INTRODUCTION
Dental caries or cavities represent damage to the hard tissues of the tooth caused by organic acids produced from carbohydrate fermentation by endogenous Streptococcus mutans in the oral cavity (Hossain et al., 2021; Peres et al., 2019). Because of the negative effects on human well-being, dental caries is a significant oral health problem in people of all ages. It is also considered a highly prevalent infectious disease. Over two billion people worldwide suffer from dental caries (Peres et al., 2019). A dramatic increase in the incidence of dental caries has been reported in developing and developed countries.
Xylitol, a five-carbon sugar polyol found naturally and artificially manufactured from plant materials, has been developed as a self-applied preventive agent for dental caries (Janakiram et al., 2017). It can interfere with the growth of cariogenic bacteria and their adhesion to the tooth surface. Moreover, it inhibits tooth demineralization and decreases plaque formation, which are primary factors associated with caries etiology. A recent meta-analysis revealed the benefits of xylitol in dental caries prevention. Regarding inhibition against bacterial growth, the recommended daily dose of xylitol for managing dental caries is 6–10 g (Alshibani et al., 2022).
Xylitol-containing oral products, such as toothpaste, chewing gum, and mouth rinse, have been developed (Gargouri et al., 2018; Janakiram et al., 2017), but the xylitol content in these commercial products is approximately 5% w/w or lower. In addition to the optimum concentration of xylitol in commercial products, delivery systems that allow the tooth to be exposed to xylitol for longer periods are necessary to achieve clinical benefits. The effective mode of delivering xylitol remains unclear, and little research has been carried out to develop dental gels and pastilles containing high concentrations of xylitol.
In this study, we developed dental gels and pastilles containing up to 30% and 55.6% w/w xylitol, respectively, and evaluated the physicochemical characteristics of these formulations. Several formulations of xylitol dental gels and pastilles were prepared accordingly. In addition, stability studies were carried out to evaluate the effects of temperature and humidity on the physicochemical stabilities of the optimized formulations.
MATERIALS AND METHODS
Materials
Xylitol Pharmaceutical Secondary Standard (Lot. LRAA9077) and Xylitol BP (Lot. 117050506, purity 99.56 %) were purchased from Sigma-Aldrich, USA, and Finechem Group, China, respectively. Gelatin was obtained from Shandong Hengxin Biotech, China. Carrageenan, carboxy methyl cellulose (CMC), xanthan gum, methyl paraben, sunset yellow, and brilliant blue were acquired from the Chemipan Corporation, Thailand. Hydroxy propyl methyl cellulose 4000 (HPMC 4000) and propyl paraben were purchased from S. Tong Chemicals, Thailand. Sodium ascorbyl phosphate was obtained from DSM Nutritional Products AG., Switzerland. Citric acid was purchased from Krungthepchemi, Thailand. Acetonitrile was obtained from RCI Labscan Limited, Thailand. Buffers (pH 2.01, pH 4.01, and pH 7.01) were obtained from Mettler Toledo, InLab® Solutions, Switzerland.
Formulation of xylitol dental gels
The formulated xylitol dental gels (G1–G16) and their compositions are presented in Table 1. The gels were prepared by a beaker method. First, CMC, HPMC 4000, or xanthan gum was dispersed in purified water. Next, mixtures of xylitol, preservative, and flavoring agent were added to the gel bases. The final weight of the prepared mixtures was adjusted with purified water. After mixing, homogeneous mixtures of xylitol gels were stored in glass bottles with rubber stoppers until further evaluation.
Formulation of xylitol pastilles
Table 2 shows the composition of each pastille formulation. The development and optimization of xylitol pastilles consisted of two phases. Formulations P1–P10 and P11–P12 were prepared in first and second phase experiments, respectively. Gelatin was dissolved in warm water prior to adding xylitol. Carrageenan, flavoring agents, and colorants were subsequently added. The weight of the pastilles was adjusted to 100 g with purified water. The well-mixed mixtures were poured into molds and allowed to set at room temperature. The pastilles were stored in polypropylene sachets until further evaluation.
Evaluation of the physicochemical characteristics of xylitol dental gels and pastilles
Physical appearance
The prepared dental gels and pastilles were visually inspected for color, texture, homogeneity, and clearness within 24 h after preparation. The formulations displaying acceptable appearance were selected for further analysis.
pH measurement
The pH values of the dental gels were measured using a pH meter (Mettler Toledo, SevenCompact™ pH/Ion S220, Switzerland) equipped with a pH combined glass electrode (Inlab® Routine Pro, Switzerland) at room temperature. The pH values of the pastilles were measured by dissolving a sample pastille in 10 ml of ultrapure water to measure pH. The acceptable pH values of the formulations were in the range of 5.0–7.0.
 | Table 1. Formulations of xylitol dental gels. [Click here to view] |
 | Table 2. Formulations of xylitol pastilles. [Click here to view] |
Viscosity test
The viscosity of the dental gels was measured using a cone and plate Brookfield Digital Viscometer (Brookfield, Germany)with a suitable spindle speed at room temperature. Commercial fluoride varnish, Duraphat®, was used as a reference because of its acceptable viscosity for dental use. The gels exhibiting comparable viscosity to that of Duraphat® were selected.
Adhesiveness test
The adhesiveness of the dental gels was determined using a modified method (Nair et al., 2021; Zhang et al., 2019). The gels were attached onto a ceramic surface before purified water was added dropwise to the samples at a rate of 0.65 ml/min using a peristaltic pump. The adhesive time, defined as the duration that the gels were attached to the ceramic surface before washing, was recorded.
Dissolution test
The release of xylitol from the pastille base was determined using a Vankel dissolution tester apparatus II (Agilent Technologies, Thailand) equipped with small volume vessels employing 50 ml of artificial saliva at 37 ± 0.5°C as a dissolution medium. The paddles were operated at 11 ± 2 mm above the vessel bottom (at the middle of medium height) and at 150 rpm. The dissolution time was set to 60 minutes. The percentage of dissolved xylitol in the medium was calculated. A dissolution of no less than 85% within 60 minutes was considered acceptable.
Xylitol content assay
Quantitative determination of xylitol in the dental gels and pastilles was carried out using high-performance liquid chromatography (HPLC). Standard xylitol and samples were dissolved in ultrapure water and filtered through a 0.45 μm syringe filter prior to HPLC analysis. The final xylitol concentration was approximately 10 mg/ml. The samples were prepared and measured in triplicate. The chromatographic separation was achieved using an NH2 column (250 mm × 4.6 mm, 5 μm particle) in an Agilent instrument equipped with a refractive index detector (1260 Infinity II, USA). The mobile phase consisted of acetonitrile:water (85:15), filtered through a 0.45 μm membrane filter and sonicated to degas for 30 minutes before use at a flow rate of 1.0 ml/min. The column and the detector temperatures were set to 35°C and 40°C, respectively. A 20 μl sample was injected to measure xylitol concentration. The analytical method was verified for specificity, linearity, range, accuracy, and repeatability. The xylitol analytical method exhibited linearity in a range of 5–15 mg/ml with an R2 value greater than 0.99. The accuracy of the method was in the range of 98.82%–101.17%. In addition, repeatability was shown by an RSD percentage less than 0.66.
Stability evaluation
Heating–cooling test
In each heating–cooling cycle, the temperature was set to 40°C for 24 hours and 4°C for 24 hours. The optimized gel formulations were stored for seven consecutive cycles (Larrea-Wachtendorff et al., 2022). Then, the physical appearance, pH value, and viscosity of the gels were determined.
Syneresis test
The optimized pastille formulations were stored individually in glass bottles with a rubber stopper for 24 h, and the temperature was maintained at 8 ± 1°C and 25 ± 5°C (Gomaa and Ayoub, 2021; Kadhim and Ali, 2019). Observation of syneresis was performed.
Stability test
The gels and pastilles were stored under a temperature and humidity of 40 ± 2°C/75 ± 5% RH (accelerated conditions) and 30 ± 2°C/75 ± 5% RH (long-term conditions) for 3 months. Changes in the physicochemical characteristics of the formulations and the xylitol content were determined at 0, 1, 2, and 3 months.
Statistical analysis
Data are reported as the mean ± standard deviation (SD). The SPSS® Statistics version 22 software (SPSS Co., Ltd., Bangkok, Thailand) was used, and analysis of variance was carried out. A p-value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Formulation of xylitol dental gels
The relatively high concentration of xylitol was considered in the formulated gels based on prior work that suggested the effectiveness of 10% and 20% w/w xylitol varnishes and solutions were comparable to the 5% fluoride product (Janakiram et al., 2017). We postulated that the new formulations containing 20% and 30% w/w xylitol could promote clinical benefits. Polymers, including HPMC, CMC, and xanthan gum, were used as gelling agents. Citric acid was used as a flavoring agent, and either paraben concentrate or sodium benzoate was used as a preservative.
Physicochemical characteristics of dental gels
Table 3 presents the physicochemical characteristics of the dental gels. The color of the xylitol dental gels was yellowish. The homogeneity of the gels depended on the gelling agents. Formulations containing 4% w/w HPMC 4000 were nonhomogeneous. This phenomenon may be explained by the interaction between xylitol and HPMC 4000. Because of the polyol nature of xylitol, it attracts water more efficiently than HPMC 4000. Therefore, the small numbers of free water molecules are insufficient to completely hydrate HPMC 4000, leading to a phase separation in the formulations. The viscosities of the formulations containing 4% w/w CMC were very thick in appearance, whereas formulations containing 1% and 2% w/w xanthan gum were thin. Furthermore, the different preservatives exhibited different effects on gel clearness. The turbid gels were observed in formulations containing 0.2% w/w sodium benzoate. A plausible explanation is that xylitol decreases the solubility of sodium benzoate and the undissolved portion results in gel turbidity. Meanwhile, formulations containing 0.5% w/w paraben concentrate were apparently translucent.
The pH values of the gel formulations were in the range of 5.56–6.12. The viscosities of the formulations containing 2% w/w xanthan gum were comparable to Duraphat® (625.18 ± 58.62 cps). Thus, the gels were considered to show an optimal viscosity for dental use. Formulations containing 1% w/w xanthan gum exhibited lower viscosities than Duraphat®. The gel formulations containing 4% w/w CMC were too viscous. The adhesive time of the gels containing 1–2% w/w xanthan gum was in the range of 5–10 min. As a result, 20% and 30% w/w xylitol dental gels were successfully developed, and formulations G7 and G15 were considered optimized gel formulations with respect to the acceptance criteria.
 | Table 3. Physicochemical characteristics of xylitol dental gels. [Click here to view] |
Xylitol and the gel base are water-soluble. The gel formulation is expected to dissolve and release xylitol in saliva. In addition, the friction between the tooth surface and cheek bulge enhances the dissolution of the gel formulation. It was hypothesized that xylitol would be easily washed away and swallowed. Therefore, the adhesiveness test was designed to show the duration of the gel formulation adhering to the tooth surface. The long contact time increased the possibility of xylitol forming a complex with calcium in the tooth, which could retard tooth demineralization, the same mechanism as the fluoride varnish.
Formulation of xylitol pastilles
Each pastille formulation contained a combination of gelatin and carrageenan as gelling agents to optimize the pastille’s texture. Either citric acid or sodium ascorbyl phosphate was used as a flavoring agent. Lemon oil was used as a flavoring agent, whereas brilliant blue and sunset yellow were used as colorants.
Physicochemical characteristics of pastilles
The physicochemical characteristics of the formulated xylitol pastilles are presented in Table 4. Increased gelatin content resulted in a different physical appearance of the pastilles. Formulations containing 6% and 8% w/w gelatin were fluidlike, whereas the others formed pastilles. However, formulations containing 14% w/w gelatin appeared difficult to chew, and there was an icing crust on the pastille surface. Formulations containing 10% and 12% w/w gelatin showed a physical appearance that met the acceptance criteria, but they were discarded because of xylitol recrystallization. Subsequently, the additional two formulations (P11 and P12) were prepared. They possessed a smooth surface, homogeneous texture, translucency, and chewability. The percentage of dissolved xylitol in the artificial saliva was 98.71 ± 2.11% for P11 and 101.15 ± 1.01% for P12, which were within the limits for immediate release pastilles. Therefore, formulations P11 and P12 were considered optimized pastille formulations for this study.
The criteria for the pastille dissolution test might be impractical in real-life situations since the consumers would chew and swallow the pastille in a couple of minutes. The pastilles were designed as a chewable pastille with a gummy texture. During the chewing process, teeth come into contact with the pastilles for 1–2 minutes. Xylitol in small chunk of pastilles would be dissolved in saliva and form a complex with calcium in the tooth.
Stability evaluation
After gel formulations G7 and G15 were treated using a heating–cooling cycle method, no changes in physical appearance were evident. The pH and viscosities of the gels were in the range of 5.82–5.95 and 783.45–866.68 cps, respectively, throughout the seven cycles. Similarly, no evidence of syneresis was observed in pastille formulations P11 or P12. This may be attributed to the high concentration of gelling agents added to the formulations (Kadhim and Ali, 2019).
Table 5 shows that the physical appearance of G7 and G15 was not changed in long-term stability studies. The gel viscosities of G7 and G15 decreased over time, and the pH of G15 significantly changed, but the values were within the acceptance limits. The results of both storage conditions were consistent. In addition, the xylitol content in G15 significantly decreased at 3 months compared with the other observation times (p < 0.05) after storage at 40 ± 2°C/75 ± 5% RH and 30 ± 2°C/75 ± 5% RH. This may result from the high amount of xylitol added to the gels, which exhibits a high degree of hygroscopicity (Rad et al., 2019). In addition, xylitol undergoes oxidation (Jofre et al., 2022); however, the xylitol content in the formulations still met the criteria ranging from 90.0% to 110.0%. No alteration in xylitol content in G7 was observed during storage at 40 ± 2°C/75 ± 5% RH and 30 ± 2°C/75 ± 5% RH for 3 months. This indicated that the increased xylitol content up to 30% w/w might affect the stability. The percentage label amount of xylitol in each batch was plotted for the assessment of shelf-life. A least-square linear regression analysis was employed (Komesmuneeborirak et al., 2020). Then, a lower one-sided 95% confidence interval was constructed with the linear regression line. The shelf-life was considered the time when the lower one-sided 95% confidence line intersected with the lower acceptance criterion. Using this technique, the shelf-lives of G7 and G15 were estimated to be 2.5 and 3.6 months, respectively (Fig. 1).
 | Table 4. Physicochemical characteristics of xylitol pastilles. [Click here to view] |
 | Table 5. Physicochemical stabilities of xylitol dental gels and pastilles. [Click here to view] |
 | Figure 1. Xylitol concentration-time profile for G15 (X). The solid line and the dashed line represent a linear regression line and the lower one-sided 95% confidence limit of the mean around the linear regression line, respectively. [Click here to view] |
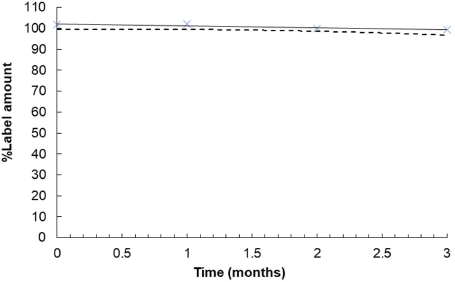 | Figure 2. Xylitol concentration-time profile for P11 (X). The solid line and the dashed line represent a linear regression line and the lower one-sided 95% confidence limit of the mean around the linear regression line, respectively. [Click here to view] |
As shown in Table 5, the impact of environmental factors on the stability of the pastilles was evident. The percentage of dissolved xylitol of the pastilles gradually decreased, but the values remained acceptable. After storage at 30 ± 2°C/75 ± 5% RH for 3 months, the physical appearance of the pastilles did not differ from the baseline. This was in agreement with the content uniformity assay. There was no reduction in xylitol content in P11 and P12, which was in the range of 90.0%–110.0% throughout the three-month period. We further postulated that citric acid and sodium ascorbyl palmitate in P11 and P12, respectively, did not affect the stability of the formulations and they may be used as flavoring agents for the xylitol pastilles. On the contrary, both P11 and P12 became physically unstable when they were stored at 40 ± 2°C/75 ± 5% RH for 1 month. As the pastilles were more likely to melt at high temperatures, control of delivery, handling, and storage conditions should be taken into consideration. Using the least-square linear regression analysis with the lower one-sided 95% confidence interval, the shelf-lives of P11 and P12 were estimated to be 2.7 and 5 months, respectively (Fig. 2 and 3).
 | Figure 3. Xylitol concentration-time profile for P12 (X). The solid line and the dashed line represent a linear regression line and the lower one-sided 95% confidence limit of the mean around the linear regression line, respectively. [Click here to view] |
CONCLUSION
Dental gels and pastilles containing a relatively high concentration of xylitol were successfully formulated. The gelling agents affected the characteristics of the prepared formulations considerably. The optimized formulations exhibited acceptable physicochemical characteristics and stability. With the promise of xylitol for caries prevention, xylitol dental gels (G15) and pastilles (P12) may represent effective therapeutic agents. Further preclinical and clinical studies should be carried out to determine patient satisfaction, safety, and efficacy of the finished products.
FUNDING
This Research is funded by TSRI Fund No. CU_FRB640001_01_33_6.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alshibani N, Shalabi M, AlMugbel K, AlSaqer E, AlFarraj N, Allam E. Xylitol content and acid production of chewing gums available in the markets of Saudi Arabia. Saudi Dent J, 2022; 34(2):121–8. CrossRef
Gargouri W, Kammoun R, Elleuche M, Tlili M, Kechaou N, Ghoul-Mazgar S. Effect of xylitol chewing gum enriched with propolis on dentin remineralization in vitro. Arch Oral Biol, 2020; 112:104684. CrossRef
Gomaa E, Ayoub MM. Vardenafil oral jellies as a potential approach for management of pediatric irritable bowel syndrome. Saudi Pharm J, 2021; 29(9):955–62. CrossRef
Hossain MS, Alam S, Nibir YM, Tusty TA, Bulbul SM, Islam M, Hossain MS. Genotypic and phenotypic characterization of Streptococcus mutans strains isolated from patients with dental caries. Iran J Microbiol, 2021; 13(4):449–57. CrossRef
Janakiram C, Deepan Kumar CV, Joseph J. Xylitol in preventing dental caries: a systematic review and meta-analyses. J Nat Sci Biol Med, 2017; 8(1):16–21. CrossRef
Jofre FM, Bordini FW, de Andrade Bianchini I, de Souza Queiroz S, da Silva Boaes T, Hernández-Pérez AF, de Almeida Felipe MdG. 8-Xylitol and sorbitol: production routes, challenges and opportunities in biorefineries integration. In: Chandel AK, Segato F (eds.). Production of top 12 biochemicals selected by USDOE from renewable resources. Elsevier, Amsterdam, The Netherlands, pp 233–68, 2022. CrossRef
Kadhim Z, Ali, W. Preparation and evaluation of granisetron chewable pediatric oral jelly. Int. J. Drug Deliv. Technol, 2019; 9(3):347–51. CrossRef
Komesmuneeborirak P, Werawatganone P, Muangsiri, W. Amphotericin B topical extemporaneous preparations for the treatment of non-dermatophytic onychomycosis. Asian J Pharm Clin Res, 2020; 13(12):135–9. CrossRef
Larrea-Wachtendorff D, Del Grosso V, Ferrari G. Evaluation of the physical stability of starch-based hydrogels produced by high-pressurepProcessing (HPP). Gels, 2022; 8(3):152; doi:10.3390/gels8030152 CrossRef
Nair AB, Shah J, Jacob S, Al-Dhubiab BE, Patel V, Sreeharsha N, Shinu, P. Development of mucoadhesive buccal film for rizatriptan: In Vitro and In Vivo evaluation. Pharmaceutics, 2021; 13(5):728; doi:10.3390/pharmaceutics13050728 CrossRef
Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño CC, Kearns C, Benzian H, Allison P, Watt RG. Oral diseases: a global public health challenge. Lancet, 2019; 394(10194):249–60. CrossRef
Rad AH, Pirouzian HR, Konar N, Toker OS, Polat DG. Effects of polyols on the quality characteristics of sucrose-free milk chocolate produced in a ball mill. RSC Adv, 2019; 9:29676–88. CrossRef
Zhang C, Liu Y, Li W, Gao P, Xiang D, Ren X, Liu D. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: in vitro and in vivo studies. Pharm Dev Technol, 2019; 24(1):118–26. CrossRef