ± 0.73 nm and 221.56 ± 0.32 nm, respectively. The PDI values of AAL, CAAL, and SAAL were 0.054, 0.315, and 0.478, respectively, indicating a slight series of vesicle size dispersal. A practical method for evaluating the surface electrical charge of a particle, which shows the potential stability of a vesicular system, is ζ potential measurement. A distinctive quality of the ζ potential was reported to be a surface charge of −20 to +20 mV, followed by flocculation exceeding repulsive forces. The optimized formulations of AAL, CAAL, and SAAL showed ζ potentials of −15.35 mV, 22.8 mV, and −14.8 mV. This ζ potential resulted in a high level of physical stability. The positive charge of CAAL favors cohesion to the cell membrane and facilitates penetration into the mucous membrane (Bannunah et al., 2014).
Percentage entrapment eficiency (%EE)
The drug AA entrapped in CAAL was 74.2 ± 0.1% due to the stable chitosan coat and larger vesicle size. On the contrary, AAL and SAAL were found to be 64.3 ± 0.4% and 69.78 ± 0.2% (n = 3), respectively. The optimized formulation of CAAL had a significantly higher percentage of entrapment of the drug due to its stable vesicular form. The drug is insoluble in the external solvent phase but shows higher affinity toward phospholipid membranes, which results in enhanced entrapment efficiency of the liposome. The free drugs were kept untouched in the formulation to prevent drug loss (Hooresfand et al., 2015; Vijayakumar et al., 2017).
Surface morphology
TEM
The morphological evaluation, such as fusion and aggregation of the colloidal suspension or thickness of the bilayer in multilaminar vesicles, was determined using TEM images. TEM images of optimized AAL, CAAL, and SAAL (Fig. 3) showed well-formed, separate vesicles free from aggregation. When optimized liposomes were swirled in distilled water, they revealed a nanovesicle structure with no aggregation or degradation (Jiang et al., 2016; Ravi et al., 2018).
AFM
The high-resolution three-dimensional image of AFM detects the repulsive or attractive surface forces between the vesicles that could scan and visualize the sample’s surface texture, size, and morphology. The AFM image of the formulations (Fig. 4) revealed well-formed, discrete vesicles with no sign of accumulation or decomposition. The formation indicates an improved dissolution profile than the pure drug and shows better stability (Hossain et al., 2015).
Investigation of drug release
An in -vitro drug release study was conducted with a modified Franz diffusion cell to compare the percentage of drugs released from the formulations. The obtained data can be correlated with the in -vitro and in -vivo drug release values. The drug release profile of AAL, CAAL, and SAAL in pH 7.4 phosphate buffer for 24 hours was determined and compared with the pure AA suspension shown in Figure 5. This investigation demonstrated that AA rapidly releases 99.60 ± 0.43% in about 8 hours. However, compared to AA, AAL and SAAL demonstrated sustained release and improved drug release rates, with 97.00 ± 0.56% and 86.42 ± 0.38% release in 18 hours, respectively. However, the drug release of CAAL was found to be 87.42 ± 0.43% in 24 hours, which was a better-sustained drug release compared to all other forms of the liposome. The physicochemical alteration and complexation between AA and SPC and chitosan are responsible for the drug release from the optimized CAAL. The mechanism of drug discharge involves layer swelling and dispersion, and erosion of the polymer layer due to the hydrophilic nature of chitosan. Compared to the uncoated liposome of pure drugs, it possibly enhanced the solubility and wettability of the complex (Dash et al., 2010; Jain et al., 2012).
Investigation of drug permeation
The drug absorption in the intestinal region of the rat can be effectively determined using everted and noneverted intestinal sac models. However, some significant advantages are associated with the noneverted sac model, such as lower sample volume requirements and less intestinal morphological damage, because of the absence of eversion. The study measured the transport mechanisms and correlated them with in -vivo drug absorption. This study analyzed the %permeation rate of AA and optimized AAL, SAAL, and CAAL, as shown in Figure 6 (n ≥ 3). The amount of drugs permeated throughout 8 hours from AA was found to be 13.97 ± 0.5%, whereas optimized CAAL was found to be better (71.63 ± 3.47%) than the optimized SAAL (54.06 ± 2.23%) and AAL (43.81 ± 1.74%) and exhibited substantial deviation in permeation rate. It probably occurred because of the bioadhesive property of the chitosan that led to an increase in accommodating time and extended adsorption rate in the mucosal region. Moreover, optimized CAAL is more stable in simulated intestinal pH than AA, optimized AAL, and SAAL.
Stability studies
The term “stability studies” refers to an ingredient’s physical and chemical integrity during its shelf life. Liposomes are thermodynamically unstable systems. The major problem involves vesicles that might fuse, increasing their size and breaking the liposomes, followed by drug loss in storage (Taira et al., 2004). The stability data of AAL, CAAL, and SAAL at 4 ± 2°C, 24°C, and 37 ± 2°C at 60% ± 2% RH were analyzed, respectively. Since phospholipid in liposomes deteriorates at higher temperatures high room temperature), stability cannot be carried out at those temperatures. The evaluated parameters, such as vesicle size (nm), drug content (%), and entrapment efficiency (%), were observed for up to 3 months, and AAL showed increased vesicle size in all three different temperatures. It was in the range of 246.3–272.6 nm. After the third month of storage, there was no significant difference in vesicle size between CAAL and SAAL (274.2 nm and 253.6 nm, respectively). The entrapment efficiency of AAL showed a decrease in room temperature and a slight decrease in 24 ± 2°C and 4 ± 2°C, and there was a considerable change in the range of 68.03%–52.37 %. Throughout the study, CAAL and SAAL showed higher entrapment efficiency, i.e., 76.26% and 69.26%. The drug content in AAL decreased in the range of 77.28%–62.12%. However, drug content in CAAL and SAAL did not vary at 76.26% and 63.26%, respectively. Therefore, it was proved that coated liposomal formulations have much better stability than uncoated liposomes.
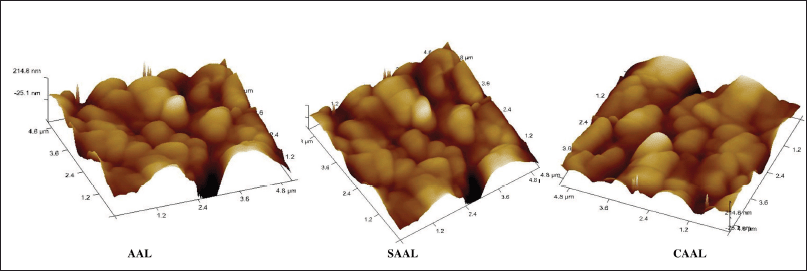 | Figure 4. AFM images of the optimized AAL, CAAL, and SAAL formulations. [Click here to view] |
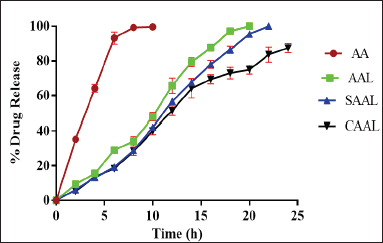 | Figure 5. Drug release profiles of AAL, CAAL, and SAAL in pH 7.4 phosphate buffer for 24 hours were determined and compared with the pure AA suspension. [Click here to view] |
Liposomal stability study in SGF
The liposomal formulations of CAAL and SAAL were compared to uncoated liposomes to ensure the integrity of the vesicles in SGF. The turbidity units of CAAL were found to be 15.8 ± 0.002 NTU, whereas the turbidities of SAAL and AAL were 41.3 ± 0.003 and 46.7 ± 0.001 NTU, respectively, indicating vesicle leakage. The hydrochloric acid present in the gastric fluid causes an acidic pH with the hydrolysis of disulfide bonds of the liposome. The enzyme pepsin interacts with the SAAL and AAL, transferring hydrogen ions [H+] responsible for the breakdown of the phospholipid layer by disturbing liposomal integrity. Both the formulations were unstable in gastric pH, so the study proved that AAL and SAAL formulations are not suitable for oral administration (Gaber et al., 2017).
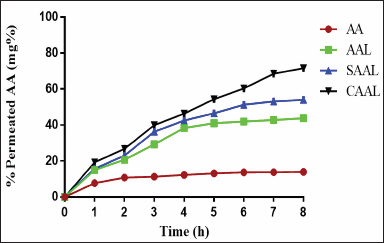 | Figure 6. %Permeation rate of AA and optimized AAL, SAAL, and CAAL. [Click here to view] |
DISCUSSION
This investigation involved the fabrication of liposomes using the thin-film hydration method and was optimised using factorial design and characterised. The selection of the lipids was made based on the solubility of the AA. The liposomes were optimised using a factorial design using two independent variables, viz., the concentration of phospholipid and cholesterol on dependent variables such as EE% and the vesicle size of the liposomes. Similarly, DSPE-mPEG was used to make Asiatic acid liposomes (AAL), chitosan-coated Asiatic acid liposomes (CAAL), and Stealth Asiatic acid liposomes (SAAL). The prepared optimised formulations were evaluated for compatibility by Fourier transform infrared spectroscopy (FTIR), liposomal vesicle size, drug entrapment and drug content studies, polydispersibility index (PDI), and surface morphology studies by TEM and AFM, in vitro drug release percentage, and stability evaluation.
The compatibility study was performed using FTIR, which resulted in AA displaying compatibility with other ingredients utilised in the fabrication of liposomes. There was no shifting, stretching, or bending at the peak. The chemical interaction of AA molecules with the phospholipids was observed and compared with pure compounds in terms of changes in a specific region. A practical method for evaluating the surface electrical charge of a particle, which shows the potential stability of a vesicular system (liposomes), is ζ potential measurement. This ζ potential resulted in a high level of physical stability. The positive charge of CAAL favours cohesion to the cell membrane and facilitates penetration into the mucous membrane. The physicochemical alteration and complexation between AA and SPC and chitosan are responsible for the drug release from the optimised CAAL. Compared to the uncoated liposome of pure drugs, it possibly enhanced the solubility and wettability of the complex. Additionally, the release study demonstrated that CAAL reduced the drug release compared to SAAL, AAL, and AA, which may be due to the electrostatic interaction between chitosan and the surface liposomes. (Mengoni et al., 2017) The sustained drug release will help reduce the drug dosing frequency and avoid acute or chronic toxicity to healthy tissues. The ex-vivo permeation study revealed that CAAL showed higher permeability through the biological membrane than SAAL, AAL, and AA. This may be because chitosan’s properties can significantly open up the tight
junctions of epithelial cells. Additionally, it probably occurred because of the bio-adhesive property of the chitosan that leads to an increase in residence time and extended adsorption rate at a mucosal site. (Li et al., 2009) Moreover, optimised CAAL is more stable in simulated intestinal pH than AA, the optimised AAL, and SAAL.
The different fabricated nanocarriers can be significantly utilised for efficient brain targeting to treat or prevent Alzheimer’s disease. The present investigation gives proof of concept, which can be further studied in the form of pharmacokinetics, biodistribution, and pharmacodynamic studies to determine the efficiency of AA in the prevention and treatment of Alzheimer’s disease. The results indicated that CAAL displayed greater efficiency towards reduction in AA release from the liposomes and increased permeation through the intestinal membrane of rats compared to SAAL, AAL, and pure AA.
CONCLUSION
Nowadays, liposomal vesicular drug delivery is gaining more importance in recent times due to its lipophilic enhanced drug delivery in the nanoscale colloidal form. The study was designed to prepare and evaluate AA in three liposomal forms, i.e., conventional, chitosan-coated, and stealth liposomes. FTIR, TEM, and AFM confirmed the formation of liposomes. A better drug release rate was found and prolonged in CAAL and SAAL compared to AAL and AA. CAAL showed better stability in SGF than SAAL and AAL, which showed poor stability and more turbidity. It indicates the degradation of phospholipid layers in the presence of acidic pH. Hence, AAL and SAAL formulations are not suitable for oral drug delivery concerning CAAL. For 3 months, stability study data of AAL, CAAL, and SAAL showed that vesicle size, drug content, and percentage entrapment efficiency of CAAL and SAAL were consistent without any deviation compared to AAL. This study made an effort to develop better surface-modified lipid carriers and evaluate them in-vitro characterization. However, extensive studies are required to authenticate the possible outcome of the developed formulation.
CONFLICT OF INTEREST
The authors declare that they have no financial or other conflict of interest.
AUTHORSHIP CONTRIBUTION
All the authors substantially contributed to this research article in accordance with the ICMJE guidelines.
ACKNOWLEDGMENTS
The authors would like to thank the support of Manipal College of Pharmaceutical Sciences and NGSM Institute of Pharmaceutical Sciences.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aisha AFA, Majid AMSA, Ismail Z. Preparation and characterization of nano liposomes of Orthosiphon stamineusethanolic extract in soybean phospholipids. BMC Biotechnol, 2014; 14:23; doi:10.1186/1472-6750-14-23 CrossRef
Bannunah AM, Vllasaliu D, Lord J, Stolnik S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: effect of size and surface charge. Mol Pharmaceutics, 2014; 11:4363–73; doi:10.1021/mp500439c. CrossRef
Borhan MZ, Ahmad R, Rusop M, Abdullah S. Green extraction: enhanced extraction yield of asiatic acid from Centella asiatica (L.) nanopowders. J Appl Chem, 2013; 2013:1–7; doi:10.1155/2013/460168. CrossRef
Buboltz JT, Feigenson GW. A novel strategy for the preparation of liposomes: rapid solvent exchange. Biochim Biophys Acta (BBA) – Biomembranes, 1999; 1417:232–45; doi:10.1016/S0005-2736(99)00006-1 CrossRef
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010;67:217–23.
Fadel O, el Kirat K, Morandat S. The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochimica et Biophysica Acta, 2011; 1808:2973–80; doi:10.1016/j.bbamem.2011.08.011. CrossRef
Fatouh AM, Elshafeey AH, Abdelbary A. Galactosylated chitosan coated liposomes of ledipasvir for liver targeting: chemical synthesis, statistical optimization, in-vitro and in-vivo evaluation. J Pharm Sci, 2021; 110:1148–59; doi:10.1016/j.xphs.2020.10.002 CrossRef
Gaber DM, Nafee N, Abdallah OY. Myricetin solid lipid nanoparticles: Stability assurance from system preparation to site of action. Eur J Pharm Sci, 2017; 109:569–80; doi:10.1016/j.ejps.2017.08.007 CrossRef
Ghule MM, Bhoyar GS. Formulation and evaluation of modified liposome for transdermal drug. J Dev Drugs, 2018; 07:1–3; doi:10.4172/2329-6631.1000186 CrossRef
Hebbar S, Dubey A, Ravi GS, Kumar H, Saha S. RP-HPLC method development and validation of asiatic acid isolated from the plant Centella asiatica. Int J Appl Pharm, 2019; 11:72–8; doi:10.22159/ijap.2019v11i3.31525 CrossRef
Hooresfand Z, Ghanbarzadeh S, Hamishehkar H. Preparation and characterization of rutin-loaded nanophytosomes. Pharm Sci, 2015; 21:145–51; doi:10.15171/PS.2015.29 CrossRef
Hossain S, Hashimoto M, Katakura M, Al Mamun A, Shido O. Medicinal value of asiaticoside for Alzheimer’s disease as assessed using single-molecule-detection fluorescence correlation spectroscopy, laser-scanning microscopy, transmission electron microscopy, and in silico docking. BMC Complement Altern Med, 2015; 15:118; doi:10.1186/s12906-015-0620-9 CrossRef
Jain S, Kumar D, Swarnakar NK, Thanki K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials, 2012; 33:6758–68; doi:10.1016/j.biomaterials.2012.05.026 CrossRef
Jiang Q, Yang X, Du P, Zhang H, Zhang T. Dual strategies to improve oral bioavailability of oleanolic acid: Enhancing water-solubility, permeability and inhibiting cytochrome P450 isozymes. Eur J Pharm Biopharm, 2016; 99:65–72; doi:10.1016/j.ejpb.2015.11.013 CrossRef
Kapp RW. Clarke’s analysis of drugs and poisons. In: Moffat AC, Osselton MD, Widdop B (eds.). 3rd edition, Pharmaceutical Press, London, UK, 2004. ISBN: 0-853-69473-7. Volume I: 480 pages; Volume II: 1176 pages. Price: $545.00. Int J Toxicol, 2006; 25:81–2; doi:10.1080/10915810500527093. CrossRef
Khan H, Ali M, Ahuja A, Ali J. Stability testing of pharmaceutical products-comparison of stability testing guidelines. Curr Pharm Anal, 2010; 6:142–50. CrossRef
Laouini A, Jaafar-Maalej C, Limayem-Blouza I, Sfar S, Charcosset C, Fessi H. Preparation, characterization and applications of liposomes: state of the art. J Colloid Sci Biotechnol, 2012; 1:147–68; doi:10.1166/jcsb.2012.1020 CrossRef
Le-Vinh B, Le N-MN, Nazir I, Matuszczak B, Bernkop-Schnürch A. Chitosan based micelle with zeta potential changing property for effective mucosal drug delivery. Int J Biol Macromol, 2019; 133:647–55; doi:10.1016/j.ijbiomac.2019.04.081 CrossRef
Li N, Zhuang C, Wang M, Sun X, Nie S, Pan W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm, 2009; 379:131–8; doi:10.1016/j.ijpharm.2009.06.020 CrossRef
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin–phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm, 2007; 330:155–63; doi:10.1016/j.ijpharm.2006.09.025 CrossRef
Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci, 2009; 30:592–9; doi:10.1016/j.tips.2009.08.004 CrossRef
Mengoni T, Adrian M, Pereira S, Santos-Carballal B, Kaiser M, Goycoolea F. A Chitosan—based liposome formulation enhances the in vitro wound healing efficacy of substance P neuropeptide. Pharmaceutics, 2017; 9:56; doi:10.3390/pharmaceutics9040056 CrossRef
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Kumara Swamy M, Sharma S, Habtemariam S, Shin H. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol, 2018; 16:71; doi:10.1186/s12951-018-0392-8 CrossRef
Panwar Preety, Pandey Bhumika, Lakhera P C SKP. Preparation, characterization, and in vitro release study of albendazole-encapsulated nanosize liposomes. Int J Nanomed, 2010; 5:101; doi:10.2147/IJN.S8030 CrossRef
Raval N, Mistry T, Acharya N, Acharya S. Development of glutathione-conjugated asiatic acid-loaded bovine serum albumin nanoparticles for brain-targeted drug delivery. J Pharm Pharmacol, 2015; 67:1503–11; doi:10.1111/jphp.12460 CrossRef
Ravi GS, Charyulu RN, Dubey A, Prabhu P, Hebbar S, Mathias AC. Nano-lipid complex of rutin: development, characterisation and in vivo investigation of hepatoprotective, antioxidant activity and bioavailability study in rats. AAPS PharmSciTech, 2018; 19:3631–49; doi:10.1208/s12249-018-1195-9 CrossRef
Sailor G, Seth A, Parmar G, Chauhan S, Javia A. Formulation and in vitro evaluation of berberine containing liposome optimized by 32 full factorial designs. J Appl Pharm Sci, 2015; 5:023–8; doi:10.7324/JAPS.2015.50704 CrossRef
Sikarwar MS, Sharma S, Jain AK, Parial SD. Preparation, characterization and evaluation of marsupsin–phospholipid complex. AAPS PharmSciTech, 2008; 9:129–37; doi:10.1208/s12249-007-9020-x CrossRef
Taira MC, Chiaramoni NS, Pecuch KM, Alonso-Romanowski S. Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Deliv, 2004; 11:123–8; doi:10.1080/10717540490280769. CrossRef
Tatode AA, Patil AT, Umekar MJ. Application of response surface methodology in optimization of paclitaxel liposomes prepared by thin film hydration technique. Int J Appl Pharm, 2018; 10:62; doi:10.22159/ijap.2018v10i2.24238 CrossRef
The Merck Index Online—chemicals, drugs and biologicals. n.d. Available via https://www.rsc.org/merck-index (Accessed 26 April 2022).
Vasanth S, Dubey A, Ravi GS, Lewis SA, Ghate VM, El-Zahaby SA, Hebbar S. Development and investigation of vitamin c-enriched adapalene-loaded transfersome gel: a collegial approach for the treatment of acne vulgaris. AAPS PharmSciTech, 2020; 21:61; doi:10.1208/s12249-019-1518-5 CrossRef
Vijayakumar A, Baskaran R, Jang YS, Oh SH, Yoo BK. Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. AAPS PharmSciTech, 2017; 18:875–83; doi:10.1208/s12249-016-0573-4 CrossRef
Wang A, Ahmad A, Ullah S, Cheng L, Ke L, Yuan Q. A cheap and convenient method of liposome preparation using glass beads as a source of shear force. AAPS PharmSciTech, 2017; 18:3227–35; doi:10.1208/s12249-017-0812-3 CrossRef
GRAPHICAL ABSTRACT
 | [Click here to view] |