INTRODUCTION
Etazolate is a pyrazolopyridine-derived compound that exhibits a wide range of pharmacological properties (Alzoubi et al., 2018; Megat et al., 2021; Siopi et al., 2013). Etazolate enhances GABAergic signaling through positive allosteric modifications of GABA(A) receptors, which could explain its anxiolytic activity (Marcade et al., 2008; Thompson et al., 2002). Etazolate is also a selective phosphodiesterase-4 inhibitor, and thus it can be used to manage mood and cognitive disorders (Ankur et al., 2013; Jindal et al., 2015; Wang et al., 2014). For example, using animal models, etazolate showed positive results in the treatment of neuropsychiatric disorders such as depression and anxiety associated with traumatic brain injury via normalization of oxidative stress and restoring the Cyclic adenosine monophosphate (cAMP) signaling pathway (Jindal et al., 2017). In addition, etazolate was shown to stimulate soluble N-terminal amyloid precursor protein fragment (sAPPalpha) secretion and subsequent elevations in the soluble amyloid precursor protein that can protect neurons from the development of beta-amyloid and Alzheimer’s disease (Marcade et al., 2008). Moreover, etazolate has been shown to improve spatial learning and memory performance related to the homing task in aged rats (Drott et al., 2010). A pilot phase IIA, randomized, double-blind, multicenter clinical trial that included 159 patients with Alzheimer’s disease demonstrated the safety and tolerability of etazolate and its ability to alter disease progression (Vellas et al., 2011). Similarly, data generated from phase 1 clinical trials of Alzheimer’s disease showed that the etazolate has an acceptable safety profile with fewer side effects than other phosphodiesterase-4 inhibitors (Desire et al., 2008). This made etazolate an attractive agent to be implicated in Alzheimer’s disease treatment (Drott et al., 2010; Vellas et al., 2011). Additionally, etazolate was shown to protect against memory impairment anxiety- and depression-like behaviors induced by posttraumatic stress disorder (Alzoubi et al., 2017, 2019) and Parkinson’s disease (Alzoubi et al., 2018).
The majority of studies with etazolate have focused on the clinical uses of this drug (Alzoubi et al., 2018; Jindal et al., 2017; Marcade et al., 2008; Megat et al., 2021). However, studies that examine the genotoxicity and cytotoxicity of etazolate to human cells are needed. Previous studies showed that some pyrazolopyridine derivatives might have DNA-binding affinity (El-Gohary and Shaaban, 2018; El-Gohary et al., 2019; Suntharalingam et al., 2010). In addition, some pyrazolopyridine derivatives showed antitumor and antiproliferative activity and could interfere with cell cycle progression (Baviskar et al., 2013; Butu et al., 2014, 2015; Gavriil et al., 2016; Rodino et al., 2014). Therefore, the current investigation aims to evaluate the genotoxicity and cytotoxicity of different concentrations of etazolate in cultured human lymphocytes using the chromosomal aberrations (CAs) assay, the oxidative DNA damage 8-hydroxy-2-deoxyguanosine (8-OHdG) assay, and the mitotic index (MI) assay.
`
MATERIALS AND METHODS
Blood sampling
Five healthy young men (aged 20–27 years) donated blood for laboratory experiments. Blood donors were nonsmokers and nonalcoholics and had not taken any medications or supplements for at least 3 months before donating blood (Rababa et al., 2021). Blood samples were collected in lithium heparin tubes and cultured fresh within 1 hour of donation (Al-Eitan et al., 2020).
Ethical approval
The study was approved by the Institutional Review Board of King Abdullah University Hospital (Approval No. 23/93/2016). Informed consent was taken from the donors before blood sampling.
Etazolate preparation
Etazolate hydrochloride (Cas No. 35838-58-5) was obtained from Bio-Apex (Houston, TX, Catalog No. B6304) as a powder. The drug was freshly prepared before each experiment by dissolving it in sterile distilled water to obtain a 10 mM stock solution. The stock solution was sterilized using a Millipore filter (0.2 μM) before being used to treat cultured blood lymphocytes. Cultures were treated with different doses of etazolate (final concentrations: 0.01, 1, 10, and 50 µM). These doses were within the range that was previously used with cultured cells (Barnes et al., 1983; Marcade et al., 2008; Mehta and Ticku, 1987).
Human lymphocyte cultures
Freshly drawn blood (1 ml) was inoculated into a tissue culture flask containing 9 ml PB-MAX Media (Thermo Fisher Scientific, MA). Cultures were incubated at 37°C, 5% CO2, and appropriate humidity for 72 hours (Alqudah and Al-Ashwal, 2018).
CAs assay
Cultured cells were treated with different concentrations of etazolate and the positive control (cisplatin, Sigma-Aldrich, USA; final concentration 0.4 μg/ml) in the last 24 hours of the 72-hour incubation period (Azab et al., 2019). After cell incubation, harvesting was initiated by arresting the cultured cells for 2 hours in the mitotic phase with 0.1 µg/ml colcemid (Sigma-Aldrich) (Mhaidat et al., 2016). Cultures were then transferred to 15 ml conical tubes and centrifuged at 500 × g for 5 minutes. The supernatant was removed, and cells were then resuspended in 9 ml of prewarmed hypotonic solution (0.56% KCL) and then incubated at 37°C for 20 minutes. Swelled cells were collected by centrifugation at 500 × g for 5 minutes, the supernatant was removed, and cells were then fixed in methanol: acetic acid (3:1) for 10 minutes at room temperature (Khabour et al., 2016). The cells were then washed with the above fixative 3×, and finally, cells were resuspended in about 1.5 ml of the fixative. The suspended cells were then dropped onto prechilled glass slides to obtain metaphase chromosomal spreads. Chromosomes were then stained by immersing the slides in slide jars containing 5% Giemsa stain (Sigma-Aldridge, USA) in a phosphate buffer for 7 minutes. Slides were then air-dried, and metaphases were visualized using a Nikon medical microscope at 1,000 × magnification. CAs were examined in 500 cells per treatment (100 cells from each donor). CAs were categorized into breaks/exchanges and gaps (Khabour et al., 2013).
MI assay
The MI is a measure of the degree of cellular proliferation and is used to measure cell division (Romansik et al., 2007). The MI was assessed by random analysis >5,000 cells per drug concentration (at least 1,000 cells per concentration per donor) along with scoring of cells that were in the metaphase stage. The MI was expressed as the ratio of the number of metaphases seen over the total number of intact cells seen (Al-Sweedan et al., 2012).
The 8-OHdG assay
8-Hydroxyl-2-deoxy guanosine is a naturally occurring hydroxyl radical-damaged guanosine that is generated during cellular processes (Esmadi et al., 2016). The 8-OHdG test is widely used to assess oxidative DNA damage induced by exposure to chemical agents and disease conditions (Urbaniak and Boguszewska, 2020; Valavanidis et al., 2009). Lymphocytes were cultured and incubated as described for the CAs assay (see above). After 72 hours of incubation, cultured cells were centrifuged at 500 × g for 5 minutes, and the supernatant was removed. The cell pellets were then washed 5× with 9 ml of RPMI 1,640 medium each, and then the cells were resuspended in 1 ml of RPMI 1,640 medium (Alzoubi et al., 2012). Thereafter, the cell suspension was treated with etazolate or the positive control (cisplatin) for 6 hours at 37°C. Tubes were then centrifuged at 500 × g, and 8-OHdG levels were quantified in the supernatant using an enzyme-linked immunoassay (ELISA) kit from Abcam (Cambridge, UK; Catalog No. ab201734) according to the protocol supplied with the kit. Plates were read at 450 nm using an automated ELISA reader (Bio-Tek, Winooski, VT). A standard for known concentrations of 8-OHdG was provided by the kit and was used to calculate the 8-OHdG biomarker in samples.
Statistical analysis
Statistical analysis was conducted using the GraphPad Prism software (version 5). All parameters were compared using analysis of variance and Tukey’s post hoc test. A p < 0.05 indicates a statistical difference.
RESULTS
Table 1 shows the frequency of gap aberrations after lymphocyte cultures were treated for 24 hours under different concentrations (0.1, 1, 10, and 50 μM) of etazolate. None of the examined etazolate concentrations increased the frequency of gap aberrations observed in the control group (p > 0.05). On the other hand, the positive control cisplatin at 0.4 μg/ml caused significant increases in the frequency of gap aberrations by several folds.
When considering CAs (breaks/exchanges), results similar to those of gap aberrations were obtained (Table 2). Thus, etazolate did not induce genotoxicity in cultured human lymphocytes at concentrations up to 50 μM as examined using the CA assay.
Figure 1 shows the level of 8-OHdG in cultured lymphocytes treated with different concentrations of etazolate. 8-OHdG levels were not significantly different between the etazolate groups and the control group (p > 0.05). The treatment of cultures with cisplatin, the positive control, caused significant (p < 0.01) increases in 8-OHdG at a concentration of 0.4 μg/ml. Thus, etazolate did not induce oxidative DNA damage as assessed by the 8-OHdG biomarker.
The MI assay was used to evaluate the antiproliferative potential of etazolate in cultured human lymphocytes. Cultures treated with different concentrations of etazolate showed similar mitotic indices to those of the control group (Fig. 2, p > 0.05). On the other hand, the positive control, cisplatin, at 0.4 µg/ml significantly reduced the MI (p < 0.05). Thus, etazolate did not have antiproliferative effects on cultured human lymphocytes at all examined concentrations.
DISCUSSION
In the current study, the genotoxicity and antiproliferative effects of etazolate were evaluated in cultured human lymphocytes. The results showed that etazolate at concentrations up to 50 μM did not cause CAs or oxidative DNA damage as measured in cultured human lymphocytes by the CAs and 8-OHdG assay, respectively. In addition, etazolate did not affect the MI of cultured cells.
Etazolate is a neuroprotective agent that showed promising results in the treatment of Alzheimer’s disease, depression, anxiety, and memory impairments (Alzoubi et al., 2018; Ankur et al., 2013; Drott et al., 2010; Jindal et al., 2015). Etazolate is a pyrazolopyridine derivative, and previous studies showed that some pyrazolopyridine-derived compounds could interact with DNA (El-Gohary and Shaaban, 2018; El-Gohary et al., 2019; Suntharalingam et al., 2010). In addition, some pyrazolopyridine derivatives showed antitumor and antiproliferative activity and could block the progression of the cell cycle (Baviskar et al., 2013; Gavriil et al., 2016). Furthermore, some studies have reported the ability of phosphodiesterase inhibitors to modulate DNA repair systems via alterations of the cellular cAMP-Protein Kinase A pathway (Ghorbani et al., 2015; Parkkonen et al., 2008; Sabisz and Skladanowski, 2008). These reports prompted us to evaluate the potential genotoxic effect of etazolate in cultured human cells using the CAs assay and the 8-OHdG assay. These assays are widely used, sensitive, and robust indicators of genotoxicity (Mateuca et al., 2006; Urbaniak and Boguszewska, 2020; Valavanidis et al., 2009). According to the present results, etazolate is not genotoxic to cultured lymphocytes at the examined concentrations (range 0.01 to 50 µM). The CAs are primarily caused by agents that directly interact with DNA or interfere with its replication. Regarding 8-OHdG, it can be generated by chemical agents that trigger oxidative stress. According to the previous literature, not all pyrazolopyridine derivatives can interact with DNA (El-Gohary and Shaaban, 2018; El-Gohary et al., 2019; Suntharalingam et al., 2010). In addition, etazolate may possess antioxidant and neuroprotective properties as shown using animal models (Alzoubi et al., 2018; Alzoubi et al., 2017; Jindal et al., 2015, 2017). Thus, etazolate appears to be a promising and safe neuroprotective agent. Further studies using other cell types and/or other in vivo genotoxicity models are needed to confirm the present findings.
 | Table 1. Frequencies (mean ± S.E.) of gap aberrations induced by treatments. [Click here to view] |
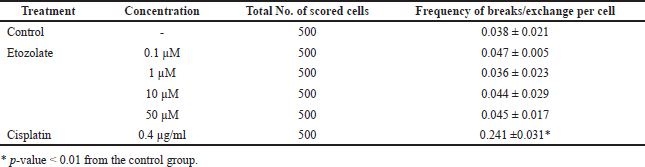 | Table 2. Frequencies (mean ± S.E.) of chromosomal breaks/exchanges induced by treatments. [Click here to view] |
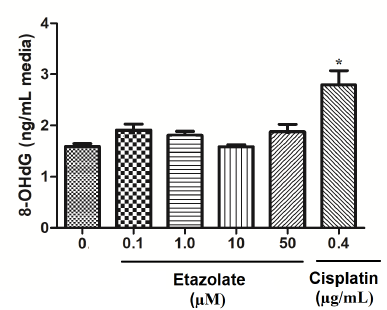 | Figure 1. Induction of 8-OHdG in cultured human lymphocytes by different treatments. Cultures were treated with different concentrations of etazolate or cisplatin for 6 hours. Data are expressed as mean ± SD. * indicates a significant difference from the control group (p < 0.01). [Click here to view] |
 | Figure 2. Changes in the MI of cultured human lymphocytes induced by different treatments. Cultures were treated with different concentrations of etazolate or cisplatin for 24 hours. Data are expressed as mean ± SD. * indicates a significant difference from the control group (p < 0.05). [Click here to view] |
In the current study, etazolate at the concentrations examined did not impact the proliferative capacity of cultured human lymphocytes. In a study on Leishmania donovani promastigotes, etazolate was shown to have an antiproliferative effect at a dose of 24 µM (Saha et al., 2020). In addition, in an animal study on mice epidermal mitosis, etazolate has been shown to inhibit mitosis at concentrations between 10 and 100 µM (Birnbaum et al., 1976). On the other hand, etazolate has been shown to delay the apoptosis and cell death of intestinal epithelial cells induced by chemical agents (Joseph et al., 2005). Thus, the potential antiproliferative effect of etazolate can vary according to the cell type and/or animal model used.
In the current study, an in vitro culture system was used. Previous studies have shown that metabolic activation within the body may significantly impact the genotoxicity and the cytotoxicity of chemical compounds (Ku et al., 2007; Oliveira et al., 2021; Zhou et al., 2007). Therefore, in vivo studies are needed to confirm the present in vitro findings.
CONCLUSION
In the current study, using the CAs, 8-OHdG, and MI assays, etazolate was found to be neither genotoxic nor cytotoxic to cultured human lymphocytes. More in vivo studies are needed to confirm these results.
ACKNOWLEDGMENTS
The authors would like to thank the subjects for donating their blood to conduct the study experiments.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
The study was funded by a grant number (57/2016) from the Deanship of Scientific Research at Jordan University of Science and Technology to KA and OK.
ETHICAL APPROVALS
The study was approved by the Institutional Review Board of King Abdullah University Hospital (Approval No. 23/93/2016).
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
AUTHORS’ CONTRIBUTION
All authors contributed to the study design, writing of the grant proposal, conduction of experimental work, data analysis, and interpretations, participated in drafting and/or revising the manuscript, and approved the final version.
DATA AVAILABILITY
Data will be available upon request via e-mailing the corresponding author.
REFERENCES
Al-Eitan LN, Alzoubi KH, Al-Smadi LI, Khabour OF. Vitamin E protects against cisplatin-induced genotoxicity in human lymphocytes. Toxicol In Vitro, 2020; 62:104672. CrossRef
Al-Sweedan SA, Khabour O, Isam R. Genotoxicity assessment in patients with thalassemia minor. Mutat Res, 2012; 744(2):167–71. CrossRef
Alqudah MAY, Al-Ashwal FY, Alzoubi KH, Alkhatatbeh M, Khabour O. Vitamin E protects human lymphocytes from genotoxicity induced by oxaliplatin. Drug Chem Toxicol, 2018; 41(3):281–6. CrossRef
Alzoubi K, Khabour O, Hussain N, Al-Azzam S, Mhaidat N. Evaluation of vitamin B12 effects on DNA damage induced by pioglitazone. Mutat Res, 2012; 748(1-2):48–51. CrossRef
Alzoubi KH, Al Subeh ZY, Khabour OF. Evaluating the protective effect of etazolate on memory impairment, anxiety- and depression-like behaviors induced by post traumatic stress disorder. Brain Res Bull, 2017; 135:185–92. CrossRef
Alzoubi KH, Mokhemer E, Abuirmeileh AN. Beneficial effect of etazolate on depression-like behavior and, learning, and memory impairment in a model of Parkinson’s disease. Behav Brain Res, 2018; 350:109–15. CrossRef
Alzoubi KH, Al Subeh ZY, Khabour OF. Molecular targets for the interactive effect of etazolate during post-traumatic stress disorder: Role of oxidative stress, BDNF and histones. Behav Brain Res, 2019; 369:111930. CrossRef
Ankur J, Mahesh R, Bhatt S. Anxiolytic-like effect of etazolate, a type 4 phosphodiesterase inhibitor in experimental models of anxiety. Indian J Exp Biol, 2013; 51(6):444–9.
Azab B, Alassaf A, Abu-Humdan A, Dardas Z, Almousa H, Alsalem M, Khabour O, Hammad H, Saleh T, Awidi A. Genotoxicity of cisplatin and carboplatin in cultured human lymphocytes: a comparative study. Interdiscip Toxicol, 2019; 12(2):93–7. CrossRef
Barnes DM, White WF, Dichter MA. Etazolate (SQ20009): electrophysiology and effects on [3H]flunitrazepam binding in cultured cortical neurons. J Neurosci, 1983; 3(4):762–72. CrossRef
Baviskar AT, Banerjee UC, Gupta M, Singh R, Kumar S, Gupta MK, Kumar S, Raut SK, Khullar M, Singh S, Kumar R. Synthesis of imine-pyrazolopyrimidinones and their mechanistic interventions on anticancer activity. Bioorg Med Chem, 2013; 21(18):5782–93. CrossRef
Birnbaum JE, Sapp TM, Tolman EL. Cyclic AMP-phosphodiesterase and epidermal mitosis. The J Invest Dermatol, 1976; 67(2):235–9. CrossRef
Butu A, Rodino S, Golea D, Butu M, Butnariu M, Negoescu C, Dinu–Pirvu CE. Liposomal nanodelivery system for proteasome inhibitor anticancer drug bortezomib. Farmacia, 2015; 63(2):224–9.
Butu M, Butnariu M, Rodino S, Butu A. Study of zingiberene from lycopersicon esculentum fruit by mass spectometry. Digest J Nanomater Biostruc, 2014; 9(3):935–41.
Desire L, Buee L, Lambeng N, Drouin D, Schweighoffer F. O3-05-02: EHT-0202: a neuroprotective and procognitive alpha-secretase stimulator targeted towards Alzheimer disease therapy. Alzh Dement, 2008; 4(4):T168. CrossRef
Drott J, Desire L, Drouin D, Pando M, Haun F. Etazolate improves performance in a foraging and homing task in aged rats. Euro J Pharmacol, 2010; 634(1-3):95–100. CrossRef
El-Gohary NS, Shaaban MI. Design, synthesis, antimicrobial, antiquorum-sensing and antitumor evaluation of new series of pyrazolopyridine derivatives. Euro J Med Chem, 2018; 157:729–42. CrossRef
El-Gohary NS, Hawas SS, Gabr MT, Shaaban MI, El-Ashmawy MB. New series of fused pyrazolopyridines: synthesis, molecular modeling, antimicrobial, antiquorum-sensing and antitumor activities. Bioorgan Chem, 2019; 92:103109. CrossRef
Esmadi FT, Khabour OF, Abbas K, Mohammad AE, Obeidat RT, Mfady D. Synthesis, characterization and biological activity of some unsymmetrical Schiff base transition metal complexes. Drug Chem Toxicol, 2016; 39(1):41–7. CrossRef
Gavriil ES, Lougiakis N, Pouli N, Marakos P, Skaltsounis AL, Nam S, Jove R, Horne D, Gioti K, Pratsinis H, Kletsas D, Tenta R. Synthesis and antiproliferative activity of new pyrazolo[3,4-c]pyridines. Med Chem, 2016. CrossRef
Ghorbani A, Jeddi-Tehrani M, Saidpour A, Safa M, Bayat AA, Zand H. PI3K/AKT and Mdm2 activation are associated with inhibitory effect of cAMP increasing agents on DNA damage-induced cell death in human pre-B NALM-6 cells. Arch Biochem Biophys, 2015; 566:58–66. CrossRef
Jindal A, Mahesh R, Bhatt S. Etazolate, a phosphodiesterase-4 enzyme inhibitor produces antidepressant-like effects by blocking the behavioral, biochemical, neurobiological deficits and histological abnormalities in hippocampus region caused by olfactory bulbectomy. Psychopharmacology (Berl), 2015; 232(3):623–37. CrossRef
Jindal A, Mahesh R, Bhatt S, Pandey D. Molecular modifications by regulating cAMP signaling and oxidant-antioxidant defence mechanisms, produce antidepressant-like effect: a possible mechanism of etazolate aftermaths of impact accelerated traumatic brain injury in rat model. Neurochem Int, 2017; 111:3–11. CrossRef
Joseph RR, Yazer E, Hanakawa Y, Stadnyk AW. Prostaglandins and activation of AC/cAMP prevents anoikis in IEC-18. Apoptosis, 2005; 10(6):1221–33. CrossRef
Khabour OF, Enaya FM, Alzoubi K, Al-Azzam SI. Evaluation of DNA damage induced by norcantharidin in human cultured lymphocytes. Drug Chem Toxicol, 2016; 39(3):303–6. CrossRef
Khabour OF, Saleh N, Alzoubi KH, Hisaindee S, Al-Fyad D, Al-Kaabi L, Dodeen A, Esmadi FT. Genotoxicity of structurally related copper and zinc containing Schiff base complexes. Drug Chem Toxicol, 2013; 36(4):435–42. CrossRef
Ku WW, Bigger A, Brambilla G, Glatt H, Gocke E, Guzzie PJ, Hakura A, Honma M, Martus HJ, Obach RS, Roberts S. Strategy for genotoxicity testing--metabolic considerations. Mutat Res, 2007; 627(1):59–77. CrossRef
Marcade M, Bourdin J, Loiseau N, Peillon H, Rayer A, Drouin D, Schweighoffer F, Désiré L. Etazolate, a neuroprotective drug linking GABA(A) receptor pharmacology to amyloid precursor protein processing. J Neurochem, 2008; 106(1):392–404. CrossRef
Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M. Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie, 2006; 88(11):1515–31. CrossRef
Megat S, Hugel S, Journée SH, Bohren Y, Lacaud A, Lelièvre V, Doridot S, Villa P, Bourguignon JJ, Salvat E, Schlichter R, Freund-Mercier MJ, Yalcin I, Barrot M. Antiallodynic action of phosphodiesterase inhibitors in a mouse model of peripheral nerve injury. Neuropharmacology, 2021; 205:108909. CrossRef
Mehta AK, Ticku MK. Characteristics of flunitrazepam binding to intact primary cultured spinal cord neurons and its modulation by GABAergic drugs. J Neurochem, 1987; 49(5):1491–7. CrossRef
Mhaidat NM, Alzoubi KH, Khabour OF, Alawneh KZ, Raffee LA, Alsatari ES, Hussein EI, Bani-Hani KE. Assessment of genotoxicity of vincristine, vinblastine and vinorelbine in human cultured lymphocytes: a comparative study. Balkan J Med Genet, 2016; 19(1):13–20. CrossRef
Oliveira JT, Franco FN, Tecchio KB, Gonçalves A, Barbosa CS, Ribeiro R, Viana GHR, Santos V, Santos FVD. Metabolic activation enhances the cytotoxicity, genotoxicity and mutagenicity of two synthetic alkaloids with selective effects against human tumour cell lines. Mutat Res Genet Toxicol Environ Mutagen, 2021; 861–862:503294. CrossRef
Parkkonen J, Hasala H, Moilanen E, Giembycz MA, Kankaanranta H. Phosphodiesterase 4 inhibitors delay human eosinophil and neutrophil apoptosis in the absence and presence of salbutamol. Pulm Pharmacol Ther, 2008; 21(3):499–506. CrossRef
Rababa HA, Alzoubi KH, Khabour OF, Ababneh M. Ameliorative effect of metformin on methotrexate-induced genotoxicity: an in vitro study in human cultured lymphocytes. Biomed Rep, 2021; 15(1):59. CrossRef
Rodino S, Butu M, Negoescu C, Caunii A, Cristina R, Butnariu M. Spectrophotometric method for quantitative determination of nystatin antifungal agent in pharmaceutical formulations. Digest J Nanomater Biostruc, 2014; 9(3):1215–22.
Romansik EM, Reilly CM, Kass PH, Moore PF, London CA. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Vet Pathol, 2007; 44(3):335–41. CrossRef
Sabisz M, Skladanowski A. Modulation of cellular response to anticancer treatment by caffeine: inhibition of cell cycle checkpoints, DNA repair and more. Curr Pharma Biotechnol, 2008; 9(4):325–36. CrossRef
Saha A, Bhattacharjee A, Vij A, Das PK, Bhattacharya A, Biswas A. Evaluation of modulators of cAMP-response in terms of their impact on cell cycle and mitochondrial activity of Leishmania donovani. Front Pharmacol, 2020; 11:782. CrossRef
Siopi E, Llufriu-Dabén G, Cho AH, Vidal-Lletjós S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Etazolate, an α-secretase activator, reduces neuroinflammation and offers persistent neuroprotection following traumatic brain injury in mice. Neuropharmacology, 2013; 67:183–92. CrossRef
Suntharalingam K, Gupta D, Sanz Miguel PJ, Lippert B, Vilar R. Synthesis, structural characterisation and quadruplex DNA binding studies of a new gold(III) pyrazolylpyridine complex. Chemistry (Weinheim an der Bergstrasse, Germany), 2010; 16(12):3613–6. CrossRef
Thompson SA, Wingrove PB, Connelly L, Whiting PJ, Wafford KA. Tracazolate reveals a novel type of allosteric interaction with recombinant gamma-aminobutyric acid(A) receptors. Mol Pharmacol, 2002; 61(4):861–9. CrossRef
Urbaniak SK, Boguszewska K. 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG) and 8-hydroxy-2’-deoxyguanosine (8-OHdG) as a potential biomarker for gestational diabetes mellitus (GDM) development. Molecules, 2020; 25(1):202. CrossRef
Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. Journal of environmental science and health Part C, Environ Carcinogen Ecotoxicol Rev, 2009; 27(2):120–39. CrossRef
Vellas B, Sol O, J Snyder P, Ousset PJ, Haddad R, Maurin M, Lemarié JC, Désiré L, Pando MP. EHT0202 in Alzheimer’s disease: a 3-month, randomized, placebo-controlled, double-blind study. Curr Alzheimer Res, 2011; 8(2):203–12. CrossRef
Wang C, Zhang J, Lu Y, Lin P, Pan T, Zhao X, Liu A, Wang Q, Zhou W, Zhang HT. Antidepressant-like effects of the phosphodiesterase-4 inhibitor etazolate and phosphodiesterase-5 inhibitor sildenafil via cyclic AMP or cyclic GMP signaling in mice. Metab Brain Dis, 2014; 29(3):673–82. CrossRef
Zhou SF, Xue CC, Yu XQ, Wang G. Metabolic activation of herbal and dietary constituents and its clinical and toxicological implications: an update. Curr Drug Metab, 2007; 8(6):526–53. CrossRef