INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia. DM can occur due to impaired insulin secretion or increased cell resistance to insulin (ADA, 2009). The World Health Organization noted that, in 2019, around 1.5 million deaths were directly caused by diabetes. Another 2.2 million deaths in 2012 were due to high blood glucose levels (WHO, 2021). The increase in diabetes cases is difficult to overcome due to inadequate DM therapy management. The treatment of DM is only to decrease the blood glucose levels, not to treat DM completely (Adu et al., 2019). Thus, DM complications continue to develop, one of which is diabetic foot ulcer (DFU) in 15–25% of the total DM patients (Yazdanpanah et al., 2018). DFU manifests diabetic neuropathy due to decreased blood perfusion to the periphery, causing tissue necrosis and peripheral ischemia (Alexiadou and Doupis, 2012; Volmer-Thole & Lobmann, 2016).
Since DFU is associated with the leading cause of morbidity and mortality in DM, effective treatment for DFU patients is needed. Wound care in DFU patients requires special handling not to cause infection and the risk of amputation (Everett and Mathioudakis, 2018). Diabetic ulcer dressing conventionally cleans the wound using normal saline or 0.9% NaCl solution, added with povidone-iodine, and wrapped with dry gauze to protect and prevent infection. However, using this method has several risks, including the gauze becoming dry and sticking to the wound and causing pain, and the new cells will also be damaged. Previous studies have exhibited that the development of wound care methods and wound dressings has shifted to the modern approach, such as alginate dressing, foam dressing, and hydrogel (Dumville et al., 2013; Saco et al., 2016). However, these methods should be controlled of humidity because it contributes to wound healing. Besides, less humid conditions will cause cell death and no matrix tissues are formed.
Nowadays, natural products derived from animals, minerals, and plants are relatively safe if the chemical content is determined. Therefore, the utilization of natural resources, including aquatic biota, has been widely studied for active component exploration (Fawwaz et al., 2018; 2019). One source of medicinal ingredients derived from animals is snakehead fish (Channa striata) which contains amino acids and albumin, and other bioactive ingredients that play a role in wound healing (Rahayu et al., 2016). Snakehead fish is a source of protein that has been used empirically in wound healing (Sahid et al., 2018). This fish lives in the river, especially in Indonesia. The snakehead fish mucus contains essential amino acids, including arginine, glycine, lysine, proline, glucosamine, D-glucuronic acid, and carnosine which are needed to heal wounds (Baie and Sheikh, 2000). Arginine is the sole precursor of nitric oxygen, a molecular signal involved in the immune response, angiogenesis, epithelialization, and tissue formation (Malone-Povolny et al., 2019). In addition, arachidonic acid, which is the most prominent fatty acid in snakehead fish, acts as a precursor of prostaglandins and thromboxane, both of which play an active role in the blood clotting process (Palta et al., 2014; Smith et al., 2015).
Transdermal patch technology can be an alternative in DFU treatment (Ramirez-Acuña et al., 2019). The ability to transfer the drugs systemically and locally is a distinct advantage of the patch. In addition, the patch is very convenient for patients, avoids first-pass metabolism of the drug, improves patient compliance, and avoids dose wastage. This method is also very suitable as a replacement therapy for patients who cannot take medicines orally. Therefore, we aim to develop and evaluate the patch for transdermal delivery containing albumin of snakehead fish mucus in wound healing of diabetic rats.
Here, we formulated the transdermal patch containing the snakehead fish mucus albumin in various concentrations. The patch was evaluated for its characteristics and the wound healing activity on diabetic mice.
MATERIALS AND METHODS
Chemicals
The solvents and other chemicals were of analytical grade. Deionized water was obtained through a Millipore-Q50 Ultrapure water system (Sartorius). Alloxan monohydrate and biuret reagent were obtained from Sigma Chemicals Co. (St. Louis, MO). Vipalbumin® as pure albumin product was obtained from Royal Medicalink Pharmalab Co. (Makassar, Indonesia).
Instrumentation
Analytical instrument used a UV-Vis spectrophotometer (LAMDA 365, PerkinElmer Inc. USA). Lyophilization of extract used vacuum freeze dryer (LGJ 10, Armfield Limited, UK). All glassware used were of analytical grade.
Sample preparation
The snakehead fish (C. striata) were obtained commercially in Makassar, Indonesia. The fresh snakehead fish were cleaned using clean water and placed in a stainless container. The fish mucus was collected from the skin by evaporation. The corpus was freeze-dried, and then the mucus was steamed at a temperature of 40°C–50°C until forming an extract. The extract was freeze-dried, and the albumin content was measured by UV-Vis spectrophotometry.
Determination of total albumin
The standard curve for determining total albumin content was carried out by preparing biuret reagent in series of 10, 9, 8, 7, 6, 5, 4, 3, 2, and 1 ml. Each of these volumes was added to 10 ml of 2,000 M NH3. Each mixture was measured on a UV-Vis spectrophotometer at a wavelength of 550 nm. The linearity of the standard series was carried out through analysis. The linearity equation was used to determine the amount of total albumin in the sample. The snakehead fish extract was weighed as much as 1.0 g, and then 50 ml of distilled water was added. The mixture was incubated for 2 hours at 25°C and filtered. As much as 4.0 ml of sample was supplemented with 6 ml of biuret reagent and set at 25°C for 30 minutes. The mixture was measured on a UV-Vis spectrophotometer at a wavelength of 550 nm (Fuadi et al., 2017).
Formulation of patch transdermal
According to the previous study, the patch formulation was conducted with slight modification (Cherukuri et al., 2017). The formula can be seen in Table 1. Firstly, the extract of snakehead fish mucus was dissolved in 15 ml of distilled water. The mixture of hydroxypropyl methylcellulose (HPMC) in 8 ml of ethanol, mucilage of sodium carboxymethyl cellulose (Na-CMC), and propylene glycol were added to the sample solution. The mixture was stirred until homogeneous. If there are bubbles during stirring, the preparation must be left to stand for 24 hours to remove the bubbles. Furthermore, the mold that already contained the formula was put into an oven at a temperature of 40°C for 12 hours (Cherukuri et al., 2017).
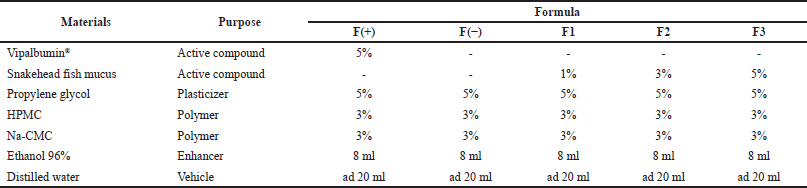 | Table 1. The formula of patch transdermal from the snakehead fish mucus extract. [Click here to view] |
F(+)= Positive control (Formula with Vipalbumin®).
F(−)= Negative control (Formula without active compound).
F1= Formula with snakehead fish mucus extract 1%.
F2= Formula with snakehead fish mucus extract 3%.
F3 = Formula with snakehead fish mucus extract 5%.
Patches’ moisture was maintained by placing the patches in a desiccator silica gel at 25°C for 2 × 24 hours. The evaluation of moisture during storage was calculated according to the following equation (Keleb et al., 2010). The desired moisture is less than 10% (Nurmesa et al., 2019).
The uniformity of the patch was evaluated by weighing each of the three patches at random, and then the weight variation was recorded. The patch weight requirement is that nothing deviates from 5 to 10% of the average weight.
Animals
The male Wistar rats (Rattus norvegicus), weight 200–300 g, were purchased commercially and housed in a cage with food and water availability. The temperature (23?C–25?C) was maintained with a 12 hour light/dark cycle. Rats were grouped into five groups consisting of three rats each; positive control, negative control, treatment by 1%, 3%, and 5% of the extract. Wound observation was carried out for 8 days to see the healing rate of diabetic ulcers in the rats. The study was conducted following laboratory guidelines for using animals and was approved by the Health Research Ethics Committee, Universitas Mega Rezky, Makassar, Indonesia, with reference number: 001.C/07.091056/VII/2021.
Alloxan-induced diabetic model
Diabetic rats were prepared following the previous study with slight modification. Alloxan monohydrate was weighed and solubilized with 0.2 ml saline individually for each animal according to their weight. At a dose of 150 mg/kg body weight, the alloxan was injected intraperitoneally. After 1 hour of alloxan administration, the animals were fed ad libitum, and a 5% dextrose solution was also given to overcome the hyperglycemic phase. The animals’ blood glucose was measured after 48 hour by a glucometer. The diabetic rats (glucose level >300 mg/dl) were separated and divided into five different groups for experimental study, with each group containing three rats (Ighodaro et al., 2017; Kannur et al., 2006).
Wound induction
Rats were exposed to anesthetic doses of ethyl ether by inhalation for 10 min. The back hairs of the rats were shaved, and the skin surface was cleaned with 70% alcohol. The incision (2 cm long and 2 mm deep) was made under aseptic conditions with surgical blade no. 11 (Arun et al., 2016; Kokan et al., 2009). The normal and diabetic rats were randomly grouped into five groups. The rats in group I was positive control treated by Vipalbumin®; group II was negative control; groups III, IV, and V were treated by the transdermal patch containing snakehead mucus extracts of 1%, 3%, and 5%, respectively.
Wound healing activity
The wound size was measured 2, 4, 6, and 8 days after treatment. The rate of wound healing was measured as the percentage of reduction from the original wound size every single day (Supplementary Figures S1–S5) (Jahandideh et al., 2017). After disinfecting the wound by ethanol 70%, a digital camera was used to take the pictures from an equal distance of the wound and at a right angle to its surface. The wounds were bandaged again after taking the pictures. The percentage of wound healing was calculated using the following equation (Jahandideh et al., 2017):
Statistical analysis
All of the presented data are mean ± SD of at least three independent experiments. Statistical analysis was performed by GraphPad Prism 8.0 (San Diego, California). Differences between groups were analyzed by one-way analysis of variance, followed by Tukey’s multiple comparisons test. The level of statistical significance was set to p < 0.001.
RESULTS AND DISCUSSION
Albumin is a protein with a vital function for the body. One of the main functions of albumin is to maintain oncotic pressure so that the supply of nutrients and oxygen is maintained. Hypoalbuminemia is often associated with DM because hyperglycemia is a manifestation of chronic endothelial dysfunction (Dang et al., 2005). Previous studies have found that changes in endothelial function lead to the accumulation of plasma proteins in the interstitial compartment (Plante et al., 1995). This condition causes microalbuminuria, which means reduced blood albumin levels (Basi and Lewis, 2006). Imbalance in blood albumin levels impacts the supply of nutrients and oxygen, which certainly interferes with the wound healing process. Therefore, in this study, we selected diabetic rats as animal models to investigate the effect of albumin-containing patches on wound healing. To compare the wound healing activity of the patch, we applied the patch containing albumin to the normal rats (without DM).
In this study, we used patch containing albumin because we assumed that the topical form would maintain blood albumin levels and initiate wound healing. Previous studies found that albumin promoted NF-κB signaling which, in turn, induced the transduction and transcription of factors involved in wound healing. Albumin infusion plays an essential role in accelerating the wound healing process, as it can contribute to correcting the hypoalbuminemia state (Utariani et al., 2020).
Total albumin of snakehead fish mucus extract
The determination of albumin levels in snakehead fish mucus has never been done, so it is interesting to investigate. Our study successfully determined the albumin levels in snakehead fish mucus. We found that albumin levels in mucus extracts were much higher than before the extraction process. The comparison of albumin levels in snakehead fish mucus can be seen in Table 2. The albumin content in mucus is closely related to the albumin content in the meat of snakehead fish. Some studies exhibited that snakehead fish contains large amounts of albumin (Baie and Sheikh, 2000; Ramadhanti et al., 2021).
Formulation of patch transdermal
The patch was prepared and characterized as the established procedure. The evaluation was visually carried out, including shape, color, and surface texture. The chemical properties were also evaluated, as shown in Table 3. The organoleptic evaluation showed that the higher the concentration of the active substance used the higher the affect of the shape and color of the patch. Furthermore, all formulas produce good characteristics. The pH measurement by digital pH meter exhibited that the pH of the patch was in the range of tolerated pH for the skin. The requirement for the pH of skin formula to avoid irritation is 4.5–6.5. The pH for all formulas showed acceptable value. Therefore, the formula of the patch is safe for transdermal usage.
Moisture evaluation aims to determine the water content in the patch formula that has been made to protect the active ingredients from microbial growth. The moisture test results showed that all formulas met the humidity requirements listed in Table 3. The humidity requirements for the patch formula should be less than 10%. There is a difference in the percentage of each formula because the concentration of the active ingredients used is different from each other.
The weight uniformity test exhibited that the weights of all formulas were not significantly different from each other, as we can see in Table 4. The difference in weight was due to the amount of active substance used, which affects the weight of each formula. The evaluation of the weight uniformity was conducted to ensure that no significant amounts of weight are lost in the manufacturing process.
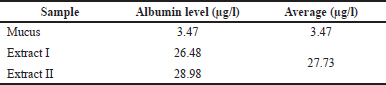 | Table 2. The albumin level of snakehead fish mucus extract. [Click here to view] |
Wound healing activity
The experimental rats acclimatized were checked for initial blood glucose level, and then alloxan was induced for 3 days. Alloxan works by destroying pancreatic beta cells (Szkudelski, 2001). Alloxan will accumulate in pancreatic beta cells through glucose transport 2 into the cytosol, which will generate reactive oxygen species with a reaction cycle that produces a dialuric acid reaction that will undergo a redox cycle (Song et al., 2015). The redox cycle then forms superoxide radicals which mutate to produce hydrogen peroxide, and the final stage will undergo an iron-catalyzed reaction process to form hydroxyl radical compounds. These hydroxy radicals will damage pancreatic beta cells, resulting in insulin-dependent diabetes mellitus. The use of alloxan as a diabetogenic is also because this drug quickly causes permanent hyperglycemia within 2–3 days (Ighodaro et al., 2017). After 3 days of alloxan administration, the measurement was repeated to compare the initial glucose levels. If rats’ blood glucose levels increase over 300 mg/dl, then the rats are considered diabetic (Sikarwar and Patil, 2010).
The percentage of wound healing can be calculated based on comparing the initial wound length and the wound after treatment (Table 5). From the percentage of wound healing of diabetic rats, the most remarkable healing on the sixth and eighth days was seen in the group of patches containing snakehead fish mucus extract 5% (F3 formula). This result was in line with the previous study showing that the greater the concentration of snakehead fish extract given, the greater the wound healing activity of experimental animals (Ramadhanti et al., 2021). A cream containing albumin from snakehead fish exhibited that 2% snakehead fish cream for 12 days can accelerate wound recovery (Tungadi et al., 2011). Baie and Sheikh (2000) found that topical albumin treatment could enhance wound healing in the animal experiment by increasing tensile strength and increasing fibroblast count and hydroxyproline level.
Wound healing activity testing on normal rats was carried out to see the difference in wound healing in diabetic rats and normal rats. The data obtained in Table 6 shows a significant difference between the time required for wound healing in normal and diabetic rats. On day 8 for the F3 formula, wounds in normal rats had reached 100% healing, while wounds in diabetic rats had only reached 50% (p < 0.05).
The wound healing activity of the patch exhibited that with the length of time the patch was used, the percentage of wound healing in rats increased, as shown in Figure 1. The formula F3 of 58% showed the highest rate on the eighth day. The activity of the positive control group, formula F(+), showed no significant difference from the action of formula F3 on the eighth day of treatment. These data indicate that the wound healing ability of formula F3 is the same as that of formula F(+). On the contrary, formula F3 and F2’s wound healing activity differed significantly from the negative control formula F(−) with p > 0.001 and p > 0.05, respectively.
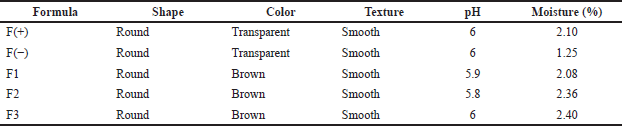 | Table 3. Evaluation of patch containing snakehead fish mucus extract. [Click here to view] |
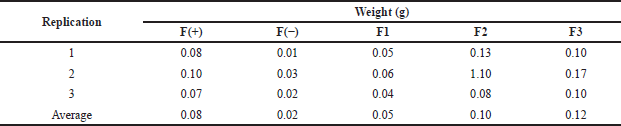 | Table 4. Weight uniformity data of patch containing snakehead fish mucus extract. [Click here to view] |
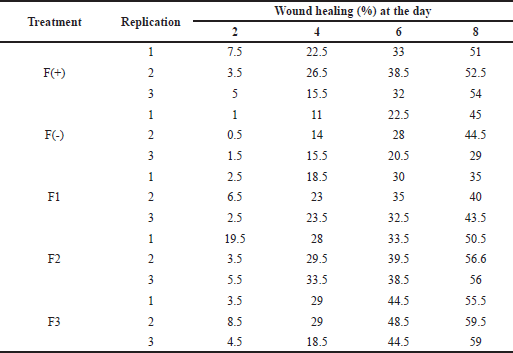 | Table 5. The wound healing effect of the patch from the snakehead fish mucus extract toward diabetic rats. [Click here to view] |
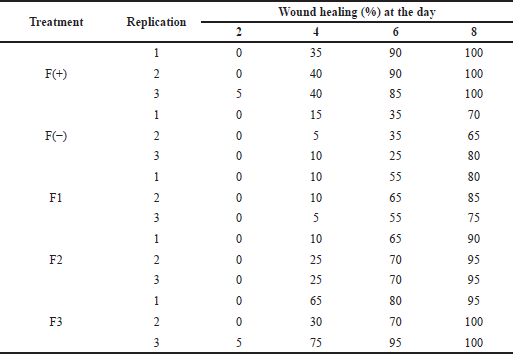 | Table 6. The wound healing effect of the patch from the snakehead fish mucus extract toward normal rats. [Click here to view] |
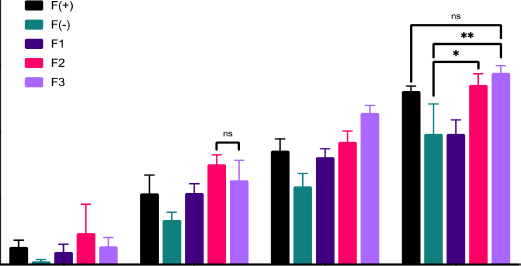 | Figure 1. Wound healing activity of patch containing snakehead fish mucus extract. [Click here to view] |
Although wound healing is a natural physiological response to tissue wounds, it is a complex phenomenon and involves a complex interplay between multiple cell types, mediators, cytokines, and the vascular system. The healing process starts from vasoconstriction of blood vessels and platelet aggregation to stop bleeding. After that, the inflammatory cells come to release various mediators and cytokines to stimulate angiogenesis, thrombosis, and re-epithelialization (Ozgok Kangal and Regan, 2021). The characteristics of rats on the first day after treatment showed wound enlargement due to the inflammatory phase. On the third and fifth days, the wound healing phase proliferation is characterized by the formation of granulation tissue in the wound, collagen formation, and fibroblasts (Ansar and Ghosh, 2016). The next phase is the maturation phase consisting of re-absorption of excess tissue, shrinkage due to gravity, and forming new tissue. The healing phase ended when all signs of inflammation had disappeared, as shown on observations on the seventh day to the ninth day, where all the wounds in the mice had healed (Ansar and Ghosh, 2016; Utariani et al., 2020).
Albumin plays a vital role in wound healing in this study because albumin can activate epidermal growth factor receptor (EGFR) expression and upregulate NF-κB signaling. By the activation of EGFR could accelerate corneal epithelialization. In addition, the EGFR stimulates tyrosine kinase activity that activates gene transcriptions, DNA synthesis, and cell proliferation (Fawwaz et al., 2020; Shu et al., 2019).
High essential amino acid components and fatty acids in snakehead fish albumin play a major role in wound healing (Haniffa et al., 2014; Zuraini et al., 2006). Both of these components work through the initiation of collagen synthesis and re-epithelialization. Other studies say that Arginine is an amino acid substance that plays an important role in healing (Molnar et al., 2014). The polyunsaturated fatty acids (PUFA) also play a role in the immune response in wound healing. PUFA works as a substrate of prostaglandin, thromboxane, leukotrienes, and lipoxin synthesis (Guo and DiPietro, 2010). The deficiency of these components will slow down the healing process (Jais et al., 1994).
CONCLUSION
The patch transdermal from the snakehead fish mucus extract was formulated with the acceptable characteristics. The formula F3 with 5% extract showed the best activity toward wound healing of diabetic rats on the eight day of treatment. Therefore, the formulation of patch transdermal containing snakehead fish mucus extract 5% can accelerate wound recovery. However, further research is needed to support the current data.
ACKNOWLEDGMENT
This work was supported by Universitas Megarezky and Universitas Hasanuddin, Makassar, South Sulawesi, Indonesia. Special thanks to the Future of Science Research Group for providing statistical analysis software GraphPad Prism 8.0.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
The institutional animal ethics committee approved the study protocol at Health Research Ethics Committee, Universitas Mega Rezky, Makassar, Indonesia, with reference number: 001.C/07.091056/VII/2021.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
REFERENCES
Adu MD, Malabu UH, Malau-Aduli AEO, Malau-Aduli BS. Enablers and barriers to effective diabetes self-management: a multi-national investigation. PLoS One, 2019; 14(6):e0217771. CrossRef
Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther, 2012; 3(1):4. CrossRef
Ansar W, Ghosh S. Inflammation and inflammatory diseases, markers, and mediators: role of CRP in some inflammatory diseases. In: Ansar W, Ghosh S (eds.). Biology of C reactive protein in health and disease, Springer India, New Delhi, India, pp 67–107. CrossRef
Arun M, Satish S, Anima P. Evaluation of wound healing, antioxidant, and antimicrobial efficacy of Jasminum auriculatum Vahl. Leaves. Avicenna J Phytomed, 2016; 6(3):295–304.
American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes Care, 2009; 32(Suppl 1):S62–7. CrossRef
Basi S, Lewis JB. Microalbuminuria as a target to improve cardiovascular and renal outcomes. Am J Kidney Dis, 2006; 47:927–46. CrossRef
Baie SH, Sheikh KA. The wound healing properties of Channa striatus-cetrimide cream-- tensile strength measurement. J Ethnopharmacol, 2000; 71(1–2):93–100. CrossRef
Cherukuri S, Batchu UR, Mandava K, Cherukuri V, Ganapuram KR. Formulation and evaluation of transdermal drug delivery of topiramate. Int J Pharm Investig, 2017; 7(1):10–7. CrossRef
Dang L, Seale JP, Qu X. High glucose-induced human umbilical vein endothelial cell hyperpermeability is dependent on protein kinase C activation and independent of the Ca2+-nitric oxide signalling pathway. Clin Exp Pharmacol Physiol, 2005; 32:771–6. CrossRef
Dumville JC, O’Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev, 2013; 2013(6):Cd009110. CrossRef
Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci, 2018; 1411(1):153–65. CrossRef
Fawwaz M, Vemilia P, Mutmainnah I, Baits M. Scylla serrata forskal as natural source of glucosamine hydrochloride. Marmara Pharm J, 2019; 23(2):259–66. CrossRef
Fawwaz M, Baits M, Saleh A, Irsyaq MR, Pratiwi RE. Isolation of Glucosamine HCl from Penaeus monodon. Int Food Res J, 2018; 25(5):2173–6.
Fawwaz M, Mishiro K, Nishii R, Sawazaki I, Shiba K, Kinuya S, Ogawa K. Synthesis and fundamental evaluation of radioiodinated rociletinib (CO-1686) as a probe to lung cancer with L858R/T790M Mutations of Epidermal Growth Factor Receptor (EGFR). Molecules, 2020; 25(12):2914. CrossRef
Fuadi M, Santoso H, Syauqi A. Albumin level test of snakehead fish (Channa striata) in different salinity environment. Bioscience-Tropic, 2017; 3(1):23–30.
Guo S, DiPietro LA, Factors affecting wound healing. J Dent Res, 2010; 89(3):219–29. CrossRef
Haniffa MAK, Jeya Sheela PA, Kavitha K, Jais AMM. Salutary value of haruan, the striped snakehead Channa striatus a review. Asian Pac J Trop Biomed, 2014; 4:S8–15. CrossRef
Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina, 2017; 53(6):365–74. CrossRef
Jais AMM, McCulloch R, Croft K. Fatty acid and amino acid composition in haruan as a potential role in wound healing. Gen Pharmacol Vasc Syst, 1994; 25(5):947–50. CrossRef
Jahandideh M, Hajimehdipoor H, Mortazavi SA, Dehpour A, Hassanzadeh G. Evaluation of the wound healing activity of a traditional compound herbal product using rat excision wound model. Iran J Pharm Sci, 2017; 16(special issue):153–63.
Kannur DM, Hukkeri VI, Akki KS. Antidiabetic activity of Caesalpinia bonducella seed extracts in rats. Fitoterapia, 2006; 77(7–8):546–9. CrossRef
Keleb E, Sharma R, Mosa EB, Zaljahwi AZ. Transdermal drug delivery system- design and evaluation. Int J Adv Pharm Sci, 2010; 1:201–11.
Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol, 2009; 124(2):311–5. CrossRef
Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv Healthc Mater, 2019; 8(12):e1801210. CrossRef
Molnar JA, Underdown MJ, Clark WA. Nutritionand chronic wounds. Adv Wound Care, 2014; 3(11):663–81. CrossRef
Nurmesa A, Nurhabibah, Najihudin A. Formulasi dan Evaluasi Stabilitas Fisik Patch Transdermal Alkaloid Nikotin Daun Tembakau (Nicotiana tobacum Lin) dengan Variasi Polimer dan Asam Oleat. Jurnal Penelitian Farmasi Herbal, 2019; 2(1): 1-8. CrossRef
Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth, 2014; 58(5):515-23. CrossRef
Plante GE, Chakir M, Lehoux S, Lortie M: Disorders of body fluid balance: a new look into the mechanisms of disease. Can J Cardiol, 1995; 11:788–802.
Rahayu P, Marcelline F, Sulistyaningrum E, Suhartono MT, Tjandrawinata RR. Potential effect of striatin (DLBS0333), a bioactive protein fraction isolated from Channa striata for wound treatment. Asian Pac J Trop Biomed, 2016; 6(12):1001–7. CrossRef
Ramadhanti NA, Sandhika W, Widodo ADW. The effect of snakehead fish (Channa striata) extract on inflammation reaction of skin wound tissue in Rattus novergicus Wistar strain. Periodical Dermatol Venereol, 2021; 33(1):48–54. CrossRef
Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, Flores-Morales V, Martinez-Fierro ML. Diabetic foot ulcers: current advances in antimicrobial therapies and emerging treatments. Antibiotics (Basel), 2019; 8(4). CrossRef
Saco M, Howe N, Nathoo R, Cherpelis B. Comparing the efficacies of alginate, foam, hydrocolloid, hydrofiber, and hydrogel dressings in the management of diabetic foot ulcers and venous leg ulcers: a systematic review and meta-analysis examining how to dress for success. Dermatol Online J, 2016; 22(8). CrossRef
Sahid NA, Hayati F, Rao CV, Ramely R, Sani I, Dzulkarnaen A, Zakaria Z, Hassan S, Zahari A, Ali AA. Snakehead consumption enhances wound healing? From tradition to modern clinical practice: a prospective randomized controlled trial. Evid Based Complement Alternat Med. 2018; 2018:3032790. CrossRef
Shu DY, Hutcheon AEK, Zieske JD, Guo X. Epidermal growth factor stimulates transforming growth factor-beta receptor type II expression in corneal epithelial cells. Sci Rep, 2019; 9(1):8079. CrossRef
Sikarwar MS, Patil MB. Antidiabetic activity of Crateva nurvala stem bark extracts in alloxan-induced diabetic rats. J Pharm Bioallied Sci, 2010; 2(1):18–21. CrossRef
Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Crit Rev Biochem Mol Biol, 2015; 50(4):326–36. CrossRef
Song I, Patel O, Himpe E, Muller CJF, Bouwens L. Beta cell mass restoration in alloxan-diabetic mice treated with EGF and gastrin. PLoS One, 2015; 10(10):e0140148. CrossRef
Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res, 2001; 50(6):537–46.
Tungadi R, Attamimi F, Sabu EF, Nugraha E. The acceleration of wound healing of snakehead fish cream towards rabbit’s skin wound histopathologically. Indonesian J Pharm Sci, 2011; 9(2):91–7.
Ozgok Kangal MK, Regan JP. Wound Healing [Online], 2021. Available via https://pubmed.ncbi.nlm.nih.gov/30571027/
Utariani A, Rahardjo E, Perdanakusuma DS. Effects of albumin infusion on serum levels of albumin, proinflammatory cytokines (TNF-α, IL-1, and IL-6), CRP, and MMP-8; Tissue expression of EGRF, ERK1, ERK2, TGF-β, collagen, and MMP-8; and wound healing in sprague dawley rats. Int J Inflam, 2020; 2020:3254017. CrossRef
Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci, 2016; 17(6). CrossRef
WHO. Diabetes [Online], 2021. Available via https://www.who.int/news-room/fact-sheets/detail/diabetes
Yazdanpanah L, Shahbazian H, Nazari I, Arti HR, Ahmadi F, Mohammadianinejad SE, Cheraghian B, Hesam S. Incidence and risk factors of diabetic foot ulcer: a population-based Diabetic Foot Cohort (ADFC Study)-two-year follow-up study. Int J Endocrinol, 2018; 2018:7631659. CrossRef
Zuraini A, Somchit MN, Solihah MH. Fatty acid and amino acid composition of three local Malaysian Channa spp. fish. Food Chem, 2006; 97(4):674–8. CrossRef
SUPPLEMENTARY MATERIAL
 | Figure S1. Wound size of diabetic rats as negative control F(-). [Click here to view] |
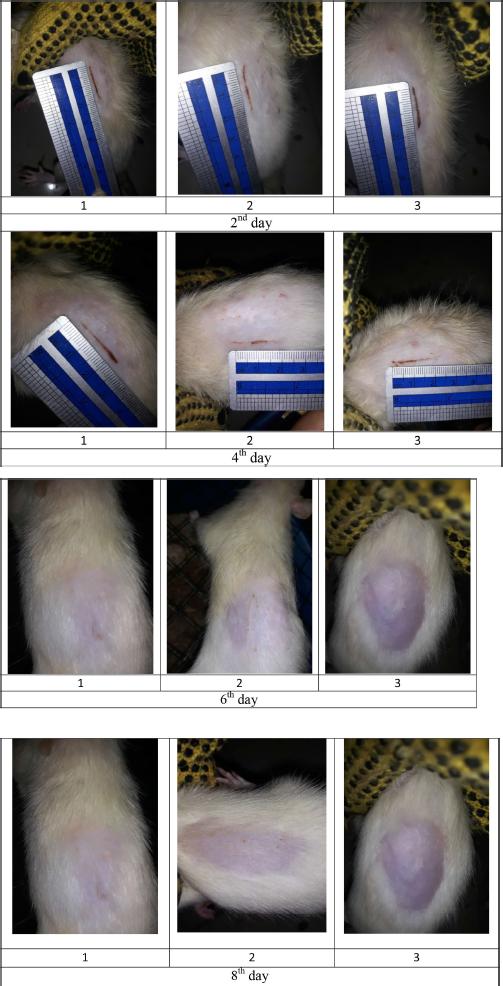 | Figure S2. Wound size of diabetic rats as positive control F(+). [Click here to view] |
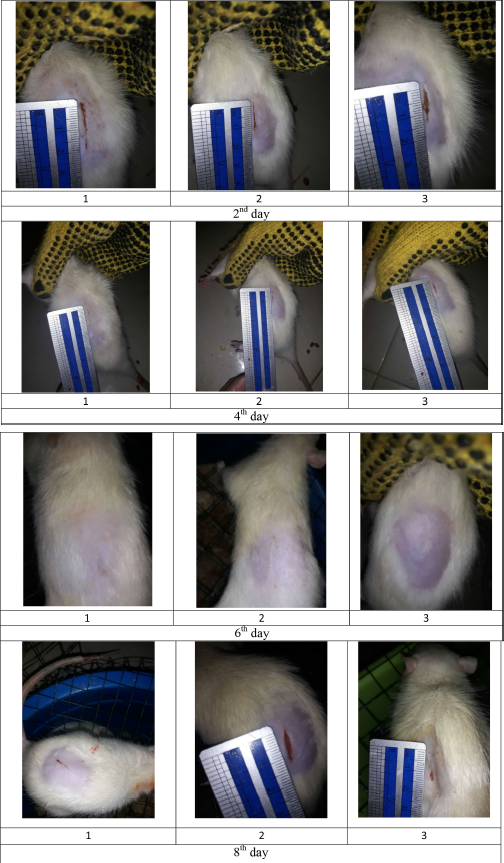 | Figure S3. Wound size of diabetic rats as treatment F1. [Click here to view] |
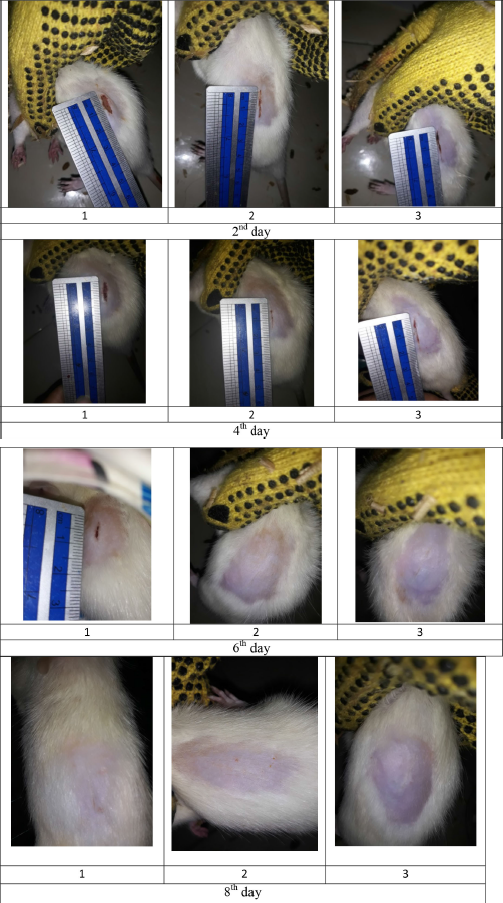 | Figure S4. Wound size of diabetic rats as treatment F2. [Click here to view] |
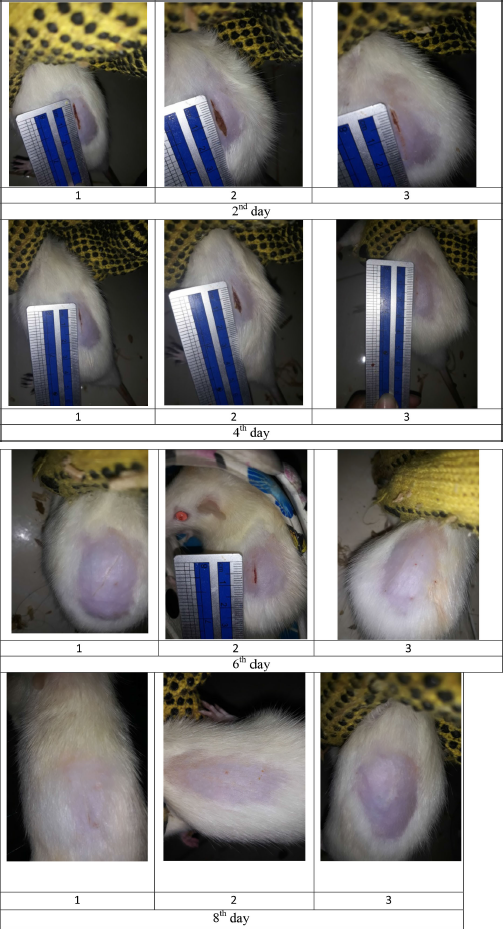 | Figure S5. Wound size of diabetic rats as treatment F3. [Click here to view] |