INTRODUCTION
Plants are an integral part of human life and have a major role in biodiversity due to renewed interest in herbal and homeopathic medicines. Plant-derived pharmaceuticals have become prominent in the marketplace, as a favored healthcare choice. The increased popularity of plant-based medicines can also be attributed to the general perception that products from nature are inherently “safer” due to the history of human use and the long association of plants and humans. Today, there is a wealth of products on the market which contain active plant material (Raskin et al., 2002).
Araucariaceae is a family of large evergreen trees, dioecious or monoecious, with relatively large pith in the trunk and resin in the cortex (Byng, 2015; Welch, 2012). The family contains 3 genera with 39 species (Mabberley et al., 1997) including Araucaria as one of its genera. This species has excellent timber, which is used for wood production and used for furniture work and flooring (Bein et al., 1996) and the resin yielded from the tree trunk is used in ulcer treatment and wound healing; in addition, its seeds are eaten, and it is a human source of edible nuts providing a source of valuable proteins such as Araucaria cunninghamii seeds which have a taste like cashew. Araucaria is an endangered species due to its exposure to extensive logging by humans for its importance as industrial crops.
Araucaria species are known to possess gastroprotective (Schmeda-Hirschmann et al., 2005), antidepressant (Vasconcelos et al., 2009), antiviral (Freitas et al., 2009), anti-inflammatory (Santi-Gadelha et al., 2006), neuroprotective (Mukherjee et al., 2007), antipyretic (Suresh et al., 1995), anticoagulant (Dhanasekaran et al., 1993), and antimicrobial (Cespedes et al., 2006) activities. The genus is native to Papua New Guinea, Australia, New Caledonia, and South America. Araucaria cunninghamii Aiton ex D. Don are grown as ornamental trees in streets, parks, and gardens and was the target of the current study.
MATERIALS AND METHODS
Plant material for solid-phase microextraction (SPME) volatiles isolation
The leaves of A. cunninghamii Aiton ex D. Don were collected from Prince Mohamed Ali Palace Garden in AL-Manyal, Giza, Egypt. The identity of the plant was authenticated by Prof. Dr. Abd El-Haleem Abd El-Motgali at Flora and Phytotaxonomy Department, Horticultural Researches Institute, Agricultural Research Center, Dokki, Cairo, Egypt. Headspace volatiles analysis using SPME was adopted from a previous study (Zini et al., 2002) with few modifications. Araucaria aerial parts were ground, and 100 mg was placed in a 1.5 ml glass vial. The 0.1 M sodium phosphate buffer solution (pH 7.2 ml) was added to the vial, which was then immediately capped and placed on a temperature-controlled tray for 30 minutes at 40°C with the SPME fiber inserted into the headspace above the plant sample. Adsorption was timed for 30 minutes. SPME fibers were desorbed at 210°C for 1 minute in the port of injection of an HP 6890A GC [Hewlett-Packard (HP), Palo Alto, CA].
Gas chromatography (GC)/mass (MS) analysis and GC/FID quantification
SPME is a rapid, sensitive technique without using a solvent for the extraction of analytes from samples; it is carried via the automatized fiber injection system coupled to chromatographic separation instrument for the extraction of volatile and semivolatile organic compounds. SPME-trapped volatile components were identified by GC (Agilent HP 6890, Agilent Technologies, Palo Alto, CA) coupled to a mass spectrometer (Agilent HP 5973, Agilent Technologies) or a flame ionization detector. The injected sample was separated on a 5% diphenyl/95% dimethylpolysiloxan capillary column column (30 m length, 0.25 mm inner diameter, and 0.25 μm film) (J&W Scientific, Santa Clara, CA). The gas chromatograph was programmed at a rate of 12°C/minute to 180°C, kept at 180°C for 5 minutes, operated under the following conditions: injector 220°C, column oven 40°C for 3 minutes, and finally ramped at a rate of 40°C/minute to 250°C and kept for 2 minutes and He carrier gas at 1 ml/minute. The HP quadrupole mass spectrometer was operated in the electron ionization mode at 70 eV and a source temperature of 180°C. Volatile components were identified using the procedure described in Farag and Wessjohann (2012) and peaks were identified by its retention indices relative to n-alkanes (C6–C20), mass spectrum matching to National Institute of Standards and Technology, and Wiley library database as well as published literature data.
Preliminary phytochemical screening
Sample of A. cunninghamii Aiton ex D. Don leaves was air-dried and ground to powder. The powdered sample was macerated in ethyl alcohol 70% for 3 days and then allowed for filtration and screened for the presence of the main classes of secondary metabolites such as flavonoids, tannins (Evans, 2002), sterols (Karnick, 1971), triterpenoids (Harborne and Baxter, 1999), saponins, cardiac glycosides, alkaloids (Fulton, 1937), anthraquinones (Fairbairn, 1949), and the presence of cyanogenic glycosides which were examined (Odugbemi, 2008) according to the approved methods of preliminary phytochemical tests.
Evaluation of antimicrobial activity
The potential antimicrobial activities of the methanolic extract of the plant species were studied through the determination of the zones of inhibition by agar well method (Cooper, 1972) against two standard Gram-positive bacteria, Bacillus subtilis ATCC-6633 and Staphylococcus aureus ATCC-6538, and two standard Gram-negative bacteria, Pseudomonas aeruginosa National Collection of Industrial Bacteria (NCIB) ATCC-9027 and Escherichia coli ATCC-8739, and the fungal strains were Candida albicans ATCC-90028 and Aspergillus niger ATCC-7966. The culture media used for bacteria were nutrient agar medium and malt extract agar medium used for cultivation of C. albicans and Czapek’s Dox agar medium was used for A. niger. The strains were first checked for purity based on standard microbiological tests and then used for their sensitivity on the test samples. The bacteria and fungi were maintained on nutrient agar medium and Czapek’s Dox agar medium, respectively. The agar media were inoculated with different test microorganisms. 100 and 200 μl of each methanolic plant extract diluted with 100 μl of dimethylformamide and the solubilized extract were placed in the well. After 24 hours of incubation at 30°C for bacteria and 48 hours of incubation at 28°C for fungi, inhibition zones were measured to the nearest mm using Vernier calipers. Standards with the concentration of 1 mg/ml were used as positive controls chloramphenicol (SEDICO Co., Egypt) for Gram-positive, cephalexin monohydrate [The Egyptian Co. for Pharmaceutical & Chemical Industries (EPCI), Egypt] for Gram-negative bacteria, and fluconazole (SEDICO Co., Egypt) for fungi.
Estimation of total phenolics content by Folin–Ciocalteu method
10 mg of each lyophilized methanolic extract of A. cunninghamii was dissolved in 10 ml 70:30 water/methanol (samples). 1 ml of each sample (1 mg/ml) was mixed with 1.5 ml of Folin–Ciocalteu; after 5 minutes, 4 ml of 20% sodium carbonate solution was added to make up to 25 ml with distilled water and was incubated for 30 minutes at room temperature, with absorbance at 765 nm. Blank was prepared in a similar way using 1 ml of 70:30 water/methanol instead of the sample.
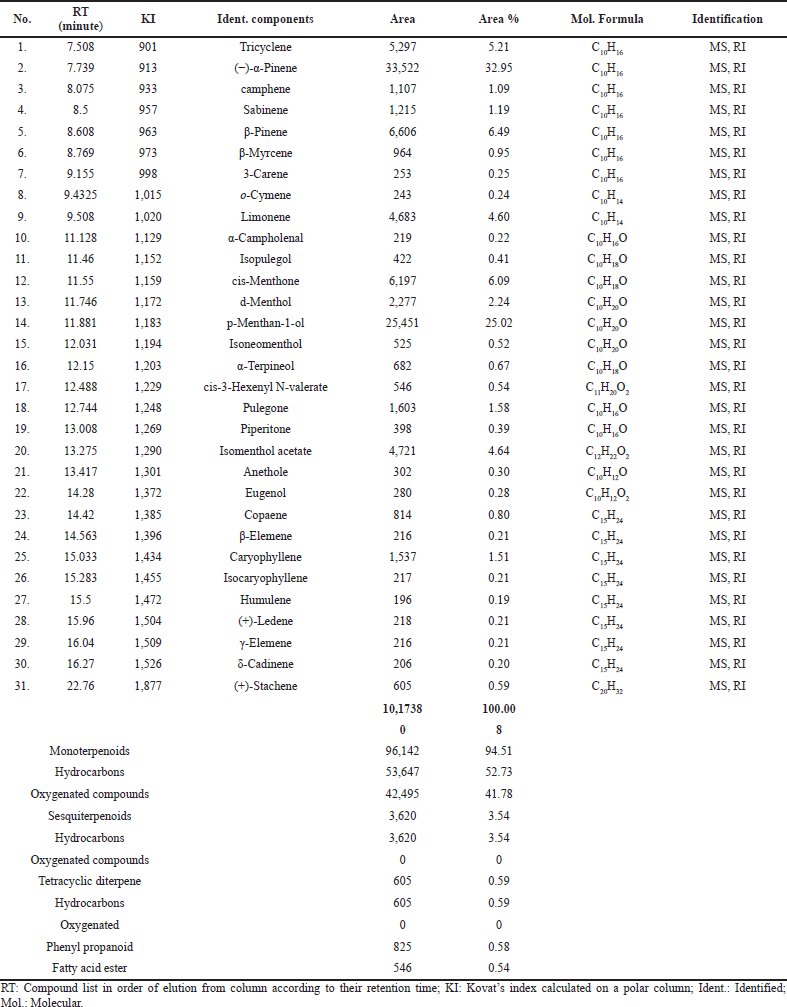 | Table 1. Relative percentage of volatile components in A. cunninghamii using SPME/GC/MS. [Click here to view] |
A standard calibration curve was prepared by weighing accurately 25 mg of standard gallic acid and dissolving in 25 ml distilled water in a volumetric flask (stock solution 1 mg/ml) and then was diluted to (80–280) μg/ml; calibration was prepared by adding 1 ml of each dilution and then 10 ml of distilled water and 1.5 ml of Folin–Ciocalteu reagent were added; after 5 minutes, 4 ml of 20% sodium carbonate solution was added to make up to 25 ml with distilled water and was incubated for 30 minutes at room temperature, with absorbance at 765 nm (Saboo et al., 2010).
All determination was performed in triplicate; the total content percentage was calculated as gallic acid equivalent (GAE).
GAE = [(C × V )/M ] × 100
where C = the conc. of gallic acid established from calibration curve mg/ml, V = volume of extract (ml), and M = the weight of dried plant extract (mg).
Evaluation of total flavonoids
10 mg of each lyophilized methanolic extract of A. cunninghamii was dissolved in 10 ml 70% methanol (samples stock). 1 ml of each sample (1 mg/ml) was added separately to 1.5 ml methanol, 0.1 ml aluminum chloride, 0.1 ml potassium acetate solution, and 2.8 ml distilled water which were added and mixed well for each sample; the absorbance was recorded after 30 minutes at 415 nm. Blank was prepared in a similar way using methanol instead of the sample. Each sample was done in triplicate (Saboo et al., 2010).
10 mg quercetin was dissolved in 10 ml methanol and then diluted to 5–100 μg/ml; to each dilution, the following reagents were added: 0.1 ml 10% AlCl3, 0.1 ml of 1 M potassium acetate, and 2.8 ml distilled water. A calibration curve was prepared by measuring the absorbance of the different dilutions at 415 nm.
RESULTS AND DISCUSSION
Analysis of volatile constituents
The SPME technique followed by GC/MS analysis of Araucaria aerial parts sample resulted in the identification of 31 different volatile constituents. Monoterpenoids content was predominant representing 94.51%, among which hydrocarbon was 52.73% followed by oxygenated monoterpenoids constituting 41.78%. Sesquiterpenoids hydrocarbon reached only 3.54% with no oxygenated sesquiterpenes. (+)-Stachene was the only tetracyclic diterpene with 0.59%. Phenylpropanoids represent 0.82%. cis-3-Hexenyl N-valerate was the only fatty acid detected with 0.54%. Major compounds detected were the monoterpene hydrocarbons (−)-α-Pinene representing 32.95% followed by oxygenated monoterpene p-menthan-1-ol with 25.02%, listed according to their retention time in Table 1, and the gas chromatogram is shown in Figure 1.
Preliminary phytochemical screening
Preliminary phytochemical screening of A. cunninghamii Aiton ex D. Don leaves was carried out to investigate the phytochemical composition of the methanolic extract of the plant. Results showed that it contains phenolic compounds, flavonoids, and saponins. While cardiac glycosides, cyanogenic glycosides, alkaloids, and anthraquinones were absent, results are tabulated in Table 2.
Antimicrobial evaluation
Methanolic extract in both concentrations 100 and 200 μg/ml showed that A. cunninghamii showed moderate activity against G+ve B. subtilis ATCC-6633 and S. aureus ATCC-6538, Table 3, which increases by using the 200 μg/ml, and showed moderate activity against G−ve P. aeruginosa NCIB ATCC-9027 and E. coli ATCC-8739 using the 200 μg/ml concentration only. The methanolic extract of the species did not exhibit any activity against A. niger ATCC-7966 fungi or against the unicellular C. albicans ATCC-90028 as shown in Table 3.
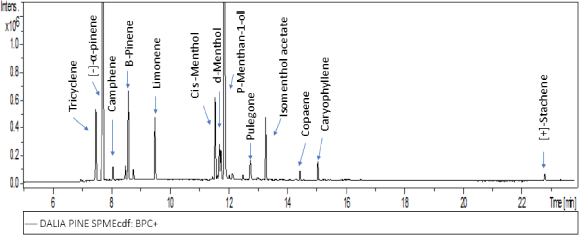 | Figure 1. GC/MS total ion chromatogram of major volatile constituents of A .cunninghamii aerial parts. [Click here to view] |
 | Table 2. Results of phytochemical screening of A. cunninghamii aerial parts. [Click here to view] |
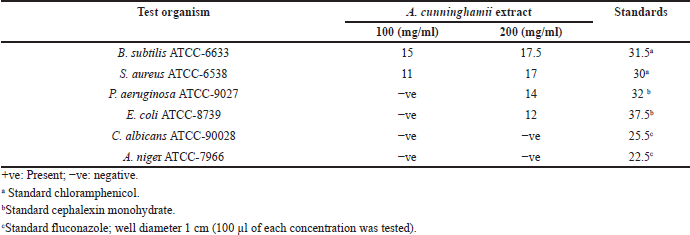 | Table 3. Antimicrobial activity studies showing mean diameter of inhibition zones of various concentrations of A. cunninghamii. [Click here to view] |
Total polyphenols and total flavonoidal content
From the following data, the estimated total polyphenolic content of the tested species was found that A. cunninghamii showed a high total polyphenolic content with 252.11 ± 0.28 mg/GAE, whereas it exhibited a high flavonoidal content of 21.65 ± 0.01 mg/μg compared to the standard quercetin.
Statistical analysis
All determinations were carried out in a triplicate manner and values are expressed as the mean ± SD. Statistical analysis was carried out by one-way analysis of variance followed by Tukey’s test. Results are expressed as means ± SEM (n = 10).
CONCLUSION
Volatiles were extracted from A. cunninghamii leaves using the headspace technique and analyzed by GC/MS. A total of 31 compounds were detected. (−)-α-Pinene was the major monoterpenoid hydrocarbon representing a third of the volatile constituents of the sample 32.95% and this is concurrent with the plant being one of the conifers, followed by the menthane monoterpenoid p-menthan-1-ol representing 25.02%, and this is the first report on A. cunninghamii volatile constituents. The antimicrobial evaluation of the methanolic extract of Araucaria against multidrug-resistant pathogens using standard microbiological techniques by agar well diffusion method for activity against B. subtilis, S. aureus, P. aeruginosa, E. coli, C. albicans, and A. niger isolated from clinical samples. The susceptibility patterns of the test isolates against the crude extract were determined at extract concentrations of 100 and 200 mg/ml, respectively. The results revealed that the extracts inhibit the growth of the tested G+ve spp. with a moderate extent followed by the G−ve spp. showing no activity for the fungus. The phytochemical screening revealed the presence of flavonoids-free and combined terpenoids and saponins. The estimated total polyphenolic content of A. cunninghamii showed a high total polyphenolic content with 252.11 ± 0.28 mg/GAE and exhibited also a high flavonoidal content of 21.65 ± 0.01 mg/μg compared to the standard quercetin. Finally, we need to save these precious species from logging to keep the natural ecosystem away from human’s danger.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Bein E, Habte B, Jaber A, Birnie A, Tengnas B. Useful trees and shrubs in Eritrea: identification, propagation and management for agricultural and pastoral communities. Technical Handbook. vol. 12, p. 42, 1996.
Byng JW. The gymnosperms handbook: a practical guide to extant families and genera of the world. Plant Gateway Ltd., 2015.
Cespedes CL, Avila JG, Garcia AM, Becerra J, Flores C, Aqueveque P, Bittner M, Hoeneisen M, Martinez M, Silva M. Antifungal and antibacterial activities of Araucaria araucana (Mol.) K. Koch heartwood lignans. Z Naturforsch C J Biosci, 2006; 61(1–2):35–43. CrossRef
Cooper R. Analytical microbiology. In: Kavanageh F (ed.), Academic Press, New York, London, vol. I & II, 1972.
Dhanasekaran S, Ravishankar S, Devi KS, Suresh B, Sethuraman M, Rajan S. Pharmacological studies of Araucaria bidwilli Hook. Anc Sci Life, 1993; 1(13):137–42.
Evans WC. Trease and Evans’ pharmacognosy. 9th edition, Saunders Elsevier, pp 553–7, 2002.
Fairbairn JW. The active constituents of the vegetable purgatives containing anthracene derivatives: part I. Glycosides and aglycones. J Pharm Pharmacol, 1949; 1(1):683–92. CrossRef
Farag MA, Wessjohann LA. Volatiles profiling in medicinal licorice roots using steam distillation and solid–phase microextraction (SPME) coupled to chemometrics. J Food Sci, 2012; 77(11):C1179–84. CrossRef
Freitas A, Almeida M, Andrighetti-Frohner C, Cardozo F, Barardi C, Farias M, Simões C. Antiviral activity-guided fractionation from Araucaria angustifolia leaves extract. J Ethnopharmacol, 2009; 126(3):512–7. CrossRef
Fulton CC. Sulfomorphid; and the purple fluorescence test, a new derivative test for morphine. J Am Pharm Assoc (1912), 1937; 26(8):726–9. CrossRef
Harborne JB, Baxter H. The handbook of natural flavonoids. John Wiley and Son,s vol. 1 & 2, 1999.
Karnick CR. Phytochemical investigations of some Dioscorea species and varieties found in India. Q J Crude Drug Res, 1971; 11(3):1761–73. CrossRef
Mabberley DJ, Cronquist A, Kubitzki K, Willis JC. The plant-book: a portable dictionary of the vascular plants. Cambridge University Press, 1997.
Mukherjee PK, Ahamed KN, Kumar V, Mukherjee K, Houghton PJ. Protective effect of biflavones from Araucaria bidwillii Hook in rat cerebral ischemia/reperfusion induced oxidative stress. Behav Brain Res, 2007; 178(2):221–8. CrossRef
Odugbemi T. A textbook of medicinal plants from Nigeria. University of Lagos Press, Lagos, Nigeria, 2008.
Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C. Yakoby N. Plants and human health in the twenty-first century. Trends Biotechnol, 2002; 20(12):522–31. CrossRef
Saboo S, Tapadiya R, Khadabadi S, Deokate U. In vitro antioxidant activity and total phenolic, flavonoid contents of the crude extracts of Pterospermum acerifolium wild leaves (Sterculiaceae). J Chem Pharm Res, 2010; 2(3):417–23.
Santi-Gadelha T, De Almeida Gadelha CA, Aragao KS, Mota MR, Gomes RC, De Freitas Pires A, Toyama M.H, De Oliveira Toyama D, De Alencar NM, Criddle DN. Purification and biological effects of Araucaria angustifolia (Araucariaceae) seed lectin. Biochem Biophys Res Commun, 2006; 350:1050–5. CrossRef
Schmeda-Hirschmann G, Astudillo L, Rodriguez J, Theoduloz C, Yanez T. Gastroprotective effect of the mapuche crude drug Araucaria araucana resin and its main constituents. J Ethnopharmacol, 2005; 101(1–3):271–6. CrossRef
Suresh B, Dhanasekaran S, Elango K, Sethuraman M, Rajan S. Anti-pyretic activity of some plants in female albino rats: a preliminary report. Anc Sci Life, 1995; 14(4):253.
Vasconcelos SM, Lima SR, Soares PM, Assreuy AM, De Sousa FC, Lobato RD, Vasconcelos G S, Santi-Gadelha T, Bezerra EH, Cavada BS. Central action of Araucaria angustifolia seed lectin in mice. Epilepsy Behav, 2009; 15(3):291–3. CrossRef
Welch HJ. The conifer manual. Springer Science & Business Media, vol. 1, 2012.
Zini CA, Augusto F, Christensen E, Caramao EB, Pawliszyn J. SPME applied to the study of volatile organic compounds emitted by three species of Eucalyptus in situ. J Agric Food Chem, 2002; 50(25):7199–205. CrossRef