INTRODUCTION
Advanced medical technologies have increased life expectancy and decreased mortality rate exploding the human population in the modern era, a significant global burning issue, adversely affecting the economic, social, and technological developments (Amit and Pankaj, 2018; Jain et al., 2015). Unwanted pregnancies raised due to inappropriate contraception or negligence sometimes become life-threatening for women (Mziray et al., 2020; Thanamool, 2013). Thus, conception and fertility control become critical issues and make the population control tops on the priority list through the use of contraceptives and family planning to improve the quality of life (Asuquo, 2012; Bandyopadhyay, 2010; Shah et al., 2016b; Sundar et al., 2013; Thanamool, 2013).
Great advances in modern medicine in recent decades have enriched today’s market with highly potent synthetic contraceptives. These drugs prevent or inhibit conception and/or modify the cyclicity of endogenous hormonal production resulting in menstrual irregularity. This cumulatively leverages the physical and mental stress and put the patient at risk of developing a variety of disorders, such as breast cancer, endometriosis, gastrointestinal disturbance, massive painful uterine contraction, etc., to name a few. This explains a need for exploring the hidden wealth of medicinal plants with better effectiveness and lesser side effects (Fajriaty et al., 2017). In Indian local communities, several plants and their parts have become crucial due to their action on the ovarian-uterine axis, biological effectiveness, factorial role in the regulation of fertility, eco-friendliness, cheapness, affordable for all segments of society, and capability to provoke changes in the configuration of the reproductive cycle (Daud et al., 2015; Mansouri et al., 2016; Singh et al., 2017).
So far, a small number of plants have been reported to have antifertility potential and out of them, only a few have been reached at clinical evaluation. Thus, hunting for herbal drugs with the capacity to disturb the endocrine function of the hypothalamic-pituitary-gonadal axis that can replace the synthetic steroidal/nonsteroidal medications as birth-control regulation strategies is in demand (Chen et al., 2021; Goyal, 2014; Udiwal et al., 2014).
Plumeria acuminata (L.) commonly known as Frangipani (Eng.) belongs to the Apocynaceae family exhibited antifertility, antipyretic, antinociceptive, antibacterial, antidiabetic, antitumor, and immune-modulatory activity (Devi et al., 2015; Farooque et al., 2012; Gupta, 2016; Gomathi et al, 2013, 2016; Singh et al., 2017; Sunitha and Mohan, 2017; Sunitha and Naga, 2018; Taid et al., 2016; Yadav et al., 2016). Active ingredients present in leaves and roots, such as stigmasterol, saponins, flavonoids, and alkaloids, are believed to be having antifertility and abortive activities (Devi et al., 2015; Gupta, 2016). Stigmasterol possesses both estrogenic and antiestrogenic effects due to its estrogen-like structure (Thanamool, 2013). Saponin, a lipid derivative triterpenoid, plays an important role in steroid biosynthesis and caused disturbances of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) release affecting the estrous cycle. Saponins possess antizygotic, blastotoxic, and anti-implantation activity in rodents (Fajriaty et al., 2017; Mziray et al., 2020). Flavonoids, which are nonsteroidal phytoestrogens, exhibit antifertility potential in male rats by decreasing the production of spermatids (Choudhary et al., 2017; Mukhram et al., 2012). It is reported that flavonoids block the cyclooxygenase-I and II which are believed to be essential for ovulation (Nayaka et al., 2014). Alkaloid is thought to possess oxytocic-like effects that affect the environment of the uterus in rodents (Kayode et al., 2007).
As there are scanty reports on the antifertility effects of the P. acuminata leaves (PAL) and P. acuminata roots (PAR) extracts, none of these have documented their possible mode of action for these extracts. The mechanism behind the extracts’ action that brings out changes in the estrous cyclicity and its length, the hormonal alterations during these changes, and histological modifications in the female reproductive organ system, are focused on in the present study. In the represented work, by emphasizing the possible antifertility role of P. acuminata leaves and roots extract in female Wistar rats, we try to explain how these extracts bring changes in the histology of female reproductive organs, hormonal levels and alter their regular estrous cyclicity.
MATERIALS AND METHODS
Identification of plant and collection of plant materials
Plant P. acuminata (L.), has been identified and authenticated by a resource person, and fully grown healthy leaves, as well as roots, were collected from the herbal botanical garden of Shree Dhanvantary Pharmaceutical Analysis and Research Centre, Surat, Gujarat (India).
Preparation of plant materials
Plumeria acuminata leaves and roots were washed thoroughly with deionized water to remove the adherent impurities like soil debris, sliced into small pieces followed by proper air-drying under shade at room temperature (25°C–30°C) for a week. The dried material was then ground to a uniform powder with the help of a suitable grinder and the powdered plant parts were then stored in an airtight container and kept in a cool dry place for further analysis.
Preparation of extracts
The ethanol extracts were prepared by soaking 1,000 g each of dry powdered plant parts in 2,000 ml of ethanol in an airtight container for about 14 days. Residual particulates and extracts were separated through filtration using Whatman Filter Paper No.1. The filtrate was concentrated to semisolid paste using a rotary evaporator with the water bath set at 50°C. The resulting paste in the form of crude ethanolic extract of P. acuminata leaves and roots, respectively, referred to as PAL and PAR, was weighed and stored in an airtight container till further used. The percentage of yield was calculated using Equation (1) (Hyacinth and Nwocha, 2011; Marin et al., 2020; Taid et al., 2016; Shah et al., 2016b).
Phytochemical analysis
Individual PAL and PAR extracts were qualitatively analyzed to identify the presence of various phytoconstituents, such as carbohydrates, alkaloids, glycosides, amino acids and proteins, phenols, tannins, flavonoids, sterols, saponins, steroids, and terpenoids by using reported standard tests (Ahirwar et al., 2010; Edwin et al., 2008; Jaber and Jasim, 2014; Jain et al., 2013).
Chemicals
Analytical grade reagents and chemicals were consumed for this analysis.
Animal studies
Wistar rats were used as an animal model for the antifertility model.
Age, sex, and housing condition of animals
The antifertility activity of the crude ethanolic extract was studied using adult female rats. The animals were housed under standard environmental conditions of temperature 22 ± 3°C, at humidity of 50 ± 20%, and a 12-hour light-dark cycle. Rats were given a standard rodent pellet chow diet and water ad libitum.
Dose selection for PAL and PAR extracts
The safe dose of the PAL and PAR extracts were determined by performing acute toxicity studies as per the OECD 423. Starting dose of 2,000 mg/kg was given to three adult female Wistar rats in the first step. Postdosing the animals were kept under special observation and screened every 30 minutes till 4 hours for any toxic response. Further till day 14, every day, the animals were monitored for the same. The absence of any mortality was confirmed by a repetition of the same dose in the second step and the LD50 value was determined.
Experimental procedure and study design
An experimental study was designed to explore the antifertility characteristics of PAL and PAR extracts. Three different doses of 100, 200, and 400 mg/kg of PAL and PAR extracts were determined and henceforth, the doses were referred to as PAL-100, PAL-200, PAL-400, PAR-100, PAR-200, and PAR-400 for the study.
Antifertility model
The effect of PAL and PAR extract on the estrous cycle was determined using nulliparous and non-pregnant adult female Wistar rats. Vaginal smears from female rats were collected through flush techniques for about 12 days (~3–4 estrous cycles) to monitor normal cyclicity. A total number of 42 rats with normal cyclicity were selected for the present model and randomized into seven different groups comprising six animals each. The normal control (Group I) group received normal saline from day 1 to day 28 (D1–D28). Test item treatment for Group II–VII was given from D1 to D28. During these 28 days, i.e., ~6–7 estrous cycles, the vaginal smear was taken on the everyday morning between 8 and 10 am from each animal and subjected to microscopic examination for distinguishing between the four-phase, i.e., estrus (E), metestrus (M), diestrus (D), and proestrus (P). On D29, after collecting blood from retro-orbital sinus, animals were euthanized by CO2 asphyxiation. White blood cells (WBC), Red blood cells (RBC), Hemoglobin (Hb), Hematocrit (HCT), Mean corpuscular volume (MCV), Mean corpuscular hemoglobin (MCH), platelet, and neutrophil counts were evaluated from the blood sample using a hematology analyzer (Siemens, Advia 2120i). FSH, LH, estrogen, and progesterone levels were quantified from serum samples using ELISA. Ovaries, uterus, vagina, oviduct, and cervix were collected, surrounding tissue was removed, blotted on filter paper, and weighed quickly. Collected organs were fixed in 10% formalin buffer, dehydrated in alcohol, and embedded in paraffin for sectioning. 6 µ thick sections were fixed on slides and subjected to the hematoxylin-eosin (H & E) staining process. The stained sections were observed for histological changes in the organs using a digital light microscope (ZEISS, Axio Lab A1) (An et al., 2020; Bhaskar et al., 2009; Byers et al., 2012; Daud et al., 2015; Mittal, 2020; Mziray et al., 2020).
Cyclicity calculations
During 28 days of dosing, different phases of estrous cycles were determined using the flush technique. Days spent in different phases of each cycle were calculated individually throughout the study and at the end of 28 days, the relative time spent in each phase was cumulatively calculated for 28 days in percentage as mentioned in the Equation (2).
Statistical analysis
All the data are expressed as mean ± standard error of mean for n = 6. Statistical analysis was performed with one-way analysis of variance followed by Dunnett’s post-test at a confidence level of 0.05 (95% confidence interval) using Graph Pad Prism Version 5.03. For comparison with normal control, differences were considered to be statistically significant when *p < 0.05, **p < 0.01, ***p < 0.001. With decreasing the p values, the difference between the two groups is more significant and there is a lesser chance that this difference is due to an error or noise.
RESULTS
Extract yield
The percentage yield from different parts of the P. acuminata was calculated from the formula given in Equtaion (1), and the values for leaves and roots are 15.3% and 13.5%, respectively.
Qualitative phytochemical screening
Qualitative analysis of the phytochemical compounds from the PAL and PAR extracts is represented in Table 1. The presence of carbohydrates, alkaloids, phenolic, sterols, flavonoids, saponins, and terpenoids was detected in both extracts. Proteins and amino acids were absent in both extracts, whereas tannins were detected from the root but absent from the leaves extract.
Acute toxicity test
In the acute toxicity test, a limit dose of 2,000 mg/kg was tested on six female rats. Three rats were used at each step. All rats were observed individually for a sign of toxicity after 30 minutes of dosing, with given special attention during the first 4 hours and then daily thereafter up to 14 days. No signs of toxicity like changes in skin and fur, eyes, mucous membranes, respiratory, circulatory, autonomic, central nervous systems, somatomotor activity, behavior patterns, and deaths were observed during 14 days period at the doses tested. The median lethal dose (LD50) was determined to be higher than the highest dose tested, i.e., 2,000 mg/kg.
Antifertility model
Effect on body weight gain
The effect of PAL and PAR extract administration on body weight change is given in Table 2. Measurement and comparison of body weight on every seventh day unveiled the slower body weight gain by animals after treatment in comparison with normal control. Although the body weight gain measured on these interim as well as terminal days was statistically significant for the treatment groups when compared with the normal control group, there was a dose-dependent decrease in this body weight gain after PAL and PAR treatment. When terminally compared with normal control, the PAL and PAR treatment resulted in lesser bodyweight gain and this decrease in body weight gain was statistically significant for all treatments.
Effect on each phase of the estrous cycle
As shown in Figure 1, a comparison of relative time span in individual phases throughout 28 days for test-item treated groups and normal control groups, revealed a statistically significant dose-dependent decrease in duration of estrus and proestrus phase after PAL and PAR treatments. Even though no dose-dependency was observed in the decreased relative time span of the metestrus phase, the decrease was statistically significant for the 400mg/kg dose of PAL and PAR extracts. On contrary to this, the earmarked observation significantly escalated the relative time span of the diestrus phase in a dose-dependent manner after PAL and PAR treatments. These disturbances in the individual phase can alter the overall cyclicity and its length as shown below. These disturbances in each phase of the estrous cycle might be due to hormonal disturbances caused by both extracts.
 | Table 1 Results of qualitative phytochemical screening of ethanolic extract of PAL and PAR. [Click here to view] |
 | Table 2 Effect of PAL and PAR extract on body weight gain during anti-fertility activity. [Click here to view] |
Effect on length and number of estrous cycles
The effect of administration of different extracts on the length and number of estrous cycles is given in Table 3. When compared to normal control, increased length of the estrous cycle has resulted in the reduced cycle numbers suggesting the reciprocal relation between cycle length and number. This dose-dependent increase in cycle length and the resulting decrease in cycle number were found statistically significant for groups treated with 200 and 400 mg/kg doses of PAL and PAR extract.
Effect on hematological parameters
As shown in Table 4, hematological parameters such as WBC, RBC, Hb, HCT, MCV, MCH, platelet, and neutrophil did not have any specific effects of PAL and PAR extract treatments when compared with the normal control group. Percentage changes in WBC, RBC, Hb, HCT, MCV, MCH, platelet, and neutrophil count were in the range of ~1%–3%, ~2%–3%, ~2%–3%, ~0%–3%, ~4%–4%, ~0%–3%, ~2%–5%, and ~4%–8%, respectively. When we compared with the normal control group , they did not exhibit any significant changes.
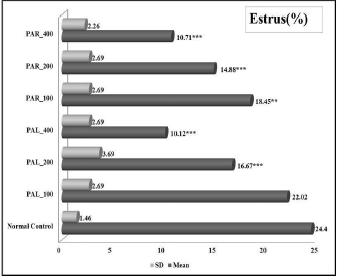 | Figure 1. (A)-(i). Effect of PAL and PAR extract on estrus phase of estrous cycle during anti-fertility model. Values are expressed as Mean ± SEM for n=6, *p<0.05, **p<0.01, ***p<0.001 vs Normal control [Click here to view] |
 | Figure 1. (A)-(ii). Microscopic image of estrus phase observed during evaluation of estrous cycle during anti-fertility model. [Click here to view] |
 | Figure 1. (B)-(i). Effect of PAL and PAR extract on metestrus phase of estrous cycle during anti-fertility model. Values are expressed as Mean ± SEM for n=6, *p<0.05, **p<0.01, ***p<0.001 vs Normal control [Click here to view] |
 | Figure 1. (B)-(ii). Microscopic image of metestrus phase observed during evaluation of estrous cycle during anti-fertility model. [Click here to view] |
 | Figure 1. (C)-(i). Effect of PAL and PAR extract on diestrus phase of estrous cycle during anti-fertility model. Values are expressed as Mean ± SEM for n=6, *p<0.05, **p<0.01, ***p<0.001 vs Normal control [Click here to view] |
 | Figure 1. (C)-(ii). Microscopic image of diestrus phase observed during evaluation of estrous cycle during anti-fertility model. [Click here to view] |
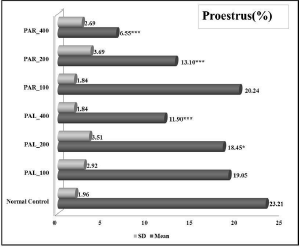 | Figure 1. (D)-(i). Effect of PAL and PAR extract on proestrus phase of estrous cycle during anti-fertility model. Values are expressed as Mean ± SEM for n=6, *p<0.05, **p<0.01, ***p<0.001 vs Normal control [Click here to view] |
 | Figure 1. (D)-(ii). Microscopic image of proestrus phase observed during evaluation of estrous cycle during anti-fertility model. [Click here to view] |
 | Table 3 Effect of PAL and PAR extract on length and number of the estrous cycle during anti-fertility activity. [Click here to view] |
Effect on reproductive organ weight
Table 5 summarizes day 29 weight data of reproductive organs, i.e., ovaries, oviducts, and uterus. In comparison to the normal control group, a statistically significant decrease in the weight of ovaries and uterus was found in both PAL and PAR extract-treated groups in a dose-dependent manner. On the other hand, no drastic change in the oviduct weight was noted.
Effect on hormone analysis
Effects of PAL and PAR extract treatments on estrogen, FSH, LH, and progesterone levels are shown in Figure 2. When compared with the normal control, a dose-dependent decrease in estrogen, progesterone, and LH levels was observed after treatment with PAL and PAR extracts. On contrary to this, a dose-dependent increase in FSH levels was noted. In most cases, this difference was statistically significant for 200 and 400 mg/kg doses of treatments. With an increased dose of PAL, there was a ~7%–43% drop in estrogen, ~13%–49% drop in progesterone, and ~6%–22% drop in LH levels. Similarly in the case of PAR with increased dose, 14%–46% drop in estrogen, ~24%–57% drop in progesterone, and 14%–35% drop in LH levels. However, the FSH level was increased by about 19%–99% in the case of PAL and around 48%–140% in the case of PAR extracts-treated groups.
 | Table 4 Effect of PAL and PAR extract on haematological parameters during anti-fertility activity. [Click here to view] |
 | Table 5 Effect of PAL and PAR extract on organs weight during anti-fertility activity. [Click here to view] |
Effect on histopathology
In histopathological studies (Fig. 3, Row 1), the ovarian section from the normal control group exhibited normal cellular morphology with plenty of primary and secondary follicles, graafian follicles, or corpora lutea. The treatment of PAL and PAR extracts has slowed down the maturation of follicles and delayed the ovulation that resulted in a follicular cyst or vacuolization, i.e., the formation of fluid-filled bubble-like structure. During the observation of oviduct sections (Fig. 3, Row 2), ciliated simple columnar cells and nonciliated peg cells of the mucosal portion from the ampulla region have been observed for the following alterations. The thickness of the mucosal layer, which was higher in the case of the normal control group due to a large number of ciliated simple columnar cells and nonciliated peg cells, was found to decrease after treatment with PAL and PAR extracts as the treatment resulted in a reduced number of ciliated simple columnar cells and nonciliated peg cells. The uterine section (Fig. 3, Row 3) from the endometrium portion was majorly focused for histological evaluation of the luminal endothelial comprising three-layer, i.e., stratum compactum, stratum spongiosum, and stratum basalis. Treatment with PAL and PAR extracts yielded notable changes in the endometrium (uterine gland and myometrium layer) as compared to the normal control group. Treatment with extracts resulted in the dilation of uterine glands, absence of pits and folds, and caused degeneration of the luminal epithelium compared to the normal control group. Cumulatively, all these effects must have controlled the enlargement of the endometrium layer making embryo implantation and growth difficult. From the cervical sections (Fig. 3, Row 4), it was observed treatment with PAL and PAR extracts has decreased the thickness of metaplastic squamous cells, columnar epithelium lining, and endo-cervical glands in a comparison with a normal control group and these changes have made the cervical region more hostile passage for sperms. Vaginal sections (Fig. 3, Row 5) of the normal control group, exhibited thick layers of nonkeratinized stratified squamous epithelium layer, which is consist of three layers, i.e., stratum corneum, stratum granulosum, and stratum germinativum, along with the elastic lamina propria as well as fibromuscular layer exhibited alterations in the same region from the groups which received test item treatments. Decreased thickness of all these layers has been seen after treatment probably due to decreased proliferation rate.
 | Figure 2. Effect of PAL and PAR extracts on hormonal levels during anti-fertility activity. Fig. 2A) Estrogen, Fig. 2B) FSH, Fig. 2C) LH, and Fig. 2D) Progesterone. Values are expressed as Mean ± SEM for n=6, *p<0.05, **p<0.01, ***p<0.001 vs Normal control [Click here to view] |
DISCUSSION
The present study focuses on the investigating antifertility efficacy of PAL and PAR extracts in laboratory animals. Drugs with antifertility potential have diverse modes of action and might target one or more organ systems in females, such as the hypothalamus, anterior pituitary, ovary-oviduct, uterus, cervix, and vagina, to disrupt the normal preovulatory functions (Amit and Pankaj, 2018). Such disruption may be attributed to the failure of hormonal balance and normal estrous cyclicity, leading to the inability of releasing mature ovum as well as failure to provide a healthy passage to ova and sperms. These can individually or cumulatively lead to fertilization failure suggesting an antifertility activity.
Alteration of the estrous cyclicity is one such method that regulates female fertility. Traditionally accurate determination of four estrous cycle stages and their durations, i.e., E, M, D, and P in the same order, is being done in rats by studying vaginal cytology. The observed mean duration of 4–5 days for a cycle length in normal control rats has been supported by the literature. Usually, the E phase lasts for 12–14 hours, the M phase for 24 hours, the D phase for 55–57 hours, and the P phase for 12–14 hours. The types of cells present in the smear can help in determining the estrous cycle phase for individual females. A large number of cornified epithelial cells are present during the E phase. A few numbers of cornfield epithelial cells and polymorphonuclear leukocytes are present during the M phase. D phase represents the longest phase, lasting more than 2 days with leukocytes and a small number of non-nucleated epithelial cells. The next phase is the P phase which is having mostly nucleated epithelial cells and at the end of the P phase, some cornified epithelial cells can be seen. At the end of the P phase or onset of the E phase, the female rat becomes sexually receptive to males during the dark period of time. Any interruption by pregnancy, pseudopregnancy, hormonal imbalance, or anestrus can alter the length of one or more phases and in turn lengthen the cycle (Byers et al., 2012; Ngadjui et al., 2015; Paccola et al., 2013; Sanabria et al., 2019).
 | Figure 3. Effect of PAL and PAR extracts on histology of reproductive organs during anti-fertility activity. Representative H & E stained photomicrographs of the (Row 1-5) ovary, oviduct, uterus, cervix and vagina respectively from diverse treatment groups. Respective treatment given to animal is mentioned on the top of each column. Ovary: Graafian follicle degeneration (GFD), Secondary follicle (SF), Primordial follicle (PF), Zona pellucida (ZP), Ovarian stroma (OS), Vacuolisation (VSL), Blood vessels (BV), Cystic follicle (CF) and antrum (ATM) visualized at 10x; Oviduct: Ciliated and peg cell (C&P), Lamina propria (LP) visualized at 40x; Uterus: Stratum compactum (SC), Stratum spongiosum layer (SS), Endometrium (EM) and Myometrium (MM), Uterine gland (UG), Dilation of uterine gland (DUG), Absence of pits and folds (APF) and Luminar epithelium degeneration (LED) visualized at 10x; Cervix: Metaplastic squamous cells (MSC), Columnar epithelium lining (CEL), Endo-cervical glands (ECG) and Decrease epithelium lining (DEL) visualized at 10x; Vagina: Stratum corneum (SC), Stratum granulosum (SG), Stratum germinativum (SGM), lamina propria layer (LPL), Fibromuscular layer (FML) and decreased proliferation of stratified squamous epithelium (DPSSE) visualized at 40x. [Click here to view] |
The reproductive function in mammals is mainly regulated by the decapeptide called gonadotropin-releasing hormone (GnRH) which is secreted by the hypothalamo-hypophyseal axis and particularly acts on the anterior pituitary gland. Stimulation of the GnRH receptor causes the anterior pituitary to release gonadotropins, i.e., FSH and LH which are essential hormones for cyclicity, steroidogenesis, and gametogenesis. FSH initiates follicular growth and LH stimulates ovarian follicles development. LH stimulates the theca cells to produce androgens, which are taken up by granulosa under FSH influence and converted into estrogens. LH also triggers ovulation and promotes corpus luteum development, which produces estrogen and progesterone which cumulatively prepare and maintain the endometrium for implantation (Mittal, 2020; Oyewopo et al., 2012; Paccola et al., 2013; Sanabria et al., 2019; Sari et al., 2016).
For healthy female rats with normal cyclicity, FSH and LH which are at lower levels during M and D phases, begin to increase in the P phase. LH reaches a peak and decreases as the cycle transits from P to E and becomes low, whereas FSH gradually increases and achieves a peak at the beginning of E and then decreases by end of the E phase. Estrogen is considered as a primary hormone as its level begins to rise at the start of the M phase, achieves a peak during the mid P phase, stimulates the gonadotropin release, triggers ovulation at the late P/early E phase, and returns to point of departure at the end of E phase. Progesterone level gradually rises and sets during both M and D phases, and by the end of the P phase, it again increases to the peak followed by a decrease in the E phase. Decreased level of progesterone follows a positive feedback mechanism which increases FSH and LH production from the anterior part of the pituitary gland. LH plays the central character in ovulation and is responsible for oocyte maturation, luteinization, and rupturing of pre-ovulatory follicles (Ganaie and Shrivastava, 2010; Marcondes et al., 2002; Van De Lagemaat et al., 2009).
Body weight gain has been reported as an important parameter that significantly regulates gonadotropin secretions from the pituitary gland and the balance of these ovarian and extra-ovarian hormones, as mentioned above, contributes to maintaining steady cyclicity in females (Yinusa et al., 2010). Therefore, a significant decrease in the body weight gain after both, PAL and PAR treatments (as observed in Table 2), might have actively contributed to altering gonadotropin levels, particularly estrogen, affecting the normal functionality of the ovary and uterus resulting in the disturbing cyclicity. Reproductive organs like the ovary, uterus, and vagina are the target organs that manifest differential sensibility to hormones as their structural and functional maintenance is completely dependent on hormones. Any changes in hormonal circulatory levels affect the internal environment of reproductive organs and thereby lead to alteration in histology and weight of organs. Thus, complex relationships within and between, these self-balanced ovarian and extra-ovarian hormones (i.e., estrogen, progesterone, FSH, and LH), as well as various events and phases of ovarian and estrous cycles (i.e., maturation of pre-ovulatory follicles and ovulation), determine the histological, physiological, morphological and biochemical changes within the reproductive organs during these phases (Kafali et al., 2004; Karateke et al., 2019; Renczes et al., 2020; Shah et al., 2016b).
In the present antifertility model, reduced estrogen levels (Fig. 2) must have disrupted the gonadotropins’ balance, which might have disturbed cyclicity by extending the duration of the mean length of the estrous cycle and reducing the number of the estrous cycles (Table 3). This reduction in estrous cycle counts can be attributed to decreased LH, progesterone, and estrogen levels which leads to as follows: (i) a prolonged percentage of D phase where follicle matures but do not ovulate, causing a failure of luteal regression at the normal time in non-pregnant females (Mair and Watson, 2019) and (ii) shortened percentage of P and E phases, where the endometrium and vaginal epithelium do not get sufficient time to prepare themselves for successful fertilization, implantation, and sustaining a pregnancy. Thus, a significant reduction in the number of the estrous cycle in females, after treatments with PAL and PAR extracts, can certainly be correlated with the fertility decrement. A shorter number of estrous cycling can cause systemic pathology characterized by profound hormonal deregulation of reproductive physiology impairing the successful ovulation, fertilization of an ovum, and/or implantation of a zygote or sometimes premature spontaneous abortion or miscarriage (Jaini et al., 2015).
After PAL and PAR treatment, estrogen, LH, and progesterone levels decreased dose-dependently, but, FSH levels increased instead. It has been documented that estrogen, produced by maturing ovarian follicles, is the primary trigger for pre-ovulatory gonadotropin surge in rodents as well as non-rodents. Estrogen induces both positive and negative feedback on gonadotropin secretion. Negative feedback on estrogen can raise the level of FSH (Mahesh and Muldoon, 1987). Decreased levels of progesterone (Fig. 2) from corpora lutea might also have affected uterine, vaginal, and cervical histology (Fig. 3). Previous studies showed that decreased levels of progesterone produced by corpora lutea affect the implantation as well as maintenance of gestation (Goyeneche et al., 2003).
Estrogen and progesterone have been reported to modulate the growth of ciliated and nonciliated cells in the oviduct (Barton et al., 2020). A decrease in the numbers of ciliated and non-ciliated cells, from the section of oviduct ampulla, after PAL and PAR treatments, might have retarded movement of sperms, ovum, and/or zygote making them unable to reach the destination causing either failure of fertilization or failure of implantation. A major noteworthy change was an alteration in the thickness of the endometrial wall of the uterus. Sloughing off of decidualized endometrial wall due to significantly decreased glandular and luminal epithelium proliferation and increased apoptosis of cells can be attributed to the low levels of progesterone as similar effects have been reported in the literature (Al-Qudsi and Linjawi, 2012). This resulted in a reduction of endometrial wall thickness making uterine conditions favorable for preimplantation loss. In the cervix, reduced proliferation of different epithelial layers can be attributed to decreased levels of progesterone. As per the studies conducted in the past, estrogen regulates the differentiation of vaginal epithelial cells, increased weight, thickening, keratinization in the squamous epithelium layer as well as in increasing density of lamina propria and fibromuscular layer (Li et al., 2017, 2018). Similarly in the vaginal region, due to hormonal imbalance, decreased proliferation of stratified squamous epithelium layer, the height of epithelium and thickness of the epithelium, and increased squamous epithelium hypoplasia were observed. All these changes in the cervix and vagina cumulatively resulted in a hostile environment for sperm transport. Thus, the above discussed histological several changes in different reproductive organs can be linked with the hormonal imbalance in the antifertility model. Studies have also reported that low levels of estrogen cause degeneration of follicles and mineralization of follicles in the ovary (Gilbreath et al., 2014). The hormonal imbalance by both extracts resulted in reduced numbers of mature follicles as well as corpora lutea, observed from the ovarian section.
CONCLUSION
Prompt quest for an innovative constituent entity with improved salutary action and the lesser side effects is always on demand. Phyto-constituents acquired from herbal preparations are essential because of their fewer or no side effects. The outcomes of the present exploration indicate that PAL and PAR extract retains antifertility properties, which are facilitated via disturbing hormonal balance, changing endometrium receptiveness, and altering the histological behavior of organs. Future investigation can be carried out to find out the exact molecular mechanism for these phyto-constituent’s modes of action through the use of advanced medical technologies. This provides a guidance corridor and opportunities for the application of such phytochemicals acting as fertility inhibitors by using up-to-date medical tools that work through various mechanisms to support healthy birth control practices (contraception).
ACKNOWLEDGMENT
The authors are honestly appreciative to VNS College of pharmacy for providing research laboratory facilities to complete the work fruitfully. Dr. Bimal Desai, HOD Department of Botany and Forestry, Navsari Agriculture University, Gujarat (India) is acknowledged for his support in recognizing and authorizing the plant species.
ETHICAL APPROVAL
All authors hereby declare that the “Committee for the Purpose of Control and Supervision of Experiments on Animals” (Compendium of CPCSEA, 2018) was followed. Authorization and ethical approvals were acquired proceeding to commencement of the research work on animals, and that the experiments were accomplished in harmony with the above guidelines. The entire animal research was executed in the department of pharmacology, VNS College of Pharmacy, Bhopal (India) with due permission from the Institutional Animal Ethics Committee (IAEC) (Reg. No. PH/IAEC/VNS/2K21/05).
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The entire work was self-funded by the first author who had a role in study design, data collection, data analysis, interpretation of data, and involvement in the writing of the report and even in the decision to submit the article for publication.
DECLARATION OF COMPETING INTEREST
This investigation research was hypothesized and implemented by the first author under the supervision of the second author. Data exploration and manuscript preparation were through by the first and second authors together. There is no conflict of interest.
AUTHORS’ CONTRIBUTIONS STATEMENT
First author: Conceptualization, Investigation, Methodology, Software. Second author: Supervision, Validation. Third author: Writing, draft preparation, reviewing, editing
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the first author upon reasonable request.
REFERENCES
Ahirwar D, Ahirwar B, Kharya M. Evaluation of antifertility activity of Trigonella foenum graecum seeds. Der Pharm Sinica, 2010; 1(3):33–9.
Al-Qudsi F, Linjawi S. Histological and hormonal changes in rat endometrium under the effect of Camphor. Life Sci J, 2012; 9(2):348–55.
Amit S, Pankaj A. Anti fertility activity of hydro alcoholic extract of Trillium Govanianum in Ethinyl estradiol induced anti fertility model in rats. Asian J Pharm Res Dev, 2018; 6(2):74–81. CrossRef
An GH, Chen XW, Li C, Zhang L, Wei MF, Chen JJ, Ma Q, Yang DF, Wang J. Pathophysiological changes in female rats with estrous cycle disorder induced by long-term heat stress. Biomed Res Int, 2020; 2020:1–10. CrossRef
Asuquo OR. Histomorphological study of the anti-fertility effect of Spondias Mombin L. in adult male rats. J Pharm Biol Sci, 2012; 3(2):29–34. CrossRef
Bandyopadhyay S. Anti-fertility activity of methanol extract of Bassia latifolia and Cajanus cajan in female albino mice ovaries. Iran J Pharmacol Ther, 2010; 9(2):83–7.
Barton BE, Herrera GG, Anamthathmakula P, Rock JK, Willie AM, Harris EA, Takemaru KI, Winuthayanon W. Roles of steroid hormones in oviductal function. Reproduction, 2020; 159(3):R125–37. CrossRef
Bhaskar VH, Profulla KM, Balakrishnan BR., Balakrishnan N, Sangameswaran B. Evaluation of the anti-fertility activity of stem bark of Crataeva nurvala buch-hum. African J Biotechnol, 2009; 8(22):6453–6. CrossRef
Byers SL, Wiles M V, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One, 2012; 7(4):1–5. CrossRef
Chen X, Hou X, Feng T, Han N, Wang J, Chang G. Anti-fertility effect of levonorgestrel and/or quinestrol on striped field mouse (Apodemus agrarius): evidence from both laboratory and field experiments. Integr Zool, 2021; 1–12. CrossRef
Choudhary M, Rani S, Sharma P, Choudhary N, Budhwaar V. Anti-fertility and abortifacient potential of hydroalcoholic leaves extract of Alstonia scholaris in female rats: an ethnomedicine used by Papua women in New Guinea. Bull Fac Pharmacy Cairo Univ, 2017; 55(1):123–7. CrossRef
Daud D, Azahar A, Zainal Abidin SSF, Tawang A. The effects of Cosmos caudatus and Piper sarmentosum aqueous extracts on male mice fertility. Int J Pharm Pharm Sci, 2015; 7:296–8.
Devi P, Kumar P, Dhamija I. Antifertility activity of medicinal plants on male and female reproduction. Int J Pharm Sci Res, 2015; 4(1):1–6.
Edwin S, Joshi SB, Jain CD. Antifertility activity of stems of Plumbago zeylanica Linn. in female albino rats. Eur J Contracept Reprod Health Care, 2008; 14(3):233–9. CrossRef
Fajriaty I, Haryanto IH, Haryanto Y. Anti-fertility effect of ethanol extract of lerak (Sapindus rarak DC) fruits in female Sprague Dawley Rats. Nusant Biosci, 2017; 9(1):102–6. CrossRef
Farooque A, Mazumder A, Mazumder R. Review on Plumeria acuminata. Int J Res Pharm, 2012; 2(2):467–9.
Ganaie JA, Shrivastava VK. Effects of gonadotropin releasing hormone conjugate immunization and bioenhancing role of Kamdhenu ark on estrous cycle, serum estradiol and progesterone levels in female Mus musculus. Iran J Reprod Med, 2010; 8(2):70–5.
Gilbreath ET, Mohankumar S, Balasubramanian P, Agnew DW, Mohankumar PS. Chronic exposure to low levels of estradiol and their effects on the ovaries and reproductive hormones: Comparison with aging. Endo Disruptor, 2014; 2(1):e967127. CrossRef
Gomathi P, Gupta M, Mazumder UK, Gebrelibanos M, Sintayehu B. Antioxidant and antitumor activity of Plumeria acuminata in Ehrlich Ascites Carcinoma Bearing Swiss Albino Mice. J Pharm Res Int, 2013; 3(4):671–85. CrossRef
Gomathi P, Shalini T, Farheen N, Sanjeevkumar A. Antidiabetic activity of Plumeria acuminata leaves on streptozotocin induced diabetic rats. Asian J Res Biol Pharm Sci, 2016; 4(3):99–104.
Goyal AK. Phytochemistry and in vitro studies on anti-fertility effect of Ficus religiosa fruits extract on uterine morphology of goat (Capra hircus). Int J Drug Dev Res, 2014; 6(2):141–58.
Goyeneche AA, Deis RP, Gibori G, Telleria CM. Progesterone promotes survival of the rat corpus luteum in the absence of cognate receptors. Biol Reprod, 2003; 68(1):151–8. CrossRef
Gupta M. Phytochemical screening of leaves of Plumeria alba and Plumeria acuminata. J Chem Pharm Res, 2016; 8:354–8.
Hyacinth AA, Nwocha UC. Antifertility activity of aqueous ethanolic extract of Hymenocardia acida stem bark in female rats. Iran J Reprod Med, 2011; 9(3):217–7.
Jaber BM, Jasim SF. Phytochemical study of stigmasterol and β-sitosterol in Viola odorata plant cultivated in Iraq. Iraqi J Biotech, 2014; 13(2):86–94.
Jain S, Choudhary GP, Jain DK. Pharmacological evaluation and antifertility activity of Jatropha gossypifolia in rats. Biomed Res Int, 2013; 1–5. CrossRef
Jain S, Choudhary, GP, Jain DK. Medicinal plants with potential anti-fertility activity: a review. Int J Green Pharm, 2015; 9(4):223–228. CrossRef
Jaini R, Altuntas CZ, Loya MG, Tuohy VK. Disruption of estrous cycle homeostasis in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol, 2015; 279:71–4. CrossRef
Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch Med Res, 2004; 35(2):103–8. CrossRef
Karateke A, Dokuyucu R, Dogan H, Ozgur T, Tas ZA, Tutuk O, Agturk G, Tumer C. Investigation of therapeutic effects of erdosteine on polycystic ovary syndrome in a rat model. Med Princ Pract, 2019; 27:515–22. CrossRef
Kayode AO, Uche CO, Grillo BD, Tolulope OO. Toxic effects of methanolic extract of Aspilia africana leaf on the estrous cycle and uterine tissues of Wistar rats. Int J Morphol, 2007; 25(3):609–14. CrossRef
Li S, Herrera GG, Tam KK, Lizarraga JS, Beedle MT, Winuthayanon W. Estrogen action in the epithelial cells of the mouse vagina regulates neutrophil infiltration and vaginal tissue integrity. Sci Rep, 2018; 8(1):1–13. CrossRef
Li T, Ma Y, Zhang H, Yan P, Huo L, Hu Y, Chen X, Zhang M, Liu Z. Estrogen replacement regulates vaginal innervations in ovariectomized adult virgin rats: a histological study. Biomed Res Int, 2017; 1–6. CrossRef
Mahesh VB, Muldoon TG. Integration of the effects of estradiol and progesterone in the modulation of gonadotropin secretion. J Steroid Biochem, 1987; 27(4–6):665–75. CrossRef
Mair T, Watson ED. The endocrine system (Chapter 11). The Equine manual, 2nd edition, pp 627–58, 2006. CrossRef
Mansouri E, Asadi-Samani M, Kooti W, Ghasemiboroon M, Ashtary-Larky D, Alamiri F, Afrisham R, Noohi ZH. Anti-fertility effect of hydro-alcoholic extract of fennel (Foeniculum vulgare Mill) seed in male Wistar rats. J Vet Res, 2016; 60(3):357–63. CrossRef
Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: Some helpful considerations. Brazilian J Biol, 2002; 62(4A):609–14. CrossRef
Marin AR, Roco IM, Okanya VK, Olin JNC, Pancho JJE. Bronchodilation effects of kalachuchi leaves (Plumeria acuminata, family: apocynaceae) of ethanol extracts on cat-induced bronchoconstriction. Eur J Pharm Med Res, 2020; 7(1):15–29.
Mittal DK. Analysis of crocus sativus (saffron) on estrous cycle and induced reversible sterility in rat. World J Pharm Pharm Sci, 2020; 9(5):1760–5.
Mukhram MA, Shivakumar H, Viswanatha GL, Rajesh S. Anti-fertility effect of flower extracts of Tabernaemontana divaricata in rats. Chin J Nat Med, 2012; 10(1):58–62. CrossRef
Mziray AM, Maina CI, Kaingu CK. Anti-fertility properties of Cissus rotundifolia Vahl. extract using female Wistar rats. Phytomedicine, 2020; 7(2):58–64. CrossRef
Nayaka HB, Londonkar RL, Andumesh MK. Evaluation of Portulaca oleracea L. for anti-fertility effect in female albino rats. Int J Pharm Pharm Sci, 2014; 6(5):86–9.
Ngadjui E, Nkeng-Efouet PA, Nguelefack TB, Kamanyi A, Watcho P. High fat diet-induced estrus cycle disruption: effects of Ficus asperifolia. J Complement Integr Med, 2015; 12(3):205–15. CrossRef
Oyewopo OA, Dare JB, Towolawi AO, Olaniyan OT, Omotoso OD, Shafe MO, Lenus SC, Leke MJ, Ariyo IA, Owolabi OJ. Cottonseed extract and anti-fertility: metabolic versus hormonal changes in rat model. World J Life Sci Med Res, 2012; 2(5):196–6.
Paccola CC, Resende CG, Stumpp T, Miraglia SM, Cipriano I. The rat estrous cycle revisited: a quantitative and qualitative analysis. Anim Reprod, 2013; 10(4):677–83.
Renczes E, Maronek M, Gaal Kovalcikova A, Vavrincova-Yaghi D, Tothova L, Hodosy J. Behavioral changes during development of chronic kidney disease in rats. Front Med, 2020; 6:1–8. CrossRef
Sanabria V, Bittencourt S, de la Rosa T, Livramento J, Tengan C, Scorza CA, Cavalheiro E, Amado D. Characterization of the estrous cycle in the Amazon spiny rat (Proechimys guyannensis). Heliyon, 2019; 5(12):1–5. CrossRef
Sari IP, Nurrochmad A, Rahayu S. Evaluation of anti-fertility effect of aqueous extract of Costus speciosus (Koen.) J.E. Smith rhizome in mice. Int J Pharm Clin Res, 2016; 8(5):440–4. CrossRef
Shah M, Singh R, Shah R, Kakar S. An overview of the current methodologies used for evaluation of anti-fertility agents. Asian Pacific J Reprod, 2016a; 5(3):175–8. CrossRef
Shah SK, Jhade D, Chouksey R. Antifertility activity of ethanolic and aqueous extracts of Aloe Vera Mill on female wistar rats: rising approaches of herbal contraception. J Pharm Sci Res, 2016b; 8(9):952–7.
Singh R, Kakar S, Shah M, Jain R. Some medicinal plants with anti-fertility potential: a current status. J Basic Clin Repro Sci, 2017; 7(1):7–19.
Sundar Rajan T, Sarathchandiran I, Kadalmani B. Evaluation of anti-fertility activity of herbal oral contraceptive suspension on male Wistar albino rats. J Pharm Res, 2013; 7(4):342–6. CrossRef
Sunitha K, Mohan GK. Immunomodulatory screening of Plumeria acuminata stem bark. World J Pharm Pharm Sci, 2017; 6(11):1172–6.
Sunitha K, Naga Rani C. Antibacterial activity of methanolic extracts of flowers of Plumeria acuminata. J Global Trends Pharm Sci, 2018; 9(1):4978–80.
Taid TC, Rajkhowa RCH, Kalita JCH. The effect of Plumeria acuminata ait on oestrous cycle and acute oral toxicity study in c3h female albino mice. Int J Dev Res, 2016; 6(12):10620–4.
Thanamool C. Evaluating the anti-fertility activity of Talinum paniculatum (Jacq.) Gaertn in female wistar rats. African J Pharm Pharmacol, 2013; 7(26):1802–7. CrossRef
Udiwal S, Jain NK, Gupta MK, Goyal S. Anti-fertility activity of Butea Monosperma Linn in albino rats. Curr Res Biol Pharm Sci, 2014; 3(4):6–11.
Van De Lagemaat R, Timmers CM, Kelder J, Van Koppen C, Mosselman S, Hanssen RGJM. Induction of ovulation by a potent, orally active, low molecular weight agonist (Org 43553) of the luteinizing hormone receptor. Hum Reprod, 2009; 24(3):640–8. CrossRef
Yadav A, Undale V, Bhosale A. Antidiabetic activity of Plumeria rubra L. in normal and alloxan induced diabetic mice. Int J Basic Clin Pharmacol, 2016; 5(3):884–889. CrossRef
Yinusa R, Adeniran A, Ibukun PO, Omowumi FA. Reproductive activities of female albino rats treated with quassin, a bioactive triterpenoid from stem bark extract of quassia amara. Niger J Physiol Sci, 2010; 25(2):95–102.