INTRODUCTION
Justicia adhatoda L. is an intense restorative plant which is utilized in treating different sicknesses, exceptionally comparable to respiratory issues, and has different organic activities (Gangwar and Ghosh, 2014). The dynamic metabolites of a restorative plant assume the main part in deciding their remedial potential. The plants’ active compounds vary with geographical locations and altitude (Chelghoum et al., 2021; Goyal et al., 2021). The environmental factors directly or indirectly affect the plants’ active constituents and their biological activities (Jugran et al., 2016). Geographical regions are positioned far from one another possess diverse climatic conditions, thereby impacting the production of the plants’ phytochemicals, and hence alter the efficacy of various biological activities, viz., antimicrobial activity, antioxidant activity, etc., (Mehdizadeh et al., 2018). Plant cells own the tendency to produce variable compounds, for instance, secondary metabolites, for their use in defense and also possess several biological activities (Bakhtiari et al., 2021; Compean and Ynalvez, 2014; Wallace, 2004). It is anticipated that the genuine and reliable medicines produced from plants in their native provenance contain adequate and best chemical compositions (Dong et al., 2011). Altitude can affect the potency of plant extracts, for instance, in a study, the antimicrobial efficiency of Satureja thymbra essential oils collected from different altitudes was tested against various pathogens, and plants from low altitude exhibited better antifungal activity, whereas those growing at a high altitude showed better antibacterial activity. Both low and high altitude essential oils displayed better anthelmintic activity in comparison to the standard drug piperazine citrate (Khalil et al., 2020). In a study, it was found that the antioxidant potential of highland populations of Hibiscus rosa-sinensis was greater in comparison to the lowland population (Wong et al., 2009). A study was conducted on the different grape stems in Portugal where the lower altitude regions were observed to have higher phenolic percentage and higher antioxidant activities (Gouvinhas et al., 2020). In another study, Zataria multiflora Boiss was investigated in Iran across its different natural habitats where it was demonstrated that the phytochemical content and biological activities were largely influenced by environmental conditions in different natural habitats (Niczad et al., 2019). Plant needles of Taxus wallichiana were gathered from various areas in Rudraprayag locale of Uttarakhand and the study exposed that the altitude considerably affected cancer prevention agent actions just as phenol, flavonoids, and other bioactive mixtures. Then again, the review divulged no relationship of tannins and taxols with altitude; however, they set up a connection with the counter microbial movement which thus suggested that the counter microbial action was primarily a result of those phytochemicals (Adhikari et al., 2020). Henceforth, considering the altitude at which a plant develops holds significance. Previously, studies have been carried out on the determination of biological activities and medicinal potential of J. adhatoda L., but this is the first report regarding the effect of different altitudes on the biological activities of J. adhatoda L.
MATERIALS AND METHODS
Sample collection and preparation
Different provenances of Jammu which varied in altitude were selected and sample collection was carried out for two consecutive years, viz. 2019 and 2020. The samples were collected before the monsoon, i.e., during the month of June. In this study, purposive sampling was performed and three different altitudes, viz. 336, 688, and 1,330 m, were selected for sample collection according to the availability of plants in the specific areas. The collected plant specimens were verified by the Botanical Survey of India, Dehradun, and submitted in the herbarium of Shoolini University under voucher numbers SUBMS/BOT-4800 (Vijaypur, 336 m), SUBMS/BOT-4801 (Harotkot, 688 m), and SUBMS/BOT-4802 (Khetriar, 1,330 m). The leaf sample collection was carried out in triplicates from each selected altitude. The mature leaves were hand harvested from each plant. Fifth and sixth mature leaves from the top of the plant were taken. Leaves were collected from all the four directions of each plant. The collected leaves were cleaned and shade dried, and after that, the leaves were crushed in a mechanical grinder to obtain ?ne powder and stored at 4°C for further analysis.
Extraction of leaves
The ethanolic extract of leaf powder samples were prepared in order to perform further experiments. A 10 g leaf powder sample was dissolved in 100 ml of 80% ethanol and further kept in a rotary orbital shaker for 48 hours. The extracts were filtered through Whatman filter paper and dried at 40°C and stored under 4°C till further use.
Microorganisms
The in vitro antibacterial activity was assessed against four different strains, viz. Escherichia coli (MTCC 82), Staphylococcus aureus (MTCC 96), Pseudomonas aeruginosa (MTCC 2453), and Klebsiella pneumonia (MTCC 39) by disk diffusion assay and subsequently antifungal activity was tested on Rosellinia necatrix (HG964402.1) and Fusarium spp. (SR266-9) by poisoned food technique.
Antimicrobial activity
Antibacterial activity was performed by disk diffusion method, following the protocol of Duraipandiyan et al. (2015). Petri plates were prepared by taking nutrient agar at a concentration of 20 ml. Nutrient agar for bacterial and fungal strains was prepared by autoclaving them at 121°C for 30 minutes. The material obtained was then poured into petri plates and allowed to solidify. A loop full of broth containing microbes was taken and uniformly spread on the nutrient agar plate. The loaded disk with different concentrations of the extract was placed on the medium. Distilled water was used for the negative control. Ampicillin served as a positive control. The plates were witnessed after incubation. Incubation was carried out for 24 hours at 37°C. The inhibition zones were produced because of the inhibitory activity of the extract and their diameters were measured and recorded.
Antifungal activity
The poisoned food technique was used for examination of antifungal efficacy by following the protocol given by Kumar and Garampalli (2015). Specific amounts of extracts were added in sterilized potato dextrose agar in petri plates. After that, actively growing mycelium disks of both the pathogens were taken and placed in the plates. Hygromycin was used in the place of positive control. Plates lacking plant extracts were reserved for negative control and all the plates were kept for incubation at a temperature of 27°C. After 7 days, the estimation of the growth of pathogen in respective plates was determined by measuring their diameter:
Antioxidant activity
The antioxidant capacity was determined by following DPPH radical scavenging assay and the method of Braca et al. (2001) was followed. Methanol was used to prepare the stock solution by adding it to the extracts. DPPH solution was also added in a specific quantity to the extracts, followed by serial dilutions. After 10 minutes, the absorbance was recorded at 515 nm using a spectrophotometer. For reference, standard ascorbic acid was used and IC50 values were also calculated.
Anti-inflammatory activity
The protocol described by Sarveswaran et al. (2017) was followed. This assay was based on protein denaturation. Denaturation is a well-established cause of inflammation. Capability of the leaf extract to prevent denaturation was studied in this assay. Bovine serum albumin was used instead of protein. Denaturation was persuaded by maintaining the reaction mixture in a water bath for 10 minutes at 70°C. The reaction mixture was made with different concentrations of the plant extract, i.e., 1,000 μl (100–500 μg/ml), 450 μl bovine serum albumin, and phosphate buffered saline, i.e., 1,400 μl. Then, the mixtures were incubated for 15 minutes at 37°C and after that they were maintained at 70°C for 5 minutes. Diclofenac sodium was used instead of the control and the absorbance was taken at 660 nm. The percentage inhibition of protein denaturation was measured with the help of following equation:
Gas chromatography–mass spectrometry (GC–MS) analysis
GC–MS analysis of J. adhatoda L. extract was performed by using an instrument, viz. Thermo Scientific (Trace 1300 GC,TSQ Duo, Triplus RSH auto sampler) equipped with Thermo Scientific column DB-5MS with the following dimensions: length = 40 m, ID = 0.15 mm, film thickness = 0.15 μm. Carrier gas = helium, and flow rate = 0.7 ml/m. Oven temperature program was as follows: 80°C (1 minute hold), 10°C/minutes, 180°C (2 minutes hold), 10°C/min, 260°C (10 minutes hold). Split flow = 50 ml/minute and split ratio = 71.4. Injector temperature = 250°C and ion source temperature = 230°C. MS Foreline temperature = 250°C at 70 eV. Mass range = 45–450. The data were interpreted using Xcalibur software and NIST/EPA/NIH Mass spectral library, version 2.2, build 2014.
Statistical analysis
Data were analyzed by one-way ANOVA and Bonferroni’s multiple comparison tests.
RESULTS AND DISCUSSION
Susceptibility of various microbes was tested against J. adhatoda L. leaf extracts from different elevations. The antibacterial efficacy of extracts was considerable against all the tested strains and the inhibition rates are described in Table 1.
All the extracts showed moderate to significant inhibitions of microbial growth in comparison to that of standard drug Ampicillin (Fig. 1).
Justicia adhatoda L. leaf extracts were able to inhibit mycelia growth better in Rosellinia in comparison to that of Fusarium (Fig. 2).
The comparison between antifungal activities from different altitudes was made and no significant result was observed. The difference between the resistances of tested fungi against J. adhatoda L. leaf extracts is described in Table 2.
The plants’ antimicrobial activity is known to be mainly because of its phytochemicals, for example, taxol, tannins, and flavanols. Previous studies have suggested that antimicrobial activity depends on the phyto-constituents of plant extracts, for instance, the antimicrobial bustle of phenolic compounds is because they have a significant impact on the permeability of the membrane and consequently the ratio of penetration in bacterial cell which in turn promotes the damage and inactivation of the cellular profile (El-Jalel et al., 2018; Moreno et al., 2006; Russo et al., 2013). Besides this, other phenol imitative compounds, such as thymol, carvacrol, etc., are known to lay the basis of cellular profile disruption, viz. inhibition of ATPase activity and further components of numerous microorganisms such as E. coli, P. aeruginosa, S. aureus, and Salmonella enteritidis (Cetin-Karaca, 2011). However, the variation in potency of J. adhatoda L. leaf extracts from different elevations did not show any significant result (Table 1), which is in agreement with a study conducted on Taxus wallichiana in Uttarakhand, where the impact of altitude on the biological activities was checked, but the antimicrobial activity did not vary with elevation, perhaps displaying a positive correlation to secondary metabolites (Adhikari et al., 2020). Justicia adhatoda L. is mainly known for its bronchodilator and antihistaminic effects that are attributed to its active alkaloid content. Various bioactive molecules present in plants take part in antimicrobial action, for instance, alkaloids, flavonoids, terpenes, etc., contain an extremely variable chemical structures with high potential antibacterial activities (Bahman et al., 2019; Gorniak et al., 2018). Bioactive constituents of plants exhibit an extraordinary spectrum of pharmacological activities, such as antibacterial, antifungal, antiprotozol, astringent, and circulatory stimulant. In a study, the alkaloids were extracted from hot methanolic extracts of J. adhatoda L. and evaluated for antimicrobial activity against clinically important bacteria, viz. E. coli, S. aureus, Proteus mirabilis, Salmonella typhi, P. aeruginosa, Candida albicans, etc., where the isolated alkaloids exhibited potent activity against the tested microorganisms (Bailey-Shaw et al., 2018; Jasim et al., 2015; Joo et al., 2010; Karthikeyan et al., 2009; Keesara and Jat, 2017; Rahaman and Sharma, 2018; Sawant et al., 2013; Shahwar et al., 2012; Singh and Sharma, 2013). Plants grow defense strategies to guard themselves from various pathogens present in the environment. They accumulate defense compounds that are chiefly secondary metabolites comprising good antimicrobial activities. It is assumed that these bioactive plant-derived secondary metabolites play a chief role in synthesizing antimicrobial agents with some pharmacological effect which in turn helps if any bacteria or fungi attacks the plants (Reichling, 2009).
Significant variations were observed among antioxidant activities of extracts from different altitudes (Fig. 3). Justicia adhatoda L. displayed the highest antioxidant activity among the extracts from a higher elevation, viz. 1330 m, in comparison to that of low and mid-altitudes, viz. 336 and 688 m, which emphasizes that environmental differences attributed to the observed variation in the antioxidant potential of J. adhatoda L. (Table 3).
Phenolic and flavonoid compounds are chiefly responsible for the antioxidant property. In a study conducted on Hedychium spicatum in Uttarakhand, all the antioxidant assays carried out on plant extracts showed significant correlations with the total phenolic compounds. The assays work on a similar mechanistic approach, i.e., transfer of electrons from the antioxidant to reduce an oxidant (Rawat et al., 2011). In adverse environmental conditions, the free radicals are generated that cause damage in their system and in order to deal with the changing environmental conditions, plants either stimulate protection mechanisms or initiate the repair mechanism (Frohnmeyer and Staiger, 2003). Oxidation is a crucial process and a prerequisite in order to run the biological processes efficiently (Jha et al., 2014). Plants possess a powerful antioxidant system (Toldi et al., 2019). The antioxidant activity of plants comes mostly from the phytochemical compounds they contain (Wojdylo et al., 2007). Change in altitude can bring about variations in antioxidant activity (Jin et al., 2017). To deal with oxidative stress, plants are equipped with defense strategies which prevent the loss caused by reactive oxygen species (Khan et al., 2016). Natural antioxidants prevent or delay oxidative stress. Free radicals may mount up as a consequence of imbalance between oxidation and antioxidation processes and ultimately cause damage to the plants’ biological scenario (Liu and Huang, 2014). Antioxidants quench free radicals as they act as electron donors and hence prevent various diseases induced because of free radicals (Nyanhongo et al., 2013). Not just altitudinal variations, seasonal variations also play a major role in bringing about change in the antioxidant levels of plants (Ahmed et al., 2017). The presence of polyphenolic compounds could be the possible reason behind the antioxidant potential of the plant (Rao et al., 2013). Besides, the secondary metabolites can also act as antimicrobial and anti-inflammatory agents (Moeini et al., 2020). In case of anti-inflammatory activity, the variation was not statistically significant among different altitudes (Fig. 4). The values are described in Table 4.
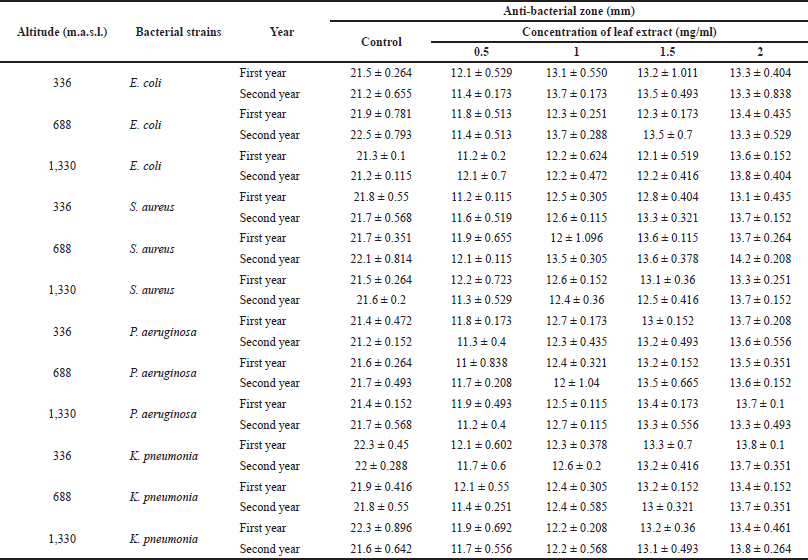 | Table 1. Antibacterial activity of J. adhatoda L. ethanolic leaf extracts collected from different provenances of Jammu against selected standard and clinical bacterial isolates. Data are represented as mean ± standard deviation. [Click here to view] |
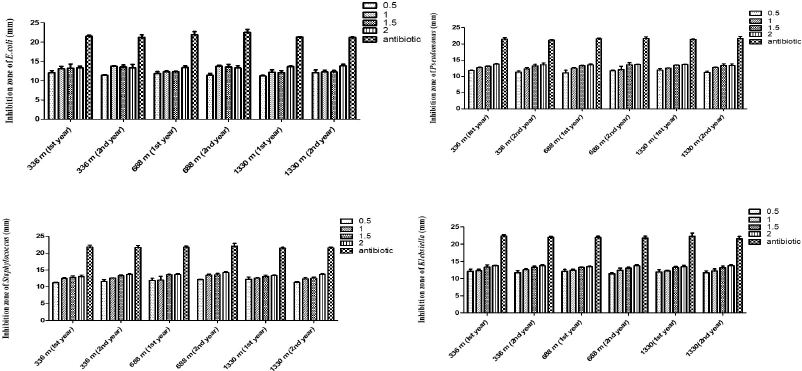 | Figure 1. Effect of different concentrations of J. adhatoda L. ethanolic leaf extract from different altitudes on (a) E. coli, (b) P. aeruginosa, (c) S. aureus, and (d) K. pneumonia. [Click here to view] |
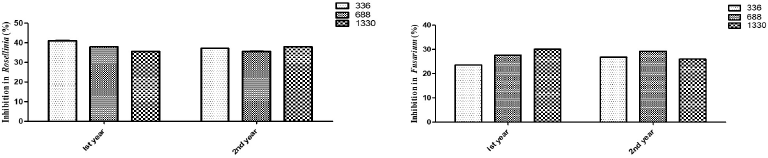 | Figure 2. Effect of different concentrations of J. adhatoda L. ethanolic leaf extract from different altitudes on (a) R. necatrix and (b) Fusarium ssp. [Click here to view] |
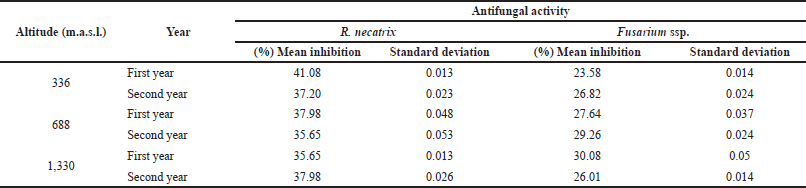 | Table 2. Antifungal activity of J. adhatoda L. ethanolic leaf extracts collected from different provenances of Jammu. [Click here to view] |
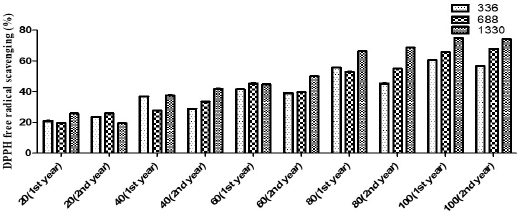 | Figure 3. Antioxidant activity of J. adhatoda L. leaf extracts from different altitudes. [Click here to view] |
The GC–MS chromatogram of ethanolic leaf extracts of J. adhatoda L. revealed nine prominent peaks at retention time: 4.27, 5.27, 6.27, 9.16, 11.11, 17.08, 17.09, 18.71, and 20.29. 1-Butanol, 3-methyl-, acetate (C7H14O2), benzaldehyde, 4-(1-phenyl-2-propenyloxy)-(C16H14O2), ethane, 1,1-dimethoxy-(C4H10O2), CYCLOPENTANE (C5H10), 1-hexadecanol (C16H34O), 1-docosene (C22H44) and 1-propanol, 3-(diethylamino)-2,2-dimethyl-, and p-amino benzoate (ester) (C16H26N2O2) are the compounds which were present in each altitudinal site. However, 1-hexyl-2-nitrocyclohexane (C12H23NO2) was present in low and mid-altitude only and 2-naphthalenamine, 5,6,7,8-tetrahydro (C13H14O2) was present only in mid-altitude. 2-Deoxy-d-ribose was the compound which was found to be present in high altitude only (Fig. 5).
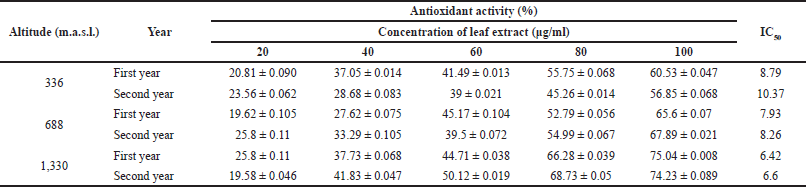 | Table 3. Antioxidant activity of J. adhatoda L. ethanolic leaf extracts collected from different provenances of Jammu using DPPH radical scavenging assay. Data are represented as mean ± standard deviation. [Click here to view] |
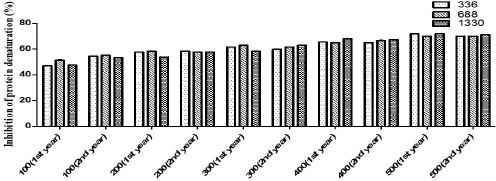 | Figure 4. Anti-inflammatory activity of J. adhatoda L. leaf extracts from different altitudes. [Click here to view] |
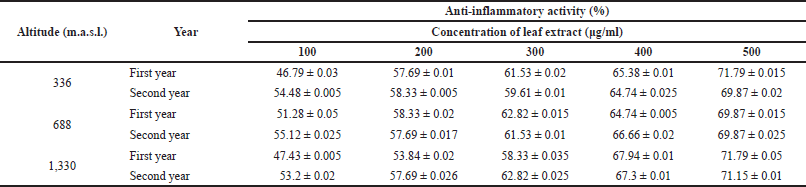 | Table 4. Anti-inflammatory activity of J. adhatoda L. ethanolic leaf extracts collected from different provenances of Jammu using inhibition of albumin denaturation technique. Data are represented as mean ± standard deviation. [Click here to view] |
Among these compounds, 1-butanol, 3-methyl-, acetate, and 1-docosene have been reported as important phytochemical compounds (Zambri et al., 2019); 1-hexadecanol is an antioxidant (Pejin et al., 2014). 1-Hexyl-2-nitrocyclohexane is an important anti-inflammatory compound. It is neuroactive and possesses analgesic property (Thirumalaisamy et al., 2018). 1-Hexyl-2-nitrocyclohexane has been reported to possess antibacterial and antifungal properties also (Dulara et al., 2019). 2-Naphthalenamine compounds have also been reported to possess anti-inflammatory, analgesic, and antimicrobial properties (Shareef et al., 2016).
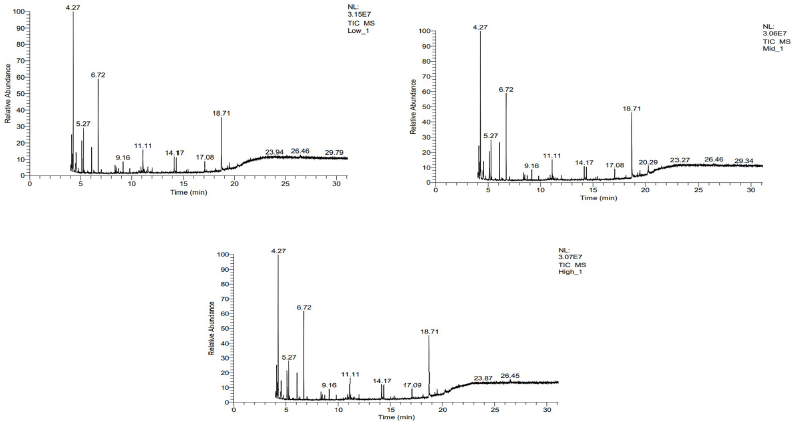 | Figure 5. Gas chromatography–mass spectrometry of the leaf extracts of J. adhatoda L. from (a) 336 m, (b) 688 m, and (c) 1,330 m altitude. [Click here to view] |
CONCLUSION
In the present study, the potent biological activities of J. adhatoda L. leaf extracts justify the usage of this plant as a medicine. However, significant elevation variations were observed among the antioxidant property only. Various biotic and abiotic stresses present in the environment act as the driving forces behind the variations observed in chemical compositions and eventually the existence of medicinal plants. The assessment of the chemical composition of plants growing at different elevations can contribute to selecting the best genotype and better altitude for the commercial cultivation of plants. Apparently, the quality and therapeutic efficacy of medicinal plants depend on their chemical composition, which in turn depends on the environmental factors.
FUNDING
The author(s) received no financial support for the research and publication of this article.
CONFLICT OF INTEREST
All authors have no conflict of interest to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adhikari P, Joshi K, Singh M, Pandey A. Influence of altitude on secondary metabolites, antioxidants, and antimicrobial activities of Himalayan yew (Taxus wallichiana). Plant Biosyst Int J Dealing All Aspects Plant Biol, 2020; 156(1):187–95. CrossRef
Ahmed Z Ben, Yousfi M, Viaene J, Dejaegher B, Demeyer K, Mangelings D, Heyden Y Vander. Seasonal, gender and regional variations in total phenolic, flavonoid, and condensed tannins contents and in antioxidant properties from Pistacia atlantica ssp. leaves. Pharm Biol, 2017; 55(1):1185–94. CrossRef
Bahman K, Milad I, Vahid S, Bibi SFB. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Res Infect Control, 2019; 8:1–28. CrossRef
Bailey-Shaw YA, Lawrence ADW, Green CE, Rodney S, Smith AM. In-vitro evaluation of the anti-inflammatory potential of selected jamaican plant extracts using the bovine serum albumin protein denaturation assay. Int J Pharm Sci Rev Res, 2018; 47(1):145–53.
Bakhtiari M, Glauser G, Defossez E, Rasmann S. Ecological convergence of secondary phytochemicals along elevational gradients. New Phytol, 2021; 229. CrossRef
Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia tarapotensis. J Nat Prod, 2001; 64(7):892–5. CrossRef
Cetin-Karaca H. Evaluation of natural antimicrobial phenolic compounds against foodborne pathogens. University of Kentucky, Lexington, KY, p 652, 2011.
Chelghoum M, Guenane H, Tahri D, Laggoun I, Marfoua FZ, Rahmani FZ, Khenifer F, Yousfi M. Influence of altitude, precipitation, and temperature factors on the phytoconstituents, antioxidant, and α-amylase inhibitory activities of Pistacia atlantica. J Food Meas Charact, 2021; 15(5):4411–25. CrossRef
Compean KL, Ynalvez RA. Antimicrobial activity of plant secondary metabolites: a review. Res J Med Plant, 2014; 8:204–13. CrossRef
Dong J, Ma X, Wei Q, Peng S, Zhang S. Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of Eucommia ulmoides. Ind Crops Prod, 2011; 34(3):1607–14. CrossRef
Dulara BK, Godara P, Barwer N. In-vivo and in-vitro phytochemical GC-MS analysis of volatile constituents of Andrographis paniculata (Burm. f.) Nees. Pharma Innov J, 2019; 8:255–61.
Duraipandiyan V, Dhabi-Al N, Balachandran C, Ignacimuthu S, Sankar C, Balakrishna K. Antimicrobial, antioxidant, and cytotoxic properties of vasicine acetate synthesized from vasicine isolated from Adhatoda vasica L. BioMed Res Int, 2015; 7–14. CrossRef
El-Jalel LFA, Elkady WM, Gonaid MH, El-Gareeb KA. Difference in chemical composition and antimicrobial activity of Thymus capitatus L. essential oil at different altitudes. Fut J Pharm Sci, 2018; 4(2):156–60. CrossRef
Frohnmeyer H, Staiger D. Ultraviolet-B radiation-mediated responses in plants. Balancing Damage and Protection. Plant Physiol, 2003; 133:1420–8. CrossRef
Gangwar AK, Ghosh AK. Medicinal uses and Pharmacological activity of Adhatoda vasica. Int J Herb Med, 2014; 2(1):89–91.Gorniak I, Rafal B, Jaroslaw K. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev, 2018; 0123456789.
Gouvinhas I, Pinto R, Santos R, Saavedra MJ, Barros AI. Enhanced phytochemical composition and biological activities of grape (Vitis vinifera L.) stems growing in low altitude regions. Sci Hortic, 2020; 265:109248. CrossRef
Goyal S, Tewari G, Pandey HK, Kumari A. Exploration of productivity, chemical composition and antioxidant potential of Origanum vulgare L. grown at different geographical locations of Western Himalaya, India. J Chem, 2021; 2021:Article ID 6683300. CrossRef
Jasim H, Hussein AO, Hameed IH, Kareem MA. Characterization of alkaloid constitution and evaluation of antimicrobial activity of Solanum nigrum using gas chromatography mass spectrometry (GC-MS). J Pharmacogn Phytother, 2015; 7(4):56–72.
Jha DK, Panda L, Ramaiah S. Evaluation and comparison of radical scavenging properties of solvent extracts from Justicia adhatoda leaf using DPPH assay. Appl Biochem Biotechnol, 2014; 174:2413–25. CrossRef
Jin XD, Wu X, Liu X. Phenolic characteristics and antioxidant activity of merlot and cabernet sauvignon wines increase with vineyard altitude in a high-altitude region. South Afr J Enol Vitic, 2017; 38(2):132–43. CrossRef
Joo SS, Kim YB, Lee DI. Antimicrobial and antioxidant properties of secondary metabolites from white rose flower. Plant Pathol J, 2010; 26(1):57–62. CrossRef
Jugran AK, Bahukhandi A, Dhyani P, Bhatt ID, Rawal RS, Nandi, SK. Impact of altitudes and habitats on valerenic acid, total phenolics, flavonoids, tannins, and antioxidant activity of Valeriana jatamansi. Appl Biochem Biotechnol, 2016; 179(6):911–26. CrossRef
Karthikeyan A, Shanthi V, Nagasathaya, A. Preliminary phytochemical and antibacterial screening of crude extract of the leaf of Adhatoda. Int J Green Pharm, 2009; 3(1):78. CrossRef
Keesara BR, Jat RK. Isolation and characterization of vasicine from Adhatoda vasica (Adusa). Int J Res Dev Pharm Life Sci, 2017; 6(2):2590–6. CrossRef
Khalil N, El-Jalel L, Yousif M, Gonaid M. Altitude impact on the chemical profile and biological activities of Satureja thymbra L. essential oil. BMC Complement Med Ther, 2020; 20(1):186. CrossRef
Khan MN, Mobin M, Abbas ZK, Mutairi KA. Impact of varying elevations on growth and activities of antioxidant enzymes of some medicinal plants of Saudi Arabia. Acta Ecol Sin, 2016; 36:141–8. CrossRef
Kumar Ramaiah A, Kumar Garampalli RH. In vitro antifungal activity of some plant extracts against Fusarium oxysporum F. sp. lycopersici. Asian J Plant Sci Res, 2015; 5(1):22–7.
Liu S, Huang H. Assessments of antioxidant effect of black tea extract and its rationals by erythrocyte haemolysis assay, plasma oxidation assay and cellular antioxidant activity (CAA) assay. J Funct Foods, 2014; 18:1095–105. CrossRef
Mehdizadeh L, Najafgholi HM, Biouki RY, Moghaddam M. Chemical composition and antimicrobial activity of Origanum vulgare subsp. viride essential oils cultivated in two different regions of Iran. J Essent Oil Bear Plants, 2018; 21(4):1062–75. CrossRef
Moeini A, Pedram P, Makvandi P, Malinconico M, Gomez G d’Ayala. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr Polym, 2020; 233:115839. CrossRef
Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radical Res, 2006; 40(2):223–31. CrossRef
Niczad A, Sharafzadeh S, Alizadeh A, Amiri B, Bazrafshan F. Variability in essential oil constituent, phenolic content, antioxidant and antimicrobial activities of different ecotypes of Zataria multiflora Boiss. from Iran. J Essent Oil Bear Plants, 2019; 22(6):1435–49. CrossRef
Nyanhongo GS, Sygmund C, Ludwig R, Prasetyo EN, Guebitz GM. An antioxidant regenerating system for continuous quenching of free radicals in chronic wounds. Eur J Pharm Biopharm, 2013; 83:396–404. CrossRef
Pejin B, Nakarada D, Novakovic M, Tesevic V, Savic A, Radotic K, Mojovic M. Antioxidant volatiles of the freshwater bryozoan Hyalinella punctata. Nat Prod Res, 2014; 28(18):1471–5. CrossRef
Rahaman L, Ghosh S, Nath N, Sen A, Sharma BK. Extracts of Adhatoda vasica Nees and vasicine inhibits biofilm formation of Klebsiella pneumoniae. J Pharm Biol Sci, 2018; 13(2):37–49.
Rao K, Munjal M, Patnayak A, Loganathan K, Kumar G. Phytochemical composition, antioxidant, antimicrobial and cytotoxic potential of methanolic extracts of Adhatoda vasica (Acanthaceae). Res Pharm Technol, 2013; 6(9):997–1002.
Rawat S, Bhatt ID, Rawal RS. Total phenolic compounds and antioxidant potential of Hedychium spicatum Buch. Ham. ex D. Don in west Himalaya, India. J Food Compos Anal, 2011; 24:574–9. CrossRef
Reichling J. Plant microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. Annu Plant Rev, 2009; 39:214–347. CrossRef
Russo M, Suraci F, Postorino S, Serra D, Roccotelli A, Agosteo GE. Essential oil chemical composition and antifungal effects on Sclerotium cepivorum of Thymus capitatus wild populations from Calabria, southern Italy. Braz J Pharmacogn, 2013; 23(2):239–48. CrossRef
Sarveswaran R, Banukie Jayasuriya WJA, Suresh TS. In vitro assays to investigate the anti-inflammatory activity of herbal extracts: a review. World J Pharm Res, 2017; 6(17):131–41.
Sawant CS, Save SS, Bhagwat AM. Antimicrobial activity of alkaloids extracted from Adhatoda vasica. Int J Pharma Bio Sci, 2013; 4(3):803–7.
Shahwar D, Raza MA, Tariq S, Riasat M. Enzyme inhibition, antioxidant and antibacterial potential of vasicine isolated from Adhatoda vasica Nees. Pak J Pharm Sci, 2012; 25(3):651–6.
Shareef HK, Muhammed HJ, Hussein HM, Hameed IH. Antibacterial effect of ginger (Zingiber officinale) roscoe and bioactive chemical analysis using gas chromatography mass spectrum. Orient J Chem, 2016; 32(2):20–40. CrossRef
Singh B, Sharma RA. Anti-inflammatory and antimicrobial properties of pyrroloquinazoline alkaloids from Adhatoda vasica Nees. Phytomedicine, 2013; 20(5):441–5. CrossRef
Thirumalaisamy R, Ammashi S, Muthusamy G. Screening of anti-inflammatory phytocompounds from Crateva adansonii leaf extracts and its validation by in silico modeling. J Gen Eng Biotechnol, 2018; 16(2):711–9. CrossRef
Toldi D, Gyugos M, Darko E, Szalai G, Gulyas Z, Gierczik K, Szekelyl A, Boldizsar A, Galiba G, Muller M, Sarkadi LS, Kocsy G. Light intensity and spectrum affect metabolism of glutathione and amino acids at transcriptional level. PLos One, 2019; 14(12):1–18. CrossRef
Wallace RJ. Antimicrobial properties of plant secondary metabolites. Proc Nutr Soc, 2004; 63(4):621–9. CrossRef
Wojdylo A, Jan O, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem, 2007; 105:940–9. CrossRef
Wong SK, Lim YY, Chan EWC. Antioxidant properties of hibiscus: species variation, altitudinal change, coastal influence and floral colour change. J Trop For Sci, 2009; 21(4):307–15.
Zambri NDS, Taib NI, Abdul Latif F, Mohamed Z. Utilization of neem leaf extract on biosynthesis of iron oxide nanoparticles. Molecules, 2019; 24(20):3803; doi:10.3390/molecules24203803 CrossRef