INTRODUCTION
Indonesia is a country with abundant biodiversity including medicinal plants. The abundance of medicinal plants encourages people to use traditional medicine based on natural ingredients. Traditional medicine is not based on scientific approaches. Traditional medicine is based on cultural knowledge, which means that the practice of traditional medicine is a part of their culture and habit. People prefer using traditional medicine with natural ingredients because they perceive natural ingredients to be safer than synthetic drugs. In addition, traditional medicine is affordable as medicinal plants are often grown in home yards (Elfahmi et al., 2014; Moreira et al., 2014).
Nyctanthes arbor-tristis (NAT) is native to South Asia. Its distribution ranges from Northern Pakistan and Nepal throughout North India to Southeast Thailand. Currently, this plant is widely cultivated in the tropics and subtropics. The genus Nyctanthes includes two types of species, namely NAT and Nyctanthes aculeata. NAT is a native plant of India and is used as a sacred plant in religious ceremonials, whereas N. aculeata is a native plant of Thailand. The scientific research and literature study on N. aculeata are still limited. In this narrative review, we provide comprehensive information on the pharmacological and phytochemical aspects of NAT and discuss its prospect as a potential source of bioactive molecules for drug discovery (Wallander and Albert, 2000).
NAT has fragrant flowers resembling jasmine. The flowers only grow in the afternoon until the evening and fall in the morning. Thus, this plant is called “night jasmine.” In Ayurveda, each part of this plant can be used to treat various diseases, including digestive problems, as an antidote to reptile venoms, as a tonic, laxative, diaphoretic, and diuretic, and as a remedy to arthritis (Rangika et al., 2015). In Indonesia, NAT is usually used as a traditional medicine to treat fever, hemorrhoids, irregular menstruation, pain, cough, and asthma. The plant parts used comprise leaves, stem bark, and flowers (Medicinal Herb Index in Indonesia, 1986). The extracts of NAT have been pharmacologically investigated as antimicrobial, analgesic, anti-inflammatory, antidiabetic, antioxidant, hepatoprotective, cough suppressant, and antimalaria agents (Chaudhary et al., 2018; Ghosh et al., 2015; Godse et al., 2016; Kakoti et al., 2013; Michael et al., 2013; Mousum et al., 2018).
Although the research on NAT is still limited, this plant has the potency to be developed as a drug or source of bioactive molecules based on its traditional use and recent scientific findings. In this review, we describe the chemical structure diversity of NAT phytoconstituents and provide the pharmacological activities of its leaves, flowers, seeds, and bark. This comprehensive review presents scientific information that motivates researchers and scientific communities to find scientific data related to NAT.
METHODS
The literature search was carried out through the PubMed and Google Scholar databases. To obtain the most recent information, we only used articles ranging from 2010 to 2021 in this study. The keywords for the database search were “Nyctanthes arbor-tristis L.; phytochemistry,” “Nyctanthes arbor-tristis L. isolation,” “Nyctanthes arbor-tristis L. study in vitro,” “Nyctanthes arbor-tristis study in vivo,” and “Nyctanthes arbor-tristis L. pharmacological study.” The data included in this work were original research articles only. Unpublished data, including theses and conference articles, were excluded from this study.
Botanical aspect of Nyctanthes arbor-tristis
NAT is a vascular plant and a member of Tracheophyta. The plant belongs to Magnoliopsida or Dicotyledoneae (which includes flowering plants with two pieces of seeds). The plant is a member of the Oleaceae family and genus Nyctanthes (Sharma et al., 2021). The term “Nyctanthes” comes from the words nykhta (night) and anthos (flower), thus referring to a night flower. NAT is also called “the sad tree” or “tree of sorrow.” NAT is often called “night jasmine.” Its flower has a fragrant aromatic odor. This woody plant reaches approximately 10 m in height and has a grey bark surrounded by shells. It usually grows and lives for up to 20 years. The leaves are ovate and tapered and sit in the opposite position, whereas the mature fruits are brown with a diameter of approximately 2 cm. The flowers grow in the armpits or terminals consisting of 2–7 chalices with rectangular stems. The flower crown is orange with a white lobe 5–15 mm long, and two stamens can be found at its top. The petals are ovoid with an orange to red color combined with white. This plant usually blossoms in the afternoon until evening (Hiremath et al., 2016; Mishra et al., 2016a; Mishra et al., 2016b; Sharma et al., 2021).
Phytoconstituents of Nyctanthes arbor-tristis
Table 1 indicates that NAT has a high diversity of phytochemicals, and it has the potential to be further explored as a bioactive molecule or a drug candidate. NAT contains various phytochemical constituents. Table 1 presents the reported phytochemical constituents from different parts of the plant (flowers, leaves, stems, and seeds). Phytochemical screening showed that NAT contains steroids, terpenes, flavonoids, iridoid glycosides, carbohydrates, and alkaloids. The ethyl acetate extract of the NAT flower contains stigmasterol, rengyolone, 2-phenylethyl β-D-glucopyranoside, and n-tetradecyl-β-D-glucopyranoside; the methanol and ethanol extracts contain crocin and crocetin (Pawar et al., 2019). The leaf contains β-sitosterol, nyctanthic acid, 1-(8-hydroxy-7-((4-nitrophenyl)(phenylamino)methyl)quinoline-3-yl) propane-2-one, 2-(8-hydroxy-7-((4-nitrophenyl)(phenylamino)methyl)quinoline-3-yl) acetic acid, arbortristoside C, calceolarioside A, arborside A, arborside B, lupeol, and betulinic acid (Ashwini and Rekha, 2019; Bhadouria et al., 2012; Chaudhary et al., 2018; Karan et al., 2019; Mishra et al., 2016b). From the seeds of NAT, an iridoid-derived compound, namely arbortristoside C, was identified from the methanolic extract (Vajravijayan et al., 2020). The stem contains 21α-hydroxyfriedel-4-(23)-en-3one, β-sitosterol, 1-triacontanol, friedel-1-ene-3-one, pelargonic acid, and lignoceric acid (Kumari et al., 2017).
Biological activities of Nyctanthes arbor-tristis
Table 2 presents the recent studies (last 10 years) on the pharmacological effects of NAT. The extracts from the leaves, flowers, seeds, and stems of NAT demonstrate various biological activities. The leaf is well studied compared with the flowers, seeds, and stem bark. Although the comprehensive information regarding the bioprospective of NAT is limited, the pharmacological activities from its different parts are discussed in this review. These pharmacological activities include antioxidant, anti-Malassezia, antihyperglycemic, antihyperlipidemia, antimalaria, hepatoprotective, antitussive, analgesic, anti-inflammatory, anticancer, antiproliferation, and antigenotoxic activities.
Antioxidant effect
Antioxidants are compounds that can prevent oxidative stress due to excessive free radicals in the body. This oxidative stress can induce lipid, lipoprotein, and DNA damage, thus triggering various diseases. The methanol extract from NAT leaves has an antioxidant activity, and these have been tested in vitro using different methods. The half-maximal inhibitory concentration (IC50) values of 1,1-diphenyl-2-picrylhydrazine (DPPH) radical scavenging, hydroxyl radical scavenging, nitric oxide scavenging, and superoxide radical scavenging assays are 57.93, 98.61, 91.74, and 196.07 µg/ml, respectively. These antioxidant activities may be correlated with the phenolic content of the extract, which reaches 78.48 ± 4.26 mg tannic acid equivalence per gram. Phenolic compounds exhibit antioxidant activity by counteracting free radicals, thus causing their free radical reduction (Michael et al., 2013).
The ethanol and aqueous extracts from the seeds and leaves of NAT showed concentration-dependent activity in a DPPH radical scavenging assay. The ethanol and water extracts of NAT leaves demonstrated stronger antioxidant activity than those of the seeds. Interestingly, betulinic acid was identified as an active antioxidant compound with an IC50 of 18.03 µg/ml with the DPPH assay. This compound might interfere with the oxidation process by donating an electron to neutralize free radicals (Karan et al., 2019; Patel and Gokhale, 2016; Sousa et al., 2021). Other studies evaluated the antioxidant activities of different NAT flower extracts (ethanol, ethyl acetate, and water extracts) using DPPH radical scavenging, superoxide radical scavenging, lipid peroxidation, and reducing power assays. The study revealed that the ethanol extract of NAT flower demonstrated the highest antioxidant activity with IC50 values of 406.37 ± 2.45, 269.66 ± 18.48, and 2004.14 ± 8.31 in DPPH, lipid peroxidation, and superoxide radical scavenging assays, respectively. This finding indicated that although the extract showed a medium degree of antioxidant activity, its reducing power is weak. The difference in the antioxidant potency may be due to the solubility of antioxidant compounds in various solvents used in the extraction process (Mishra et al., 2016a; Patel and Gokhale, 2016).
Anti-Malassezia
Malassezia is a type of eukaryotic fungus and normal microbiological flora of human and animal skin. However, Malassezia spp. can cause several diseases, including pityriasis versicolor, seborrheic dermatitis, and folliculitis (Han et al., 2017). In addition, the interaction of this fungus with the host can stimulate hypersensitivity and activate immunoglobulin E (IgE) production and T cell reactivity. It produces Mala s1, a major allergen that triggers the production of IgE and causes skin diseases (Saunders et al., 2012).
The ethanol extract of NAT leaves prevented Malassezia infection, as assessed by a microdilution method. The ethanol extract inhibited the growth of two Malassezia strains with minimum inhibitory concentrations of 1.05 and 1.47 µg/ml against M. globosa and M. restricta, respectively. The cytometric analysis demonstrated that the active compounds were β-sitosterol and calceolarioside A. Both compounds effectively bind to the specific targeting site of Mala s1 of the fungus in the molecular docking study and inhibit the production of tumor necrosis factor-α (TNF-α) in the stimulated splenocytes. NAT is considered a potential natural product for preventing Malassezia infection (Mishra et al., 2016).
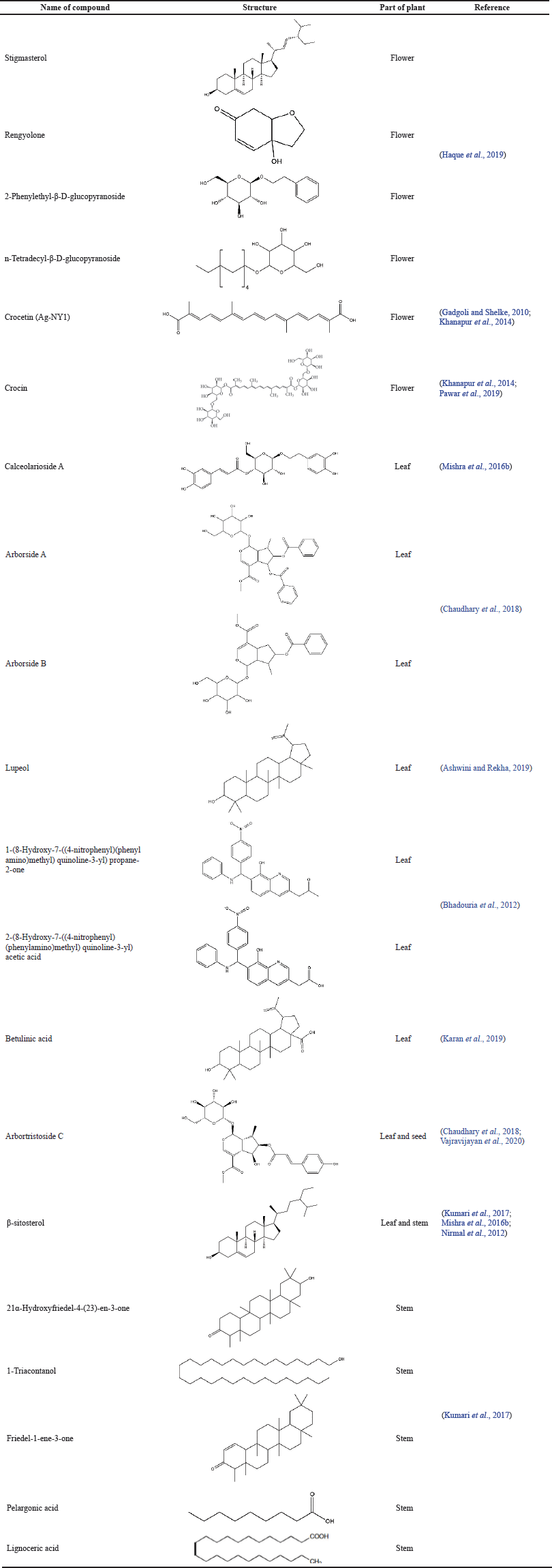 | Table 1. Reported data of compounds present in Nyctanthes arbor-tristis. [Click here to view] |
Antihyperglycemia and antihyperlipidemic
Diabetes is a disease caused by disorders of the endocrine system. This damage causes an increase in glucose levels because of the disturbance in insulin secretion. Diabetes in the long term will affect an increase in low-density lipoprotein (LDL), a decrease in high-density lipoprotein (HDL), which causes lipid dysregulation, reactive oxygen species production, and reduction of antioxidant status (Luo et al., 2019). Lipid disorders also trigger the occurrence of coronary heart disease. The ethanol extract of NAT leaves demonstrated antihyperglycemic and antihyperlipidemic activities in albino rats. It decreased blood sugar levels at the dose of 400 mg/kg given for 28 days in high-fat-diet (HFD) and streptozotocin (STZ) induced rats. The extract significantly reduced the blood sugar level from 437.4 ± 21.18 mg/dl to 191.3 ± 21.51 mg/dl (p < 0.001). Interestingly, the lipid profile was also significantly improved. The total cholesterol, triglyceride, vLDL, and LDL decreased by 36.3%, 21.6%, 21.6%, and 60.3%, respectively, whereas HDL increased compared with the control group (HFD–STZ-induced diabetic rats). The ethanol extract of NAT also decreased the malondialdehyde (MDA) level and increased the superoxide dismutase (SOD), catalase, and glutathione reductase enzyme levels. MDA is a marker of lipid peroxidase and can cause cross-linking of the polymerization of proteins, nucleic acids, and other macromolecules. Meanwhile, SOD, catalase, and glutathione reductase are enzymatic antioxidants that have an important role in the defense of the immune system. Although the active compound responsible for the antihyperglycemic and antihyperlipidemic activities remains unknown, previous studies showed that the ethanol extract of NAT leaves was able to suppress hyperglycemia-mediated oxidative stress and to inhibit inflammatory pathways upon nuclear factor kappa B activation that are crucial in diabetes and hyperlipidemia (Luo et al., 2019; Mousum et al., 2018). The inhibition of these biomarkers suggested that NAT has the potential to be a natural source for the development of immunomodulatory and chemoprevention agents.
Apart from the ethanol extract of NAT leaves, the aqueous extract of NAT flowers showed hypoglycemic and hypolipidemic activities in healthy adult imprinting control region male mice. The extract (at doses of 500 and 750 mg/kg) lowered fasting blood glucose by 49% and 39%, respectively. Additionally, the random blood glucose level was significantly reduced (32%) by the extract after 4 hours of treatment. In the absorption phase, the aqueous extract of the NAT flower also inhibited glucose absorption from the intestines and increased the diaphragmatic glucose uptake by 85% and 64%, respectively. The α-amylase enzyme activity was inhibited by 16.66%, whereas the levels of total cholesterol and triglycerides were inhibited. The level of HDL increased. Another study revealed that the antidiabetic activity of the aqueous extract of NAT was due to the capability of the extract to inhibit α-amylase glucose absorption and increase glucose transport to cell membranes. The extract mainly contained tannins and flavonoids that were able to inhibit α-amylase and prevented the hydrolysis of starch and oligosaccharides into maltose, maltotriose, and simple sugars. Thus, they might be responsible for the antihyperglycemic activity (Rangika et al., 2015; Siraj et al., 2013; Wickramaratne et al., 2016).
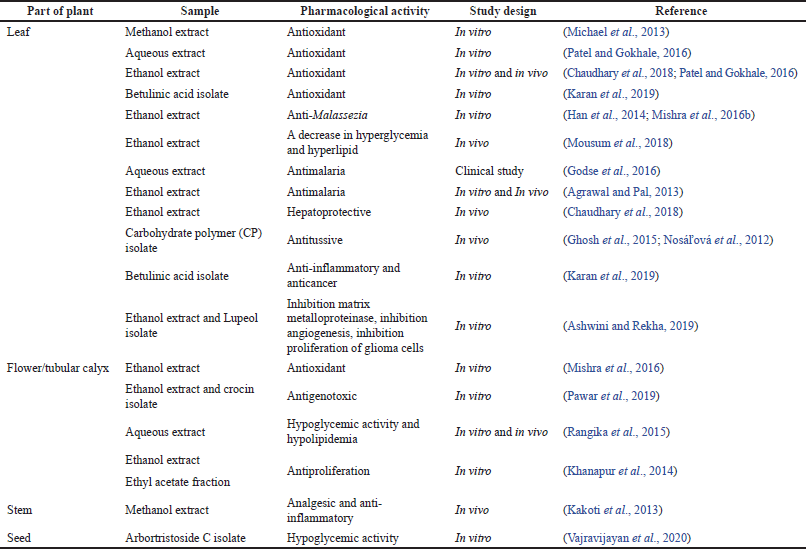 | Table 2. Summarized data of pharmacology activities from Nyctanthes arbor-tristis. [Click here to view] |
Antimalaria
Malaria is an endemic disease transmitted by mosquitos infected by a Plasmodium parasite. The current malaria drugs, namely quinine and artemisinin, are derived from medicinal plants. The main burden in malaria therapy is the progression of drug resistance. Therefore, researchers continually explore new sources, including medicinal plants, for the discovery of malaria drugs. NAT is one of the promising plants for combating malaria. A study on Swiss albino mice infected with Plasmodium berghei showed that the ethanol extract of NAT leaves reduced proinflammatory mediators (TNF-α, interleukin-6, and NO). The ethanol extract of NAT leaves contained active iridoid glycosides responsible for the antiplasmodial activity. Although the mechanism of action of the iridoid glycosides as antimalaria agents is unclear, previous studies demonstrated that these iridoid glycosides inhibited IL-6 and TNF-α production in mice induced with P. berghei (Agrawal et al., 2013; Agrawal and Pal, 2013). The ethanol extract of NAT leaves also showed a toxic effect against Plasmodium falciparum strain 3D7 (IC50: 77 ± 7 µg/mL) (Kumari et al., 2012).
The most advanced experimental data regarding the antimalarial effect of the aqueous NAT leaf extract originated from a clinical study involving 20 participants strongly suspected of having malaria. The extract was given thrice a day for 7 days to treat malaria. The results showed that 10 out of 20 patients experienced heat and parasite clearances confirmed by polymerase chain reaction. Ten patients showed persistent but decreased parasitemia. Meanwhile, 4 out of 10 patients required chloroquine for further treatment. In addition, the increase in platelet number and normalization of plasma lactic acid were observed. The levels of inflammatory cytokines, including TNF-α, were decreased (Godse et al., 2016).
Hepatoprotective
The liver is an organ that is responsible for the metabolism, secretion, storage, and detoxification of endogenous and exogenous compounds. The ethanolic extract of NAT leaves (500 mg/kg) showed a hepatoprotective effect by decreasing the aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and total bilirubin levels in Wistar male rats induced by antituberculosis drugs (isoniazid, rifampicin, and pyrazinamide). The presence of nyctanthic acid, β-sitosterol, and arborsides A, B, and C in the extract may be crucial for the hepatoprotective effect of β-sitosterol (Kim et al., 2014); no data is available regarding the hepatoprotective mechanism of action of these compounds. Further study in this area of research is needed.
Antitussive
A cough is a body protection mechanism against foreign objects that enter the respiratory tract. A cough is also a sign of body abnormality or the presence of certain diseases. Codeine and dextromethorphan are the most common drugs for suppressing a cough. However, they have major side effects due to sedation, respiratory depression, and mucus viscosity disturbance influence. The carbohydrate polymer (CP) isolated from the aqueous extract of NAT leaves reduced coughs in guinea pigs induced by citric acid by up to 66.5% at a dose of CP 50 mg/kg. The activity was slightly lower than that caused by codeine phosphate (62.1%). Previous studies showed that CP decreased airway irritation by protecting cough receptors and suppressing pathological cough reflexes. In addition, in vivo test results showed that CP had a significant effect when administered. The use of NAT for the treatment of coughs is empirically based on the traditional knowledge of Indian tribes (Ghosh et al., 2015; Nosá?ová et al., 2012).
Anti-inflammatory and analgesic
Inflammation is the body’s response to injuries caused by foreign invaders, such as viruses, bacteria, unwanted proteins, chemicals, and other agents. Inflammation also includes pathological events underlying various disorders. Currently, anti-inflammatory drugs are divided into two groups: steroids and nonsteroidal anti-inflammatory drugs (NSAIDs). However, the use of these drugs is commonly accompanied by undesired side effects. The use of NSAIDs, such as aspirin, is associated with gastric bleeding, whereas the long-term application of steroid drugs may lead to low resistance to infection, weight gain, and moon face syndrome. Alternatively, alternative anti-inflammatory agents are needed to overcome these problems. The methanolic extract of NAT stem bark was reported to have anti-inflammatory and antianalgesic activities. In the hot-plate, tail-flick, and tail-immersion assays of mice, the methanol extract of NAT stem bark (500 mg/kg) demonstrated analgesic activity. However, the analgesic activity was still lower than that of morphine, a natural opiate with potent analgesic activity (Kakoti et al., 2013).
The ethyl acetate extract of NAT leaves contains betulinic acid. The betulinic acid inhibited proinflammatory enzymes (COX-1, COX-2, and 5-lipoxygenase) with IC50 values of 10.34, 12.92, and 15.53 µg/ml, respectively. The extract was also reported to contain β-sitosterol with anti-inflammatory and analgesic activities. The anti-inflammatory activity was tested on colony-bred adult Wistar rats (150–200 g), whereas the analgesic activity was tested in the hot-plate test using acetic acid-induced writhing in mice (given intraperitoneally at 50 mg/kg). β-Sitosterol inhibited the production of proinflammatory mediators (prostaglandins and bradykinin) and exerted in vivo antinociceptive activity. In addition, an alkaloid-derived compound, namely 2-(8-hydroxy-7-((4-nitrophenyl)(phenyl amino) methyl)quinoline-3-yl) acetic acid, was identified to have anti-inflammatory activity due to its structural similarity with indomethacin, a clinically proven NSAID targeting COX enzymes (Bhadouria et al., 2012; Karan et al., 2019; Nirmal et al., 2012). From these data, NAT demonstrated anti-inflammatory and analgesic activities in several experimental models.
Anticancer
Cancer is one of the deadliest diseases in human life. Cancer progression can be initiated by angiogenesis (new formation of blood vessels to support cancer cell growth). The ethanol extract of NAT leaves at a dose of 320 µg/ml inhibited capillary formation (98.07%) in a chorioallantoic membrane experimental model. This inhibition was due to the capability of the extract to suppress secondary and tertiary vascular proliferation and inhibit angiogenesis through vascular endothelial growth factor and fibroblast growth factor pathways. These growth factors are involved in the progression of angiogenesis. Another key factor in angiogenesis, MMP (Matrix Metalloproteinase), was also inhibited by lupeol, a compound isolated from NAT leaves. Lupeol also exerted a cytotoxic activity on gliomas (brain tumors) with an IC50 of 10.75 µg/m. The mechanism of action of lupeol as an anticancer agent is mediated by its ability to suppress MMPs activity and to inhibit proliferation and angiogenesis induced by VEGF (Vascular Endothelial Growth Factor) and FGF (Fibroblast Growth Factor) (Ashwini and Rekha, 2019). Betulinic acid isolated from NAT leaves was proven effective in anticancer in vitro experiments on various types of human cancer cells. It demonstrated IC50 values of 6.53, 9.34, 14.92, 16.90, 17.07, 13.27, and 12.55 µM in HepG2, A549, HL-60, MCF-7, HCT-116, PC-3, and HeLa cells, respectively (Karan et al., 2019). The mechanism of action of betulinic acid as an anticancer agent is mediated by its activity to induce apoptosis by targeting the cell cycle at the S or G2/M phases in certain myeloma, gastric, and lung cancer cell lines. Interestingly, it was recently reported that betulinic acid also interfered with the NF-κB signaling pathway of cell survival by directly promoting ROS (Reactive Oxygen Species) overproduction leading to cell death (Park et al., 2021; Shen et al., 2019).
The ethanol extract of NAT flowers demonstrated antiproliferative activities in five types of cancer cell lines (Colo 205, Y79, K562, MCF7, and MDAMB231). Among these cell lines, Colo 205 showed the highest sensitivity against the extract (IC50: 24 ± 6.63 µg/ml), which was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Khanapur et al., 2014). The ethyl acetate fraction of the NAT flower inhibited the proliferation of primary peripheral blood mononuclear cells from chronic lymphocytic leukemia (PBMC-CLL) and PMBCs from acute myeloid leukemia (PBMC-AML) with IC50 of 60.30 and 2.99 µg/ml, respectively. Compared with normal cells, the ethyl acetate fraction of the NAT flower had selectivity index values of 2.06 and 102.50 against PBMC-AML and PBMC-CLL, respectively (Heendeniya et al., 2020). This finding indicated that the fraction has a higher selectivity against PBMC-CLL in cancer therapy. Further investigation of the bioactive compounds responsible for the anticancer activity of NAT is a promising direction.
Antigenotoxic
Genotoxicity refers to the damage of genetic information in cells caused by chemicals. This condition can lead to cell mutations and subsequent related diseases. The intensive use of NAT in traditional practices led to the need for genotoxic activity evaluation. The genotoxic activity of the ethanolic extract of the NAT flower and its constituent, crocin, was evaluated using the Ames test with the standard Salmonella assay. The experiment was carried out with and without S9 activation using Salmonella typhimurium strains TA 98, TA 100, and TA 102. The extract and crocin were nongenotoxic at doses of 125–2,000 µg with and without metabolic activation. The lack of genotoxicity data of other NAT extracts and their phytoconstituent encourages further studies to confirm the safety of complementary medicine. In addition, the genotoxicity effect of the direct and indirect interactions between extracts and phytoconstituent must be investigated (Pawar et al., 2019).
CONCLUSION
The data presented in this narrative review showed that NAT is a potential plant with a wide range of pharmacological activities. All parts of this plant, except the roots, have been investigated for their pharmacological activity and phytochemical constituents. NAT demonstrates antioxidant, anti-inflammatory, analgesic, antidiabetic, antihyperlipidemic, anticancer, antimicrobial, antigenotoxic, antimalarial, antitussive, hepatoprotective, and anti-Malassezia activities. In addition, NAT contains a variety of secondary metabolites, including steroids, terpenes, flavonoids, iridoid glycosides, and alkaloids. Further research focusing on the mechanism of the actions underlying the pharmacological effect of NAT is a promising direction. The lack of study on NAT toxicity is a major challenge for researchers to ensure the safety aspect before additional scientific evidence is provided.
ACKNOWLEDGMENTS
The authors thank the Faculty of Pharmacy, Universitas Gadjah Mada, for supporting this study (Grant No. UGM No.1.19.11/UN1/FFA.2/S1/KU PKKM/2021).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agrawal J, Pal A. Nyctanthes arbor-tristis Linn—a critical ethnopharmacological review. J Ethnopharmacol, 2013; 146:645–58; doi:10.1016/j.jep.2013.01.024 CrossRef
Agrawal J, Shanker K, Chanda D, Pal A. Nyctanthes arbor-tristis positively affects immunopathology of malaria-infected mice prolonging its survival. Parasitol Res, 2013; 112:2601–9; doi:10.1007/s00436-013-3427-y CrossRef
Ashwini P, Rekha PD. Lupeol from Nyctanthes arbor-tristis inhibits matrix metalloproteinase activity, angiogenesis and proliferation of glioma cells. Pharm Chem J, 2019; 53:160–4; doi:10.1007/s11094-019-01971-8 CrossRef
Bhadouria RS, Bhargava S, Pancholi SS. Isolation and characterization of two alkaloid from the ethanolic extract of Nyctanthes arbor-tristis L. Leaves, 2012; 2:342–4.
Chaudhary S, Gupta RK, Kumar A, Tarazi H. Hepatoprotective and antioxidant potential of Nyctanthes Arbor-tristis L. against antitubercular drugs induced hepatotoxicity. J Pharm Pharmcogn Res, 2018; 6:205–15.
Elfahmi, Woerdenbag HJ, Kayser O. Jamu: Indonesian traditional herbal medicine towards rational phytopharmacological use. J Herb Med, 2014; 4:51–73; doi:10.1016/j.hermed.2014.01.002 CrossRef
Gadgoli C, Shelke S. Crocetin from the tubular calyx of Nyctanthes arbor-tristis. Nat Prod Res, 2010; 24:1610–5; doi:10.1080/14786411003754363 CrossRef
Ghosh K, Ray S, Bera K, Ray B. Isolation and structural elements of a water-soluble free radical scavenger from Nyctanthes arbor-tristis leaves. Phytochemistry, 2015; 115:20–6; doi:10.1016/j.phytochem.2015.02.007 CrossRef
Godse CS, Tathed PS, Talwalkar SS, Vaidya RA, Amonkar AJ, Vaidya AB, Vaidya ADB. Antiparasitic and disease-modifying activity of Nyctanthes arbor-tristis Linn. in malaria: an exploratory clinical study. J Ayurveda Integr Med, 2016; 7:238–48; doi:10.1016/j.jaim.2016.08.003 CrossRef
Han N-R, Kim H-M, Jeong H-J. The β-sitosterol attenuates atopic dermatitis-like skin lesions through down-regulation of TSLP. Exp Biol Med, 2014; 239:454–64; doi:10.1177/1535370213520111. CrossRef
Han SH, Hur MS, Kim MJ, Jung WH, Park M, Kim JH, Shin HJ, Choe YB, Ahn KJ, Lee YW. In vitro anti- Malassezia activity of Castanea crenata shell and oil-soluble Glycyrrhiza extracts. Ann Dermatol, 2017; 29:321; doi:10.5021/ad.2017.29.3.321 CrossRef
Haque M, Sultana N, Abedin S, Kabir S. Stigmasterol, rengyolone, 2-phenylethyl β-D-glucopyranoside and n-tetradecyl-β-D-glucopyranoside from the flowers of Nyctanthes arbor-tristis Linn. Bangladesh J Sci Ind Res, 2019; 54:275–82; doi:10.3329/bjsir.v54i3.42680 CrossRef
Heendeniya SN, Keerthirathna LakshikaR, Manawadu CK, Dissanayake IH, Ali R, Mashhour A, Heendeniya SN, Keerthirathna LR, Manawadu CK, Dissanayake IH, Ali R, Mashhour A, Alzahrani H, Godakumbura P, Boudjelal M, Peiris DC. Therapeutic efficacy of Nyctanthes arbor-tristis flowers to inhibit proliferation of acute and chronic primary human leukemia cells, with adipocyte differentiation and in silico analysis of interactions between survivin protein and selected secondary metabolites. Biomolecules, 2020; 10:165; doi:10.3390/biom10020165 CrossRef
Hiremath V, Hiremath BS, Mohapatra S, Kumar Das A. Literary review of Parijata (Nyctanthus arbor-tristis Linn.) an herbal medicament with special reference to ayurveda and botanical literatures. Biomed Pharmacol J, 2016; 9:1019–25; doi:10.13005/bpj/1043 CrossRef
Kakoti BB, Pradhan P, Borah S, Mahato K, Kumar M. Analgesic and anti-inflammatory activities of the methanolic stem bark extract of Nyctanthes arbor-tristis Linn. BioMed Res Int, 2013; 2013:1–6; doi:10.1155/2013/826295 CrossRef
Karan BN, Maity TK, Pal BC, Singha T, Jana S. Betulinic Acid, the first lupane-type triterpenoid isolated via bioactivity-guided fractionation, and identified by spectroscopic analysis from leaves of Nyctanthes arbor-tristis?: its potential biological activities in vitro assays. Nat Prod Res, 2019; 33:3287–92; doi:10.1080/14786419.2018.1470171 CrossRef
Khanapur M, Avadhanula RK, Setty OH. In Vitro antioxidant, antiproliferative, and phytochemical study in different extracts of Nyctanthes arbortristis flowers. BioMed Res Int, 2014; 2014:1–10; doi:10.1155/2014/291271 CrossRef
Kim K-S, Yang HJ, Lee J-Y, Na Y-C, Kwon S-Y, Kim Y-C, Lee J-H, Jang H-J. Effects of β-sitosterol derived from Artemisia capillaris on the activated human hepatic stellate cells and dimethylnitrosamine-induced mouse liver fibrosis. BMC Complement Altern Med, 2014; 14:363; doi:10.1186/1472-6882-14-363. CrossRef
Kumari P, Sahal D, Jain SK, Chauhan VS. Bioactivity Guided Fractionation of Leaves Extract of Nyctanthes arbor tristis (Harshringar) against P. falciparum. PLoS One, 2012; 7:e51714; doi:10.1371/journal.pone.0051714 CrossRef
Kumari S, Singh R, Gurav NP, Mehta N. Isolation and characterization of bioactive compounds from stem of nyctanthes arbor-tristis linn. and effect of different fractions on phytopathogens. Asian J Chem, 2017; 29:787–91; doi:10.14233/ajchem.2017.20306 CrossRef
Luo Y, Peng B, Wei W, Tian X, Wu Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules, 2019; 24:1343; doi:10.3390/molecules24071343 CrossRef
Michael JS, Kalirajan A, Padmalatha C, Singh AJAR. In vitro antioxidant evaluation and total phenolics of methanolic leaf extracts of Nyctanthes arbor-tristis L. Chin J Nat Med, 2013; 11:484–7; doi:10.1016/S1875-5364(13)60088-6 CrossRef
Mishra AK, Upadhyay R, Chaurasia JK, Tiwari KN. Comparative antioxidant study in different flower extracts of Nyctanthes arbor-tristis (L.) (Oleaceae): an important medicinal plant. Braz J Bot, 2016a; 39:813–20; doi:10.1007/s40415-016-0283-x CrossRef
Mishra RK, Mishra V, Pandey A, Tiwari AK, Pandey H, Sharma S, Pandey AC, Dikshit A. Exploration of anti-Malassezia potential of Nyctanthes arbor-tristis L. and their application to combat the infection caused by Mala s1 a novel allergen. BMC Complement Altern Med, 2016b; 16:114; doi:10.1186/s12906-016-1092-2 CrossRef
Moreira D de L, Teixeira SS, Monteiro MHD, De-Oliveira ACAX, Paumgartten FJR. Traditional use and safety of herbal medicines1. Rev Bras Farmacogn, 2014; 24:248–57; doi:10.1016/j.bjp.2014.03.006 CrossRef
Mousum SA, Ahmed S, Gawali B, Kwatra M, Ahmed A, Lahkar M. Nyctanthes arbor-tristis leaf extract ameliorates hyperlipidemia- and hyperglycemia-associated nephrotoxicity by improving anti-oxidant and anti-inflammatory status in high-fat diet–streptozotocin-induced diabetic rats. Inflammopharmacology, 2018; 26:1415–28; doi:10.1007/s10787-018-0497-6 CrossRef
Nirmal SA, Pal SC, Mandal SC, Patil AN. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. Inflammopharmacology, 2012; 20:219–24; doi:10.1007/s10787-011-0110-8 CrossRef
Nosá?ová G, Capek P, Matáková T, Nosá? S, Flešková D, Jure?ek ?. Antitussive activity of an extracellular Rhodella grisea proteoglycan on the mechanically induced cough reflex. Carbohydr Polym, 2012; 87:752–6; doi:10.1016/j.carbpol.2011.08.058 CrossRef
Park C, Jeong J-W, Han MH, Lee H, Kim G-Y, Jin S, Park J-H, Kwon HJ, Kim BW, Choi Y-H. The anti-cancer effect of betulinic acid in u937 human leukemia cells is mediated through ROS-dependent cell cycle arrest and apoptosis. Anim Cells Syst, 2021; 25:119–27; doi:10.1080/19768354.2021.1915380. CrossRef
Patel S, Gokhale M. Comparative study of antioxidant activity of ethanol and aqueous extracts of different parts of Nyctanthes Arbor-tristis Linn. Pharmacogn J, 2016; 8:113–6; doi:10.5530/pj.2016.2.3 CrossRef
Pawar N, Panchal S, Kawle D, Gadgoli C, Maru G. Evaluation of genotoxic and modulatory effects of Nyctanthes arbor-tristis calyx extract and the isolated crocin in Ames’ assay. Nat Prod Res, 2019; 33:884–8; doi:10.1080/14786419.2017.1410807 CrossRef
Rangika BS, Dayananda PD, Peiris DC. Hypoglycemic and hypolipidemic activities of aqueous extract of flowers from Nycantus arbor-tristis L. in male mice. BMC Complement Altern Med, 2015; 15:289; doi:10.1186/s12906-015-0807-0 CrossRef
Saunders CW, Scheynius A, Heitman J. Malassezia fungi are specialized to live on skin and associated with dandruff, eczema, and other skin diseases. PLoS Pathog, 2012; 8:e1002701; doi:10.1371/journal.ppat.1002701 CrossRef
Sharma L, Dhiman M, Singh A, Sharma MM. Nyctanthes arbor-tristis L.: “an unexplored plant of enormous possibilities for economic revenue.” Proc Natl Acad Sci India Sect B Biol Sci, 2021; 91:241–55; doi:10.1007/s40011-020-01213-y CrossRef
Shen M, Hu Y, Yang Y, Wang L, Yang X, Wang B, Huang M. Betulinic acid induces ROS-dependent apoptosis and s-phase arrest by inhibiting the NF-κ B pathway in human multiple myeloma. Oxid Med Cell Longev, 2019; 2019:1–14; doi:10.1155/2019/5083158 CrossRef
Siraj A, Shams R, Hossain E, Salahuddin M, Tahsin F, Khalid AA, Paul SP. Assay of antidiabetic activity of Hemidesmus indicus by gut perfusion and six segment methods on Long Evans rats. Pharmacology, 2013; 3:81–7.
Sousa JLC, Gonçalves C, Ferreira RM, Cardoso SM, Freire CSR, Silvestre AJD, Silva AMS. Functionalization of betulinic acid with polyphenolic fragments for the development of new amphiphilic antioxidants. Antioxidants, 2021; 10:148; doi:10.3390/antiox10020148 CrossRef
Vajravijayan S, Nandhagopal N, Anantha Krishnan D, Gunasekaran K. Isolation and characterization of an iridoid, Arbortristoside-C from Nyctanthes arbor-tristis Linn., a potential drug candidate for diabetes targeting α-glucosidase. J Biomol Struct Dyn, 2020:1–11; doi:10.1080/07391102.2020.1813201 CrossRef
Wallander E, Albert VA. Phylogeny and classification of Oleaceae based on rps16 and trnL-F sequence data. Am J Bot, 2000; 87:1827–41; doi:10.2307/2656836 CrossRef
Wickramaratne MN, Punchihewa JC, Wickramaratne DBM. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement Altern Med, 2016; 16:466; doi:10.1186/s12906-016-1452-y CrossRef