INTRODUCTION
Azo dyes constitute 70% of dye chemistry, and their relative significance may increase in the future (Alsoghier et al., 2021; Benkhaya et al., 2020; Chen et al., 2021; Gester et al., 2020; Ben Mohamed-Smati et al., 2021; Omar et al., 2021; Prashantha et al., 2021; Rashidnejad et al., 2021; Selvaraj et al., 2021; Srinivasan and Sadasivam, 2021; Sweidan et al., 2018; Weldegebrieal, 2020). Empagliflozin (Fig. 1) is a competitive inhibitor of sodium-glucose co-transporter-2 that is orally active and has an antihyperglycemic effect (Hailat et al., 2022). It is approved for treating adults with type 2 diabetes in the EU, USA, and Japan, among other parts of the world (Frampton, 2018). This mechanism is independent of β-cell function; thus, these agents effectively treat type 2 diabetes mellitus at any disease stage (Levine, 2016; Mula-Abed and Aughsteen, 2005). Many methods have been adopted to determine empagliflozin (Ahmad et al., 2021). The liquid chromatography-mass spectrometry method was developed, optimized, and validated for simultaneous quantification of empagliflozin and metformin in human plasma using empagliflozin D4 and metformin D6 as an internal standard (Wattamwar et al., 2020). An Liquid Chromatography with tandem mass spectrometry (LC-MS-MS) method was developed to determine empagliflozin and metformin using a bridged ethylene hybrid C18 column (Ayoub and Mowaka, 2017). Another univariate spectrophotometric method and multivariate chemometric approach were developed and compared to determine empagliflozin simultaneously and metformin manipulating their zero-order absorption spectra with application to their pharmaceutical preparation (Mabrouk et al., 2019). 4-Nitroaniline forms molecular adducts with 4-aminobenzoic acid. It reacts with nitrite ion in a hydrochloric acid medium to form 4-nitrophenyldiazonium chloride, which couples with naphth-1-ol in an alkaline medium to give a purple azo dye. (Figure 2) Photocatalytic degradation of 4-nitroaniline in the presence of TiO2 suspensions in a batch and continuous annular reactor has been studied (Abed-Elmageed et al., 2020; Ayoub et al., 2021; Baveja et al., 1981; Marchewka et al., 2011; Wu et al., 2012). Sulfanilamide is an organic sulfur compound similar to p-aminobenzoic acid (PABA) with antibacterial properties. Sulfanilamide competes with PABA for the bacterial enzyme dihydropteroate synthase, thereby preventing the incorporation of PABA into dihydrofolic acid, the immediate precursor of folic acid (Dionisio et al., 2018; United States Pharmacopeial Convention, 2007).
Effect of acid, base concentration, and temperature used
The effect of acid and base on the diazotization reaction of empagliflozin (2 µgml−1) was studied by adding different acidic solutions (1 M) such as HCl, HNO3, H2SO4, and CH3COOH and basic solutions (1 M) such as KOH, NaOH, Na2CO3, and NH4OH. It was observed that CH3COOH gave low absorbance with low color stability. In contrast, HCl gave high absorbance with the highest color stability, whereas 1.0 ml of NaOH gave the maximum absorbance for the reaction of empagliflozin coupled with diazotized 3-chloro-4-nitroaniline or sulfanilamide. Therefore, 0.5 ml of 0.5 M HCl (Table 1) and 1.0 ml of 1 M NaOH solutions were preferred for the diazotization reaction of empagliflozin.
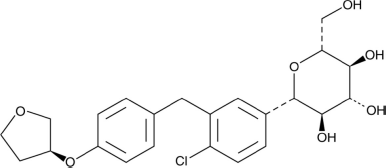 | Figure 1. Empagliflozin structure. [Click here to view] |
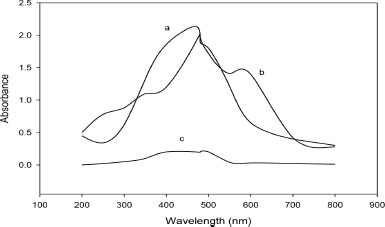 | Figure 2. Absorption spectra of the diazo-couple of nitrite with 3-chloro-4-nitroaniline against reagent blank (a), absorption spectra of the diazo-couple of nitrite with sulfanilamide against reagent blank (b), and reagent blank against double-distilled water (c). [Click here to view] |
The effect of various acids such as HCl, HNO3, H2SO4, and CH3COOH (0.5 M) on the diazotization reaction was studied under the maximum absorbance by varying the volume of different acids between 0.25 and 1.0 ml while fixing all other parameters. It was found that 0.5 ml of HCl (0.5 M) gave the highest absorbance and was preferred for the diazotization reaction of empagliflozin (Table 2).
Room temperature (25°C ± 5°C) is recommended for these diazotization reactions because losses in color intensity and stability were observed at low or high temperature.
Effect of nitrite concentration and coupling reagents
The color is at maximum intensity when using 1 ml of a 0.1 M sodium nitrite solution using the current procedure with 2 µgml−1 of empagliflozin and adding 1 ml of 0.02–0.16 M solutions of the nitrite in hydrochloric acid (0.5 M) to a series of nitrite solutions. A higher concentration did not build up the absorbance further, and at a lower concentration, no good results were obtained (Table 3).
The current procedure uses 3-chloro-4-nitroaniline or sulfanilamide as a coupling agent by taking 2 µgml−1 of empagliflozin and adding 0.25–2.0 ml of 1% 3-chloro-4-nitroaniline or sulfanilamide to a string of nitrite solutions. The firmest color was obtained with 1 ml of a 3-chloro-4-nitroaniline or sulfanilamide (1%) solution in 10.0 ml (Table 4).
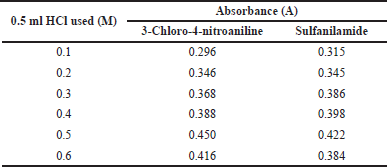 | Table 1. Acid concentration on absorbance. [Click here to view] |
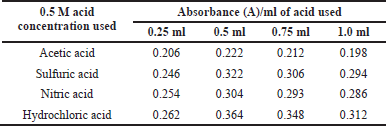 | Table 2. Different acid concentrations on absorbance. [Click here to view] |
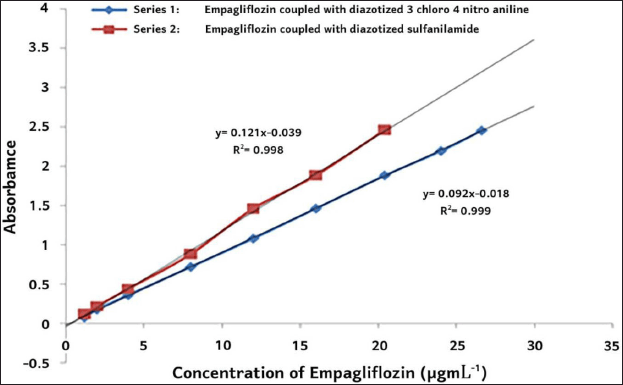 | Figure 3. Adherence to Beer’s law using empagliflozin coupled with diazotized 3-chloro-4-nitroaniline and sulfanilamide. [Click here to view] |
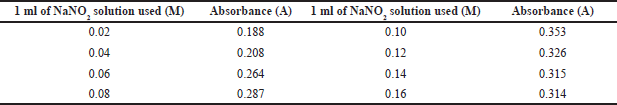 | Table 3. Effect of NaNO2 on absorbance. [Click here to view] |
Effect of interference
Some excipients generally present in the pharmaceutical preparations were examined by carrying out the determination of empagliflozin in the presence of different excipients such as glucose (1,200 µgml−1), fructose (1,000 µgml−1), lactose (800 µgml−1), starch (600 µgml−1), and urea (300 µgml−1), which did not interfere.
Analytical data
A straight line is obtained in the graph by plotting absorbance beside the concentration of empagliflozin. (Figure 3) Beer’s law is obeyed in the range of 1.2–26.6 µgml−1 of empagliflozin with 3-chloro-4-nitroaniline or 0.8–20.4 µgml−1 of empagliflozin with sulfanilamide (Figure 3). The molar absorptivity of the colored azo dye of empagliflozin coupled with diazonium salt 3-chloro-4-nitroaniline or sulfanilamide was Scheme 1 and 2 3.179 × 104 l mol−1cm−1 or 4.367 × 104 l mol−1cm−1 (Figure 3). On the other hand, Sandell’s sensitivity to the colored system with nitrite-3-chloro-4-nitroaniline or nitrite-sulfanilamide was found to be 1.149 × 10−2 µgcm−2 or 8.368 × 10−3 µgcm−2, respectively.
The detection limit (DL = 3.3 σ/S) and quantitation limit (QL = 10 σ/S) of empagliflozin coupled with diazotized 3-chloro-4-nitroaniline or sulfanilamide were found to be 0.363 and 1.100 µgml−1 or 0.270 and 0.820 µgml−1 [where σ is standard deviation (n = 5) and S is slope of the curve] and the correlation coefficient of empagliflozin with 3-chloro-4-nitroaniline or empagliflozin with sulfanilamide was 0.999 or 0.998. The better optical characteristics and statistical data were obtained under optimum conditions (Table 5).
Applications
This simple and uncomplicated method is beneficial for determining empagliflozin in different pharmaceutical samples. The results of the offered method are in good agreement with the acknowledged content. The relative standard deviation and percentage recoveries for all five samples ranged from 0.81% to 2.27% and 98.00% to 100.40% at 95% confidence. The additional ingredients present in pharmaceutical sample appearances did form, not hinder. The results (Table 6) are compared with the endorsed spectrophotometric method (Ayoub, 2016; Patil et al., 2017). These confirm no significant differences between the offered and endorsed methods. The precision and accuracy were evaluated by replicate analysis of three different samples containing empagliflozin at different concentrations.
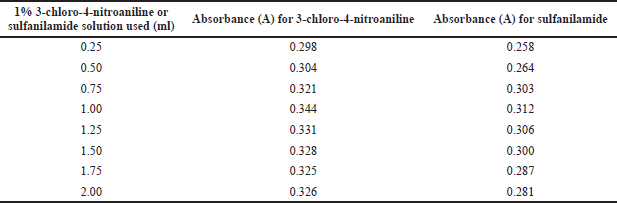 | Table 4. Effect of 3-chloro-4-nitroaniline or sulfanilamide solution on absorbance. [Click here to view] |
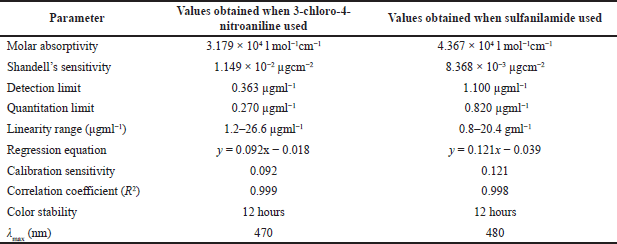 | Table 5. Optical characteristics and statistical data. [Click here to view] |
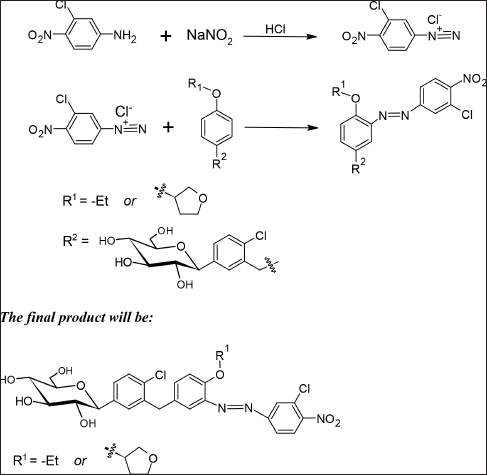 | Scheme 1. Formation of colored azo dye. [Click here to view] |
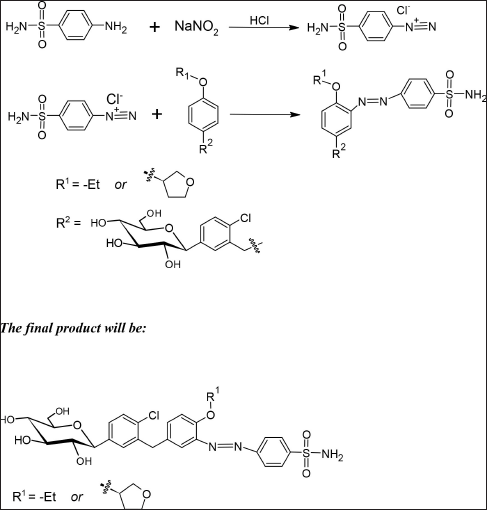 | Scheme 2. Formation of colored azo dye. [Click here to view] |
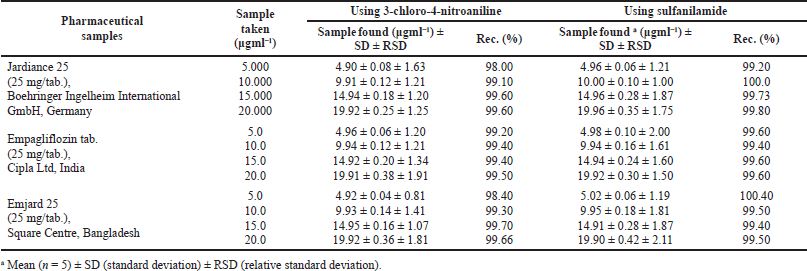 | Table 6. Determination of empagliflozin in different pharmaceutical samples using 3-chloro-4-nitroaniline or sulfanilamide as a coupling agent for three trade names of empagliflozin. [Click here to view] |
CONCLUSION
Sulfanilamide and 3-chloro-4-nitroaniline, the first spectrophotometric coupling agents used to determine empagliflozin, are inexpensive and equitably selective. Compared to other methods, this one is simple, quick, sensitive, and reproducible, has good precision and accuracy, and has high dye stability (12 hours).
As low relative standard deviation and percentage recovery values highlighted good accuracy and precision of the proposed methods, no tedious separation or solvent extraction procedures were required. There is no interference from excipients in results obtained using the proposed methods. The proposed method examined empagliflozin levels in pharmaceutical samples, which can be applied to more complex samples. For example, a blood sample to determine the blood level of empagliflozin helps in various pharmacokinetic and toxicological studies.
ACKNOWLEDGMENTS
The authors would like to thank those who helped finish this research, especially the Faculty of Pharmacy at Mutah University.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abed-Elmageed AAI, Zoromba MS, Hassanien R, Al-Hossainy AF. Facile synthesis of spin-coated poly (4-nitroaniline) thin-film: structural and optical properties. Opt Mater (Amst), 2020; 109:110378; doi:10.1016/j.optmat.2020.110378. CrossRef
Ahmad R, Hailat M, Jaber M, Alkhawaja B, Rasras A, Al-Shdefat R, Mallah EY, Abu Dayyih W. RP-HPLC method development for simultaneous estimation of empagliflozin, pioglitazone, and metformin in bulk and tablet dosage forms. Acta Pol Pharm Res, 2021; 78:305–15; doi:10.32383/appdr/139635. CrossRef
Alsoghier HM, Abdellah M, Rageh HM, Salman HMA, Selim MA, Santos MA, Ibrahim SA. NMR spectroscopic investigation of benzothiazolylacetonitrile azo dyes: CR7 substitution effect and semiempirical study. Results Chem, 2021; 3:100088; doi:10.1016/j.rechem.2020.100088. CrossRef
Ayoub B, El Zahar N, Michel H, Tadros M. Economic spectrofluorometric bioanalysis of empagliflozin in rats’ plasma. J Anal Methods Chem, 2021; 2021:1–7; doi:10.1155/2021/9983477. CrossRef
Ayoub BM. Development and validation of simple spectrophotometric and chemometric methods for simultaneous determination of empagliflozin and metformin: applied to recently approved pharmaceutical formulation. Spectrochim Acta A Mol Biomol Spectrosc, 2016; 168:118–22; doi:10.1016/j.saa.2016.06.010. CrossRef
Ayoub BM, Mowaka S. LC-MS/MS determination of empagliflozin and metformin. J Chromatogr Sci, 2017; 55:742–7; doi:10.1093/CHROMSCI/BMX030. CrossRef
Baveja AK, Nair J, Gupta VK. Extraction—spectrophotometric determination of sub-microgram amounts of nitrite using 4-nitroaniline and naphth-1-0l. Analyst, 1981; 106:955–9; doi:10.1039/AN9810600955. CrossRef
Benkhaya S, M’rabet S, El Harfi A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon, 2020; 6:e03271; doi:10.1016/j.heliyon.2020.e03271. CrossRef
Chen J, Hu H, Yang Junhan, Xue H, Tian Y, Fan K, Zeng Z, Yang J, Wang R, Liu Y. Removal behaviors and mechanisms for series of azo dye wastewater by novel nano constructed macro-architectures material. Bioresour Technol, 2021; 322:124556; doi:10.1016/j.biortech.2020.124556. CrossRef
Dionisio KL, Phillips K, Price PS, Grulke CM, Williams A, Biryol D, Hong T, Isaacs KK. Data descriptor: the chemical and products database, a resource for exposure-relevant data on chemicals in consumer products. Sci Data, 2018; 5:1–9; doi:10.1038/sdata.2018.125. CrossRef
Frampton JE. Empagliflozin: a review in type 2 diabetes. Drugs, 2018; 78:1037–48; doi:10.1007/s40265-018-0937-z. CrossRef
Gester R, Torres A, Bistafa C, Araújo RS, da Silva TA, Manzoni V. Theoretical study of a recently synthesized azo dyes useful for OLEDs. Mater Lett, 2020; 280:128535; doi:10.1016/j.matlet.2020.128535. CrossRef
Hailat M, Zakaraya Z, Al-Ani I, Al Meanazel O, Al-Shdefat R, Anwer MK, Saadh MJ, Abu Dayyih W. Pharmacokinetics and bioequivalence of two empagliflozin, with evaluation in healthy Jordanian subjects under fasting and fed conditions. Pharmaceuticals, 2022; 15:193; doi:10.3390/ph15020193. CrossRef
Levine MJ. Empagliflozin for type 2 diabetes mellitus: an overview of phase 3 clinical trials. Curr Diabetes Rev, 2016; 13:405; doi:10.2174/1573399812666160613113556. CrossRef
Mabrouk MM, Soliman SM, El?Agizy HM, Mansour FR. A UPLC/DAD method for simultaneous determination of empagliflozin and three related substances in spiked human plasma. BMC Chem, 2019; 13:1–9; doi:10.1186/s13065-019-0604-9. CrossRef
Marchewka MK, Drozd M, Janczak J. Crystal and molecular structure of N-(4-nitrophenyl)-β-alanine—its vibrational spectra and theoretical calculations. Spectrochim Acta A Mol Biomol Spectrosc, 2011; 79:758–66; doi:10.1016/j.saa.2010.08.050. CrossRef
Ben Mohamed-Smati S, Faraj FL, Becheker I, Berredjem H, Le Bideau F, Hamdi M, Dumas F, Rachedi Y. Synthesis, characterization and antimicrobial activity of some new azo dyes derived from 4-hydroxy-6-methyl-2H-pyran-2-one and its dihydro derivative. Dye Pigment, 2021; 188:109073; doi:10.1016/j.dyepig.2020.109073. CrossRef
Mula-Abed WAS, Aughsteen AA. Biochemical analysis of serum pancreatic amylase and lipase enzymes in patients with type 1 and type 2 diabetes mellitus (multiple letters). Saudi Med J, 2005; 26:1158–60.
Omar AZ, Mahmoud MN, El-Sadany SK, Hamed EA, El-Atawy MA. A combined experimental and DFT investigation of mono azo thiobarbituric acid based chalcone disperse dyes. Dye Pigment, 2021; 185:108887; doi:10.1016/j.dyepig.2020.108887. CrossRef
Patil SD, Chaure SK, Rahman MAH, Varpe PU, Kshirsagar S. Development and validation of simple UV-spectrophotometric method for the determination of empagliflozin. Asian J Pharm Anal, 2017; 7:18; doi:10.5958/2231-5675.2017.00004.7. CrossRef
Prashantha AG, Shoukat Ali RA, Keshavayya J. Synthesis, spectral characterization, DFT studies and antimicrobial activities of amino-methylbenzoic acid based azo dyes. Inorg Chem Commun, 2021; 127:108392; doi:10.1016/j.inoche.2020.108392. CrossRef
Rashidnejad H, Ramezanitaghartapeh M, Pesyan NN, Mahon PJ, Raposo MMM, Coelho PJ, Lup AN, Soltani A. A comprehensive spectroscopic, solvatochromic and photochemical analysis of 5-hydroxyquinoline and 8-hydroxyquinoline mono-azo dyes. J Mol Struct, 2021; 1223:129323; doi:10.1016/j.molstruc.2020.129323. CrossRef
Selvaraj V, Swarna Karthika T, Mansiya C, Alagar M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J Mol Struct, 2021; 1224:129195; doi:10.1016/j.molstruc.2020.129195. CrossRef
Srinivasan S, Sadasivam SK. Biodegradation of textile azo dyes by textile effluent non-adapted and adapted Aeromonas hydrophila. Environ Res, 2021; 194:110643; doi:10.1016/j.envres.2020.110643. CrossRef
Sweidan K, Zalloum H, Sabbah DA, Idris G, Abudosh K, Mubarak MS. Synthesis, characterization, and anticancer evaluation of some new N 1-(anthraquinon-2-yl) amidrazone derivatives. Can J Chem, 2018; 96:1123–8; doi:10.1139/cjc-2018-0145. CrossRef
United States Pharmacopeial Convention. USP DI. Thomson/MICROMEDEX, Greenwood Village, CO, Vol. 1, 2007.
Wattamwar T, Mungantiwar A, Halde S, Pandita N. Development of simultaneous determination of empagliflozin and metformin in human plasma using liquid chromatography–mass spectrometry and application to pharmacokinetics. Eur J Mass Spectrom, 2020; 26:117–30; doi:10.1177/1469066719879297. CrossRef
Weldegebrieal GK. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: a review. Inorg Chem Commun, 2020; 120:108140; doi:10.1016/j.inoche.2020.108140. CrossRef
Wu W, Liu G, Xie Q, Liang S, Zheng H, Yuan R, Su W, Wu L. A simple and highly efficient route for the preparation of p-phenylenediamine by reducing 4-nitroaniline over commercial CdS visible light-driven photocatalyst in water. Green Chem, 2012; 14:1705–9; doi:10.1039/c2gc35231a. CrossRef