INTRODUCTION
Mycotoxins, such as aflatoxin B1 (AFB1), are secondary metabolites produced from filamentous fungi that are toxic, carcinogenic, and immunosuppressive to animals and humans. The estimated world production of feed contaminated with fungus is approximately 5%–10% (Oswald et al., 2005; Qian et al., 2012; Tomkova et al., 2001). Aflatoxin reportedly caused up to 100,000 turkey deaths following the consumption of contaminated peanut mushrooms. Broiler feed contaminated with a mycotoxin mixture (3.5 mg/kg diet as 79% of AFB1, 16% AFG1, 4% AFG2, and 1% AFB2) can lead to weight loss and inflammation of the liver and kidneys. Aflatoxin B1 is a potent agent that causes immunosuppression in pigs at a dose of 140–280 µg/kg of feed by inhibiting DNA synthesis and immune cells, such as lymphocytes, but does not affect the humoral immune response (Perczak et al., 2018; Pierron et al., 2016).
Aflatoxin B1 suppresses the cellular immune system, in particular T lymphocytes, because of decreased complement production by the liver, phagocytosis by macrophages, and neutrophil activity (Perdigon et al., 2001). T lymphocytes affected by the toxin, as well as other lymphoid cells, such as cytotoxic T cells and natural killer cells, can promote tumor cell function directly or indirectly. The cellular components of the immune system produce cytokines for protection against tumor progression; however, cytokines may also play a role in the inflammation mechanism that causes damage to various organs (Ibrahim, 2013).
Methods such as heating, chemical treatment, or radiation can destroy and eliminate mycotoxin (Zain, 2011); however, the cost is prohibitive and it may impair the nutritional value of the feed. The decline in fungal growth may increase during feed production or storage (Munoz et al., 2010). Lactic acid bacteria (LAB) may exhibit antifungal activity, especially Lactobacillus sp (Sadiq et al., 2019). Lactobacillus pentosus and Lactobacillus brevis bacterial strains at a concentration of 3.5 × 108 colony forming unit (CFU)/ml can bind and release aflatoxin B1 by 17.4% and 34.7%, respectively, in liquid media as measured by ELISA (Hamidi et al., 2013).
Microorganisms, such as Saccharomyces cerevisiae and LAB, may be used as biopreservatives in feeds, so it is possible to extend the shelf-life and increase food safety with microflora supplementation. Antimicrobial products of microorganisms also have potential as probiotics and may improve health (Tran et al., 2020); however, there is limited data demonstrating the immunostimulating effects of LAB. Therefore, we evaluated the effect of LAB on the cellular and humoral immunity profile of mice.
MATERIAL AND METHODS
Preparation of animal
Male mice (Mus musculus), strain Balb/c aged 8–12 weeks (n = 25), with a body weight of 25–30 g, were obtained from Brawijaya University’s bioscience laboratory and divided into five groups (five mice/group). The treatment groups consisted of a positive control (mice induced with AFB1 0.2 mg/kg bw on days 15–28), negative control (healthy mice), and treatment groups, T1, T2, and T3 in which mice were administered 1 × 105 CFU/ml, 1 × 107 CFU/ml, and 1 × 109 CFU/ml of LAB on days 7–28, respectively.
Bacterial suspension preparation
Lactobacillus bulgaricus (LAB) was obtained from the Microbiology Laboratory of the Faculty of Medicine, Universitas Brawijaya, and confirmed by biochemical tests and Gram staining. The bacteria were grown on de Man, Rogosa, and Sharpe (MRS) agar media at 37°C for 24 hours. A bacterial suspension was prepared using MRS broth media and the bacterial concentrations were measured using a spectrophotometer. The bacteria were diluted with phosphate buffer saline (PBS) for the experiments.
Aflatoxin B1 preparation
Aflatoxin B1 (Sigma Company catalog: A6636®) is potent with respect to acute toxicity, mutagenicity, and carcinogenicity, and one vial of AFB1 contained 5 mg of powder. AFB1 (0.2 mg/kg BW) was diluted in 1 ml PBS, pH 7.2 (Qian et al., 2012).
Flow cytometry
The mice were sacrificed on day 29 by cervical dislocation. Spleens were harvested, placed into a petri dish containing sterile PBS, and crushed with the base of a syringe. 10 ml homogenates of the sample were prepared in a volume of 10 ml, centrifuged, and the pellets were resuspended in 1 ml PBS by pipetting (Ardiana and Rifa’i, 2015).
Then, 100 μl of the suspension was placed into a microtube; 500 μl of PBS was added; and the mixture was centrifuged at 2,500 rpm for 5 minutes at 4°C. Then, 50 µl of PE/Cy5 conjugated rat anti-mouse Cd11c, PECy5 conjugated rat anti-mouse transforming growth factor-beta (TGF-β), fluorescein isothiocyanate (FITC) conjugated rat anti-mouse CD4, PE conjugated rat anti-mouse CD8, FITC conjugated rat anti-mouse B220, and PECy5 conjugated rat anti-mouse IgG (Biolegend®, San Diego) were added to the cells and incubated for 20 minutes in 4°C (8). Afterward, 50 µl of cytofix (BD Biosciences Pharmingen) was added and incubated for 20 minutes in 4°C; wash perm solution (BioLegend®, USA) was added; and the mixture was centrifuged at 2,500 rpm at 10°C for 10 minutes. The cells were analyzed by flow cytometry (BD FACSCalibur, USA) using BD Cellquest ProTM software.
Statistical analysis
The data are presented as the relative number of immune cells (CD11c+TGFβ+, CD4+CD8+, and B220+IgG+). Data were analyzed statistically using one-way analysis of variance (ANOVA) with an error level of α = 0.05, followed by Tukey’s test.
RESULTS
CD11c+TGF-β+ cells
The results indicated that supplementation of the mice with LAB induced by AFB1 increased the relative number of CD11c+ cells that produce TGFβ+ (Fig. 1). In the positive control group, the relative number of CD11c+ expressing molecule TGF-β+ (1.75%) was different, but no significance was observed when compared with the negative control (0.91%). All treatment groups (T1, T2, and T3) administered LAB at a 105–109 CFU/ml concentration showed an increase in the relative number of CD11c+TGF-β+ cells by 2.05% 3.14%, and 3.06%, respectively, when compared to the negative and positive controls, as shown in Figure 1.
CD4+CD8+ cells
The results showed that the supplementation with LAB in mice induced with AFB1 increased the relative number of CD4+CD8+ cells in all treatment groups, but the amount did not significantly differ and was similar to that of the negative control, as shown in Figure 2. The negative control was higher when compared with the positive control.
B220+IgG+ cells
The results showed significant differences in the relative number of B220+-expressing IgG+ cells in all the treatment groups following supplementation with LAB after induction with AFB1. The negative control group (24.56%) was significantly different from the positive control group (5.76%), which showed a relatively higher number of B220+IgG+ cells compared with the positive control. There was an increase in the relative number in all treatment groups (7.44%, 10.26%, and 7.67% for T1, T2, and T3, respectively), as shown in Figure 3.
DISCUSSION
Effect of LAB on the relative number of CD11c+TGF-β+ cells
The results indicated that the relative number of CD11c+ cells that expressed TGF-β was different, but there was no significant between the treatment groups; however, a 107 CFU/ml concentration of LAB increased the average of CD11c+TGF-β+ cells in all treatments. Mycotoxin exposure decreased the relative amount of CD11c+TGF-β+ cells. Mycotoxin AF1 altered or decreased anti-inflammatory cytokine synthesis by inhibiting macrophage or T cell activation. In fact, mycotoxin inhibits the synthesis and proliferation of T cells, which prevents macrophage cells from producing anti-inflammatory cytokines. Mycotoxin is cytotoxic to lymphocytes by interfering with lymphocyte receptors or lymphocyte function (Tran et al., 2020). A study by Murugesan et al. (2015) revealed that mycotoxin was not immunogenic. It could not induce an immune response to pathogens, but interfered with mitogen-activated protein kinase signaling to modulate cell growth, apoptosis, or the immune response. This could expose an individual to a high risk of infection.
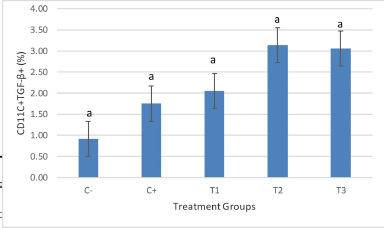 | Figure 1. Flow cytometry analysis shows that giving LAB showed an increase in the relative number of CD11c+TGF-β+ cells (p < 0.05), but was not significantly different between treatments. There was an increase in the number when compared with the positive control. The highest average increase was seen in the T2 treatment. The treatment groups are: C- (healthy mice), C+ (mice induced with AFB1), and treatment groups, T1, T2, and T3 in which mice were administered with AFB1 and 1 × 105 CFU/ml, 1 × 107 CFU/ml, and 1 × 109 CFU/ml of LAB on days 7–28, respectively. [Click here to view] |
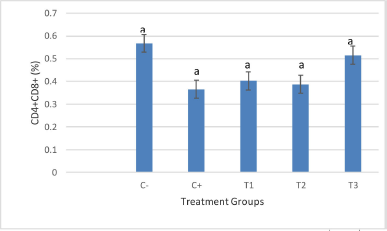 | Figure 2. Treatment with LAB showed an increase in the relative number of CD4+CD8+ cells for all treatments, which did not differ significantly (p < 0.05). The treatment groups are: C- (healthy mice), C+ (mice induced with AFB1), and treatment groups, T1, T2, and T3 in which mice were administered with AFB1 and 1 × 105 CFU/ml, 1 × 107 CFU/ml, and 1 × 109 CFU/ml of LAB on days 7–28, respectively. [Click here to view] |
Dendritic cells (DC) and CD11c markers are antigen-presenting cells (APCs) that are regulated specifically and nonspecifically by immune cells found in the lamina propria of the small intestine and gut-associated lymphoid tissues, such as the Peyer’s patches. Most dendritic cells (DCs) are present in an immature condition and are less immunogenic because of the low expression of MHC costimulators. Contact with pathogen-associated molecular patterns (PAMPs) or other signals induces the pattern recognition receptor (PRR) signal and activates the NF-κB pathway, resulting in maturation and activation of DC cells (Wells, 2011). Mature DCs may then express high MHC levels, costimulatory molecules, and cytokines which attenuate APC activation and differentiation of T cells to cause inflammation (Mohamadzadeh et al., 2005). DCs exposed to AFB1 cannot respond correctly to any invading microbes and fails to initiate antigen presentation to activated T cells that are susceptible to pathogenic agents (Mohammadi et al., 2014).
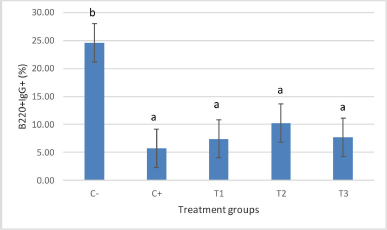 | Figure 3. Treatment with LAB showed an increase in the relative number of CD4+CD8+ cells for all treatments, which did not differ significantly (p < 0.05). The treatment groups are: C- (healthy mice), C+ (mice induced with AFB1), and treatment groups, T1, T2, and T3 in which mice were administered with AFB1 and 1 × 105 CFU/ml, 1 × 107 CFU/ml, and 1 × 109 CFU/ml of LAB on days 7–28, respectively. [Click here to view] |
The anti-inflammatory cytokine, TGF-β, inhibits the proliferation of fibroblast epithelial cells, dendritic cells, and macrophages to produce inflammatory cytokines and controls cell growth through adhesion and extracellular matrix formation (Hussain et al., 2018). Exposure to mycotoxin could stimulate CD11c+ cells to activate inflammatory pathways that TGF-β may have suppressed. Oral exposure to LAB concentrations of 105 CFU/ml could activate inflammatory cytokines, such as TGF-β, in DCs, which may inhibit CD11c+ cell activity. This was evident in the T1 and T2 group, prior to an observed decrease in the T3 group. According to Vindirelo and Alberto (2015), the higher the concentration of cell bacteria, the greater the binding capacity of AFB1 in liquid media in vitro. The concentration of bacteria that can bind AFB1 was 1010 CFU/ml for L. rhamnosus GG, L. casei Shirota, Propionibacterium freudenreichii ssp. shermanii JS, and Escherichia coli. Probiotics have an immunomodulatory effect on the release of cytokines, interleukins, tumor necrosis factor, transforming growth factor, and chemokines from immune cells that play a role in the innate and adaptive immune systems. LAB may interact with enterocytes and DCs, Th1/Th2 cytokines, or T reg cells in the intestine to stimulate the adaptive immune response into a proinflammatory or anti-inflammatory action (Azad et al., 2018; Mohamadzadeh et al., 2005).
Mycotoxin AFB1 exerts toxicity because it is readily absorbed by the intestine and rapidly binds to serum protein. AFB1 is genotoxic and immunogenic in animals (Zimmermann et al., 2014). Mycotoxin can activate the microbial intestine, and mycotoxin adsorption–desorption is highly dependent on the intestinal environment and digestive enzymes. The LAB, L. rhamnosus RC007, stimulates pH, salts, enzymes, and peristalsis at each stage of AFB1 absorption in the digestive tract. Saliva secretion results in low adsorption and high AFB1 reabsorption. Gastric fluids and intestinal fluids do not decrease the AFB1 adsorption of LAB, rather they stimulate higher AFB1 adsorption (Sadiq et al., 2019).
Metabolic LAB products inhibit aflatoxin biosynthesis. Heterofermented LAB, such as L. bulgaricus, produce a high level of acetic acid and propionic acid at acidic pH (Vinderola and Ritieni, 2015). The mechanism of action of LAB is to inactivate the fungal membrane and inhibit the absorption of amino acids and inactivated products from fungi, such as acetic acid (Perczak et al., 2018). Bacteria and yeasts may neutralize mycotoxins in the body by reshuffling, transforming, and breaking them down into nontoxic metabolic products or inactive forms (Murugesan et al., 2015). LAB binds mycotoxins to prevent further absorption by the intestine, which are then secreted with feces (Adilah et al., 2018). LAB walls contain peptidoglycans that could interact with mutagenic compounds, including mycotoxins, through binding to reduce stability and bioavailability, and stimulate the secretion of anti-inflammatory cytokines by macrophages (Niderkorn et al., 2009; Tabari et al., 2018). LAB’s capacity to bind mycotoxins would be optimal when the bacteria cells die due to a change in the cell surface. Live LABs require a long time to release mycotoxin from the body (Perczak et al., 2018). Cell wall protein denaturation may function by creating a broader area to absorb mycotoxins (Tabari et al., 2018). The proteins in the ribosomes, nucleus, chromosomes, cytosol, and cellular cytoskeleton components support the forming of the cell wall of bacteria in the exponential growth phase.
On the contrary, binding between the cell wall of LAB with mycotoxin takes place at the beginning of the end of the bacterial growth cycle (Moller et al., 2021). AFB1 could bind to the cell wall β-d-glucan through hydrogen or van der Waals bonds. Absorption of AFB1 toxin depends on the availability of the number of binding sites on the surface of microbes, and the equilibrium constant [K (eq)], which could change as a result of genetic, physical, or chemical alterations (Sadiq et al., 2019).
LAB may act as an anti-inflammatory agent, resulting in the reduction of oxidative stress from AFB1 exposure (Abbes et al., 2016). Probiotics could also stimulate T cell subsets, humoral immune cells, epithelial-associated dendritic cells, and macrophages to increase anti-inflammatory cytokine products Braat et al., 2004. The entry of LABs into the body may increase the capacity and phagocytic receptors of leukocyte cells, especially complement receptor 3 (CR3), for bluffing respiratory bursts (Bravo et al., 2019).
Effect of LAB on the relative number of CD4+/CD8+ cells
Our results showed that the administration of LAB (L. bulgaricus) had an effect on the relative number of CD4+/CD8+ immunocompetent cells in mice induced with aflatoxin B1 in the treatment groups (p > 0.05) based on a Kruskal–Wallis test. The negative control group had a higher relative number of CD4+/CD8+ T cells than the positive group. This result is consistent with that of Qian et al. (2012), in which the administration of AFB1 to mice orally for 24 hours reduced CD3 T cells in the intestinal mucosa. AFB1 caused a decrease in the cellular immune response to the specific dose and duration treatments (Zimmermann et al., 2014). A low dose of mycotoxin could induce an inflammatory response if activated by enzymes, such as inflammatory inhibitors (Hussain et al., 2018). Mycotoxin AFB1 may undergo systemic hydrolysis and further activate metabolism. Phase metabolism includes conjugation with glucuronic acid and sulfate by the whole-cell biotransformation system during immune cell communication (Tran et al., 2020).
High LAB concentrations could increase naive T lymphocyte activation and proliferation as well as memory T cells (Wells et al., 2011). Upon entering the body, an antigen is presented by DCs in the digestive tract mucosa (CD11c). Histocompatibility complex class II molecules combined with the expression of costimulatory molecules and cytokines (Gaudinoand Kumar, 2019) and activation of T lymphocytes. As a cellular defense, T cells may differentiate into a subset of T1 that activates macrophages. Macrophages and APCs induce T cells to secrete inflammatory cytokines that cause tissue damage (Mohamadzadeh et al., 2005). The T1, T2, and T3 groups had a higher relative amount of CD4+/CD8+ T cells compared with the positive control group is shown in Figure 2.
LAB may act as an antifungal agent because it contains metabolites, such as organic acids, carboxylic acids, phenolic acids, cyclic dipeptides, hydrogen peroxide, and compounds that inhibit sporulation, which may decrease mycotoxin production (Sadiq et al., 2019). LAB at concentrations of 105 CFU/ml could activate T lymphocyte cells in all groups compared with the positive control group. These results were consistent with that reported by Tsai et al. (2012) in which LAB activates the cellular adaptive immune response.
THE EFFECT OF LAB ON THE RELATIVE NUMBER OF B220+IGG+ CELLS
Our results showed that B220+ cells expressing IgG in the negative control group was higher compared with the positive group, whereas it was not significantly different among all of the treatment groups. The average of B220 cells expressing IgG increased after LAB administration. Mycotoxin can reduce immunoglobulin production in chicken feed supplemented with AFB1 (Nazarizadeh and Pourreza, 2019). LAB stimulates the humoral immune response by increased circulating antibodies and levels of plaque-forming cells in the host when exposed to mycotoxin (Abbes et al., 2015). LAB can absorb and eliminate mycotoxin to prevent intestinal absorption and reduce liver damage, which is a target of mycotoxin. Upon entering the host orally, mycotoxin stimulates the secretion of immunoglobulin A (IgA) in the digestive mucosa and antibody IgG in the circulation. IgG represents a secondary response to protect the body against foreign antigens (Chen and Tsai, 2011).
In the normal intestinal epithelium, microflora bacteria act as a barrier antigen. However, when epithelial cells are degraded by infectious and noninfectious substances, alterations in intestinal permeability and inflammation of the intestinal mucosa occur. The inflammatory response that occurs in the intestine activates IgG antibodies and causes translocation of the microflora bacteria (Paludan et al., 2020).
LAB plays a role in the body to activate the immune response by inducing the formation of Secretory IgA (SIgA) and producing vitamins (Wold, 2001). Antibody SIgA is dominant in the mucous membrane, which is the first defense immune system against a dangerous environment. SIgA antibodies play a role in neutralizing toxins, viruses, salivary exotoxins, and eliminating pathogenic microbes (Hayati et al., 2018). Lactobacillus bacteria and other probiotics are commensal microorganisms that interact with the mucosa or the immune cells. LAB stimulates specific functions of the mucosal immune system and produces secretory IgA. The presence of receptors, such as Toll-like receptors, nucleotide oligomerization domain-like receptors, and C-type lectin receptors may stimulate Lactobacillus. Lactobacillus associates with microbe-associated molecular patterns to activate APC and modulate their function through the expression of surface receptors, secretion of cytokines and chemokines, and other nonspecific immune effector cells (Mohamadzadeh et al., 2005). Our results indicate that there was an increase in the relative number of IgG+ cells resulting from LAB induction in mice exposed to mycotoxin. This is consistent with the results obtained by Tran et al. (2020) in which IgG levels in the serum of Balb/c mice increased when Lactobacillus was administered for 7 days (infected with Salmonella typhimurium bacteria). DCs play a role in the adaptive immune response. LAB stimulates DC cells to activate specific immune responses in the intestinal mucosa to maintain homeostasis, protect against pathogenic microbes, and maintain intestinal permeability (Mohamadzadeh et al., 2005). LAB increases the cellular and nonspecific humoral immune response in mice exposed to mycotoxin AFB1.
CONCLUSION
L. bulgaricus bacteria exhibited a potent effect as an immunostimulator resulting from exposure to mycotoxin AFB1.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethics committee (certified no. 012-KEP-UB-2020) of Institut BioSains.
ACKNOWLEDGMENTS
The authors thank the Faculty of Science, Universitas Brawijaya, for processing samples by flow cytometry, and the Institut BioSains, Universitas Brawijaya, for maintaining the animals.
COMPETING INTERESTS
The authors declare that there are no financial and nonfinancial conflicts of interest.
FUNDING
The Faculty of Veterinary Medicine, Universitas Brawijaya, provided funding through a DPP SPP grant.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
AUTHORS’ CONTRIBUTIONS
DQS obtained the funding; designed the study, analysis and interpretation of data; and was a major contributor in writing the manuscript. DQS, SM, and IAA analyzed the flow cytometer data, treated the animals, and collected the data. All authors read and approved the final manuscript.
REFERENCES
Abbès S, Salah-Abbès JB, Jebali R, Younes RB, Oueslati R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria. J Immunotoxicol, 2015; 13(1):46–54. CrossRef
Adilah ZN, Liew WPP, Redzwan SM, Amin I. Effect of high protein diet and probiotic Lactobacillus casei Shirota supplementation in aflatoxin B1-induced rats. Biomed Res Int, 2018; 2018:9568351. CrossRef
Ardiana O, Rifa’i M. The effect of dexamethasone treatment to humoral immunity in BALB / C mice models. J Biotropika, 2015; 3(3):112–6.
Azad MAK, Sarker M, Wan D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int, 2018; 2018:8063647. CrossRef
Braat H, Van Den Brande J, Van Tol E, Hommes D, Peppelenbosch M, Van Deventer S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr, 2004; 80(6):1618–25. CrossRef
Bravo M, Combes T, Martinez FO, Cerrato R, Rey J, Garcia-Jimenez W, Fernandez-Llario P, Risco D, Gutierrez-Merino J. Lactobacilli isolated from wild boar (Sus scrofa) Antagonize Mycobacterium bovis Bacille Calmette-Guerin (BCG) in a species-dependent manner. Front Microbiol, 2019; 10:1663. CrossRef
Chen CHC, Tsai YCC. Effects of multistrain lactic acid bacteria with probiotic properties on enhancements of IgA, IgG levels and anti- Salmonella typhimurium invasion activity. 2011; (34).
Gaudino SJ, Kumar P. Cross-talk between antigen presenting cells and t cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front Immunol, 2019; 10:360. CrossRef
Hamidi A, Mirnejad R, Yahaghi E, Behnod V, Mirhosseini A, Amani S, Sattari S, Darian EK. The aflatoxin B1 isolating potential of two lactic acid bacteria. Asian Pac J Trop Biomed, 2013; 3(9):732–6. CrossRef
Hayati M, Herman H, Rezano A. The effect of probiotic Lactobacillus casei supplementation on the secretory immunoglobulin A level in the saliva of wistar rats. Bali Med J, 2018; 7(3):727–31. CrossRef
Hussain AF, Sulaiman GM, Dheeb BI, Hashim AJ, Alrahman ESA, Seddiq SH, Khashman BM. Histopathological changes and expression of transforming growth factor beta (TGF-β3) in mice exposed to gliotoxin. J King Saud Univ – Sci, 2020; 32(1):716–25. CrossRef
Ibrahim AAE. Vitamin A downregulating Bcl-2 and TGF-α expression during colon cancer in AFB1-induced female rats. J Nat Sci Res, 2013; 3(5):67–84.
Møller GOD, Freire L, Rosim RE, Margalho LP, Balthazar CE, Franco LT, Sant’Ana AD, Corassin CH, Rattray FP, de Oliveira CAF. Effect of lactic acid bacteria strains on the growth and aflatoxin production potential of Aspergillus parasiticus, and their ability to bind aflatoxin B1, ochratoxin A, and zearalenone in vitro. Front Microbiol, 2021; 12:655386. CrossRef
Mohamadzadeh M, Olson S, Kalina W V., Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli active human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA, 2005; 102(8):2880–5. CrossRef
Mohammadi A, Mehrzad J, Mahmoudi M, Schneider M. Environmentally relevant level of aflatoxin B1 dysregulates human dendritic cells through signaling on key toll-like receptors. Int J Toxicol, 2014; 33(3):175–86. CrossRef
Muñoz R, Arena M, Silva J, González S. Inhibition of mycotoxinproducing Aspergillus nomius VSC 23 By Lactic Acid Bacteria and Saccharomyces cerevisiae. Brazilian J Microbiol, 2010; 41:1019–26. CrossRef
Murugesan GR, Ledoux DR, Naehrer K, Berthiller F, Applegate TJ, Grenier B, Phillips TD, Schatzmayr G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult Sci, 2015; 94(6):1298–315. CrossRef
Nazarizadeh, H and Pourreza J. Evaluation of three mycotoxin binders to prevent the adverse effects of aflatoxin B1 in growing broilers. J Appl Anim Res, 2019; 47(1):135–9. CrossRef
Niderkorn V, Morgavi DP, Aboab B, Lemaire M, Boudra H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria. J Appl Microbiol, 2009; 106(3):977–85. CrossRef
Oswald IP, Marin DE, Bouhet S, Pinton P, Taranu I, Accensi F. Immunotoxicological risk of mycotoxins for domestic animals. Food Addit Contam, 2005; 22(4):354–60. CrossRef
Perczak A, Goli?ski P, Bry?a M, Wa?kiewicz A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arh Hig Rada Toksikol, 2018; 69(1):32–45. CrossRef
Perdigón G, Fuller R, Raya R. Lactic acid bacteria and their effect on the immune system. Curr Issues Intest Microbiol, 2001; 2(1):27–42.
Pierron A, Alassane-Kpembi I, Oswald IP. Impact of mycotoxin on immune response and consequences for pig health. Anim Nutr, 2016; 2(2):63–8. CrossRef
Qian G, Tang L, Wang F, Guo X, Massey ME, Williams JH, Philips TD, Wang J. Physiologically based toxicokinetics of serum aflatoxin B1-lysine adduct in F344 rats. Toxicology, 2013; 303:147–51. CrossRef
Rohmawati E, Rifa’i M. Ethanol extracts of propolis ( Eep ) against lymphocyte activation cells in healthy mice (Mus Musculus) Balb / C. J Biotropika, 2014; 2(4):203.
Sadiq FA, Yan B, Tian F, Zhao J, Zhang H, Chen W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: a comprehensive review. Compr Rev Food Sci Food Saf, 2019; 18(5):1403–36. CrossRef
Tabari G, Kermanshahi H, Golian A, Heravi R. In vitro binding potentials of bentonite, yeast cell wall and lactic acid bacteria for aflatoxin B1 and ochratoxin A. Iran J Toxicol, 2018; 12(2):7–13. CrossRef
Tomková I, Šev?íková Z, Levkut M, Revajová V, ?onková E, Laciaková A, Lenhardt L. Effect of aflatoxin B1 on CD3 T cells and alkaline phosphatase in the intestine of mice. Mycopathologia, 2002; 154(1):15–9. CrossRef
Tran VN, Viktorová J, Rum T. Mycotoxins: biotransformation and bioavailability assessment using Caco-2 cell monolayer. Toxins, 2020; 12(10):628. CrossRef
Vinderola G, Ritieni A. Role of probiotics against mycotoxins and their deleterious effects. J Food Res, 2014; 4(1):10. CrossRef
Wells JM. Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact, 2011; 10(Suppl. 1):1–15. CrossRef
Wold AE. Immune effects of probiotics. Scand J Nutr, 2001; 45(2):76–85. CrossRef
Zain ME. Impact of mycotoxins on humans and animals. J Saudi Chem Soc, 2011; 15(2):129–44. CrossRef
Zimmermann C, Machado A, Cadoná F, Jaques J, Schlemmer K, Lautert C, Da cruz I, Zanette RA, Leal DBR, Santurio J. In-vitro cytotoxicity of aflatoxin B1 to broiler lymphocytes of broiler chickens. Brazilian J Poult Sci, 2014; 16(3):307–12. CrossRef