INTRODUCTION
The fruit of Morus alba (MA) L. (mulberry), from the family Moraceae, has been consumed as a food and dietary supplement for a long time and is rich in vitamin C, anthocyanins, flavonoids, and alkaloids. MA fruit and fruit extracts have shown antioxidant, antidiabetic, antiatherosclerotic, antiobesity, anticholesterol, and antibacterial activities, as well as immune enhancement, neuroprotective, and hepatoprotective properties (Chan et al., 2016; Chang et al., 2021; Hansawasdi and Kawabata, 2006; Zafar et al., 2013; Zhang et al., 2018). MA extracts are considered safe to use without severe side effects. MA leaf extract exhibited no acute toxicity in rats at doses above 5 g/kg (Abdulla et al., 2009), but mild itching was reported after topical application of MA oil for melasma treatment (Alvin et al., 2011). MA has been used as a nutraceutical product or food supplement due to the presence of several functional and pharmacological compounds in its leaves, fruit, and seeds, such as mulberroside A, rutin, chlorogenic acid, caffeic acid, quercetin, gallic acid, kaempferol, and apigenin (Chen et al., 2021; Hansawasdi and Kawabata, 2006; Mei et al., 2012).
All orally consumed substances have the potential for inter with medications (Fugh-Berman, 2000). Polypharmacy is an increasingly common event with physicians needing to prescribe multiple medications for patients with multiple conditions. In addition, people have access to a wide range of over-the-counter medications as well as health and dietary supplements. Herb-drug interactions may modulate the pharmacological and toxicological effects of either the herb or the drug. Information about MA herb-drug interactions is very limited.
Cytochrome P450 (CYP) monooxygenase enzymes, such as CYP1A2, CYP2C9, and CYP3A4, are responsible for phase I metabolism of greater than half of all clinical drugs (Zanger and Schwab, 2013). Foods or supplements can affect the metabolism of clinical drugs by interfering with the regulatory expression of CYPs. Conjugation enzymes involved in hepatic phase II metabolism including uridine diphosphate- (UDP-) glucuronosyltransferase 1A6 (UGT1A6), N-acetyltransferase 1 (NAT1), and sulfotransferase 1A1 (SULT1A1) can also play an important role in the metabolism of a variety of clinical drugs, xenobiotics, and toxicants (Kim et al., 2019; Rasool et al., 2019; Witham et al., 2017). In addition to phaseaction I and II metabolizing enzymes, some drug transporters such as organic anion transporting polypeptide 1B1 (OATP1B1) and ATP-binding cassette (ABC) transporter protein 1 (ABCB1 or P-glycoprotein) are important determinants for transporter-mediated drug interactions (Chen et al., 2019; Kayesh et al., 2021; Meszaros et al., 2013; Sorf et al., 2018).
A monolayer of human colorectal adenocarcinoma cells (Caco-2) is the most suitable in vitro model representing the human intestine for pharmacokinetic studies, especially for studies of the bioactivity of foods and herbal supplements (Brück et al., 2017; Iftikhar et al., 2020; Vaessen et al., 2017). The reduction of oxygen during normal biochemical or metabolic reactions in living cells or organisms results in the production of intermediate metabolic byproducts known as reactive oxygen species (ROS), which can cause oxidative damage to cells or tissues (Kükürt et al., 2021). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are enzyme markers of oxidative damage to hepatocytes but can also be released by other damaged cell types (Sepulveda, 2019; Udomsak et al., 2022). Hence, this study investigated the effects of MA on ROS, AST, ALT, and the mRNA expression of several important phases I and II drug-metabolizing enzymes and drug transporters in a Caco-2 monolayer model.
MATERIALS AND METHODS
Reagents
Cyanidin-3-O-glucoside (C3G) and rutin were supplied by Chengdu Alfa Biotechnology Co., Ltd. (Chengdu, China). High-performance liquid chromatography (HPLC) solvents were products of RCI Labscan Ltd. (Bangkok, Thailand). Dulbecco’s modified Eagle medium (DMEM) (+) phenol red, DMEM/F12 phenol red-free medium, Dulbecco’s phosphate-buffered saline, fetal bovine serum (FBS), 1× penicillin, streptomycin, and neomycin antibiotics (PSN), and 1× GlutaMAX® were supplied by Gibco® (Thermo Fisher Scientific, MA). Resazurin, 2,4-dinitrophenylhydrazine (DNPH), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), rifampicin, and ketoconazole, L-aspartate, L-alanine, and α-ketoglutarate were products of Sigma-Aldrich (Missouri). ReverTra Ace®, random primers, and other reagents for reverse transcription were products of Toyobo Co., Ltd. (Osaka, Japan). Primers were synthesized by Bio Basic, Inc. (Markham, Ontario, CA). Taq polymerase, RNase inhibitor, deoxynucleotide triphosphate mixture, and other reagents for polymerase chain reaction (qPCR) were products of Vivantis Technologies Sdn. Bhd. (Selangor, Malaysia). Other laboratory chemicals were obtained from commercial suppliers with the highest purity and quality.
Preparation of MA fruit powder
Seedlings of MA L. cultivar Chiangmai were provided by the Queen Sirikit Department of Sericulture, Ministry of Agriculture and Cooperatives, Thailand, and were grown in Noen-Ruang Village, Muang District, Khon Kaen, Thailand. The fruits of MA were collected in March 2021. A plant specimen (Panya, T. 8 KKU No. 25978) was deposited at the Research Institute for Human High Performance and Health Promotion, Khon Kaen University. The MA fruits were washed in cold water three times and dried in a hot air oven at 50°C until the moisture content was 10%. The dried fruits were mashed and sieved (60 mesh) before storage in a vacuum aluminum package until further analysis.
HPLC analysis
MA fruit powder, C3G, and rutin were accurately weighed and dissolved in absolute methanol before being filtered using a 0.45 µm membrane. The C3G and rutin contents of MA fruit powder were analyzed by HPLC-UV. A Hypersil ODS (4.0 × 250 mm × 5 µm, Agilent Technologies Inc., Santa Clara, CA) column was used as the stationary phase. A gradient mobile phase of acetonitrile in 0.1% phosphoric acid was increased from 5% to 80% in 25 minutes with a flow rate of 1 ml/minute. The machine (Prominence-i Model LC-2030C 3D, Shimadzu Corporation, Kyoto, Japan) was set up with dual UV detectors at 517 nm for C3G and 365 nm for rutin. The HPLC method validation and the chromatogram are shown in Table 1 and Figure 1.
Cell culture
Caco-2 cells (RBRC-RBC0988) were supplied by the Cell Bank of RIKEN BioResource Center (Saitama, Japan). The cells were maintained in DMEM (1 g/l D-glucose) with 20% FBS, L-glutamine, sodium pyruvate, 1× GlutaMAX®, and 1× PSN at 37°C with 5% CO2 and 95% relative humidity. The cells were cultured as a monolayer in 24-well plates (2.5 × 105 cells/well in 0.5 ml). At 48 hours after seeding, they were incubated with 0.1% (v/v) dimethyl sulfoxide (DMSO, control), 10 µM ketoconazole (Keto), 10 µM rifampicin (Rif), 10 µM simvastatin (Sim), 50 µM caffeine (Caf), 5 mM of paracetamol (APAP), 5 mM aspirin (ASA), 1–10 µM of C3G, 1–10 µM rutin, and/or 125–500 µg/ml of MA for 48 hours. The cells were harvested for quantitative analysis by real-time polymerase chain reaction (RT/qPCR) (n = 4–5 replicates per group with two independent experiments). In addition, the cell culture medium was collected for the determination of ROS, ALT, and aspartate transaminase (AST) levels.
Determination of cell viability
Cell viability was determined by the resazurin assay (Alamar Blue assay) as previously described (Sriset et al., 2021). Briefly, the living cells were incubated with 100 µM resazurin in 5% CO2 at 37°C for 30 minutes. The resorufin formation was measured for spectrofluorometric intensity at excitation of 530 nm and emission of 580 nm. The percentage of cell viability was calculated from an increasing rate of resoru?n compared to the nontreatment group.
 | Table 1. Reversed phase HPLC validation and rutin and C3G contents in MA extract. [Click here to view] |
Determination of ROS
The ROS level in the cell culture medium was measured using the DCFH-DA reaction as previously described (Sriset et al., 2021). The medium was incubated in the dark with 62.5 nM DCFH-DA for 40 minutes. The fluorescence intensity of 2′-7′dichlorofluorescein was measured at excitation and emission wavelengths of 484 and 530 nm, respectively, using a spectrofluorometer. The ROS level was calculated by comparison with a standard curve of hydrogen peroxide (2.5 to 20 μM).
Determination of cellular transaminases (AST and ALT)
An ALT substrate (300 mM L-alanine and 0.7 mM α-ketoglutarate) or AST substrate (10 mM L-aspartate and 1.7 mM α-ketoglutarate) was incubated with a cell culture medium or sodium pyruvate standard solution at 37C for 20–30 minutes. Then, 5 mM DNPH was added, and the mixture was left at room temperature for 20 minutes. The reaction was stopped by the addition of 1.3 N sodium hydroxide. The absorbance of the mixture was measured at 505 nm. The levels of AST and ALT were determined as international units per liter (IU/l) by comparison with a standard curve of sodium pyruvate (Sriset et al., 2021).
Quantitative determination of mRNA expression by reverse transcription (RT/qPCR)
At 48 hours after treatment, Caco-2 cells were collected using a guanidinium thiocyanate-phenol solution. Chloroform was added to extract RNA. The RNA was precipitated by isopropanol, and the pellet was washed twice with cold 70% ethanol before measuring the concentration, purity, and integrity of the RNA. Total RNA (500 ng per reaction) was reverse-transcribed using a ReverTra Ace® kit. The cDNA (20 ng per reaction) was used to determine the expression of target genes with specific primers (Table 2) using RT/qPCR with SYBR Green I probe detection. The expression of target genes was normalized to the reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The relative fold expression was calculated using the ??Ct method.
Statistical analysis
All results are presented as mean ± standard deviation (SD) from 5 to 6 samples per group. Statistical differences were analyzed using one?way analysis of variances coupled with Tukey’s procedure using IBM-SPSS version 23 software (Armonk, NY). p ≤ 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
The effect of MA fruit on cell viability, ROS, AST, and ALT
Phytochemical compounds including rutin, apigenin, chlorogenic acid, kaempferol, umbelliferone, morin, and luteolin have been isolated from the leaves, fruit, branches, bark, and roots of MA (Chu et al., 2006). Mulberroside A is a bioactive constituent previously found in the ethanolic extract of MA root (Park et al., 2011). In this study, we determined the mulberroside A, C3G, rutin, ellagic acid, chlorogenic acid, quercetin, kaempferol, galangin, genistein, luteolin, morin, myricetin, hesperidin, and silymarin contents of MA fruit powder, using HPLC (data not shown). The HPLC chromatogram (Fig. 1) indicates that rutin and C3G are the major constituents of MA. The rutin and C3G contents of MA were 7.85% ± 0.29% and 7.65% ± 0.31%, respectively (Table 1). These observations were consistent with a previous study that showed mulberry fruit contained a large amount of anthocyanins, which were mainly composed of cyanidin 3-rutinoside and C3G (Du et al., 2008). Rutin has been found in many parts of MA, including the leaves, fruit, and roots (Hunyadi et al., 2012; Zhao et al., 2015). The amount of these constituents can vary depending on the plant source, the harvest period, the method of extraction, the plant part used, and the method of analysis (Altemimi et al., 2017; Hussain et al., 2017). Since high levels of rutin and C3G were found in the MA sample, experiments in Caco-2 cells were continued with these two compounds. Previously, Zhang et al. (2013). examined the effect of 5 to 200 µM rutin on absorption and metabolism in Caco-2 cells (Zhang et al., 2013), and Ferrari et al. (2016) examined the anti-inflammatory and antioxidant activities of 20 to 40 µM C3G in Caco-2 cells (Ferrari et al., 2016). The doses of rutin and C3G (1 and 10 µM) and MA (125 to 500 µg/ml) employed in the current study were chosen to avoid cytotoxic effects and morphological changes to the Caco-2 cells while still determining their effects on the expression of metabolizing enzymes and transporters.
 | Figure 1. HPLC chromatogram of standard C3G and rutin and MA fruit. [Click here to view] |
Although Rif, rutin (10 µM), and MA (125 µg/ml) significantly reduced cell viability (Fig. 2A), the cell viability remained above 80%, which enabled us to continue the experiments, especially as MA at higher doses (250 and 500 µg/ml) did not show toxicity. All treatments did not change the ROS level (Fig. 2B). Excessive ROS production occurs in tumor cells, including Caco-2 cells. Rutin (5–100 µM) has been shown to possess ROS scavenger activity (ben Sghaier et al., 2016), and C3G (20–40 µM) improved intracellular redox status through inhibition of NF-κB signaling (Ferrari et al., 2016), in Caco-2 cells. Moreover, phytochemical-rich MA has been shown to have antioxidant and anti-inflammatory capacities (Gryn-Rynko et al., 2016). Cellular transaminases (ALT and AST) are common markers of cell injury (Pandurangan and Kim, 2015; Sriset et al., 2021). Keto and Rif markedly elevated levels of AST (Fig. 2C) and ALT (Fig. 2D) in Caco-2 cells, while all other treatments did not. Keto and Rif (at 10 µM) have also been shown to modify the expression of CYPs and transporters in Caco-2 cells (Netsch et al., 2006; Takano et al., 1998). As MA did not show toxicity in Caco-2 cells, all tested compounds were employed using these concentrations.
 | Table 2. Specific primers for RT/qPCR. [Click here to view] |
Alteration of phase I CYP expression by MA fruit
CYP1A2 is constitutively expressed in the human liver and is detectable in other tissues and cell lines such as Caco-2 cells (Netsch et al., 2006; Zanger and Schwab, 2013). CYP1A2 is responsible for the metabolism of antidepressants and antipsychotics, as well as anti-inflammatory, anesthetic, and analgesic drugs (Zhou et al., 2010). Keto extensively induced expression of CYP1A2 mRNA (p < 0.001), while Rif, C3G, and rutin did not (Fig. 3A). All doses of MA markedly elevated CYP1A2 expression.
CYP2C19 is responsible for the metabolism of proton pump inhibitors (e.g., omeprazole, esomeprazole, and pantoprazole), antidepressants (e.g., imipramine, amitriptyline, sertraline, and fluoxetine), hypnotics and sedatives (e.g., diazepam, clobazam, and phenobarbital), phenytoin, proguanil, nelfinavir, voriconazole, and clopidogrel (Uppugunduri et al., 2012). CYP2C19 is inducible by several drugs such as rifampicin, dexamethasone, and phenobarbital (Rana et al., 2010). Rif significantly induced expression of CYP2C19 mRNA, while Keto, C3G, rutin, and MA strongly suppressed CYP2C19 (Fig. 3B). Rif has previously been shown to increase CYP2C19 activity in human enterocytes (Glaeser et al., 2005). On the other hand, Keto has been reported as a CYP2C19 inhibitor (Uppugunduri et al., 2012).
CYP2D6 plays an important role in the liver and brain with reg to the metabolism of drugs such as beta-blockers, opiates, antiarrhythmic agents, neuroleptics, and antidepressants as well as neurotransmitters (Darney et al., 2021). The expression of CYP2D6 mRNA was unchanged by any of the treatments (Fig. 3C).
The most important metabolizing enzyme in phase I is CYP3A4 which metabolizes a large and diverse range of molecules covering more than half of the clinical drugs, including antihypertensive, antibacterial, antifungal, and antiviral drugs, bronchodilators, and lipid-lowering agents (Zanger and Schwab, 2013). Rif significantly increased the expression of CYP3A4 mRNA, while Keto, C3G, and MA suppressed CYP3A4 expression. Rutin did not affect CYP3A4 expression (Fig. 3D).
 | Figure 2. Effects of MA fruit, C3G , and rutin on cell viability, ROS, and cellular transaminases (AST and ALT) levels in Caco-2 cells. *p < 0.05, **p < 0.001 versus control (DMSO). Keto, 10 µM ketoconazole; Rif, 10 µM rifampicin; C3G, 1 and 10 µM C3G ; Rutin, 1 and 10 µM rutin; MA, 125, 250, and 500 µg/ml MA fruit (n = 4–5). [Click here to view] |
 | Figure 3. Effects of MA fruit, C3G , and rutin on CYP mRNA expression in Caco-2 cells. *p < 0.05, **p < 0.001 versus control (DMSO). Keto, 10 µM ketoconazole; Rif, 10 µM rifampicin; C3G, 1 and 10 µM C3G ; Rutin, 1 and 10 µM rutin; MA, 125, 250, and 500 µg/ml MA fruit (n = 4–5). [Click here to view] |
Keto has been proposed to induce CYP1A2 via activation of the aryl hydrocarbon receptor and pregnane X receptor (PXR) (Novotna et al., 2014). Moreover, Keto was reported as an inhibitor for CYP2C19 and transporters (Nikulin et al., 2017), while Rif was noted as a typical CYP3A4 inducer (Kuncharoenwirat et al., 2020). Only a few studies have examined the effect of MA and its constituents on the expression of CYPs. However, anthocyanins (including C3G) have been shown to inhibit CYP3A4 enzyme activity in human liver microsomes (Srovnalova et al., 2014), and an in silico analysis showed that rutin (quercetin-3-O-rutinoside) is metabolized by CYP1A2 and CYP2C9 (Sousa et al., 2013). More recently, Kar et al. (2015) performed an in vitro study focusing on the effect of different parts of MA on human recombinant CYP enzymes. While this approach has limitations for the study of induction, MA leaf extract inhibited the activities of human recombinant CYP3A4, CYP2D6, CYP2C9, and CYP1A2 (Kar et al., 2015), which corresponds to the present findings for CYP3A4 and CYP2C19. Therefore, potential MA-drug interactions could occur via CYP1A2 induction and/or CYP2C9 and CYP3A4 suppression.
Alteration of phase II conjugation enzyme and transporter expression by MA fruit
The conjugation reactions of phase II metabolism are also keys to the biotransformation of drugs. UGT1A6 is responsible for the biotransformation of a variety of drugs such as aspirin, carvedilol, naproxen, valproic acid, and zidovudine (Kim et al., 2019). In the present study, Rif extensively induced expression of UGT1A6 mRNA, while Keto did not (Fig. 4A). In contrast, C3G, rutin, and MA significantly suppressed UGT1A6 expression. These results correspond with previous observations that Rif induced UGT1A6 expression in the human colon (van de Kerkhof et al., 2008), and morusin, a prenylated flavonoid isolated from the root bark of MA, inhibited expression of UGT1A6, UGT1A7, and UGT1A8 in human, rat, dog, monkey, and minipig liver microsomes (Shi et al., 2016). Hence, MA and its constituents (C3G and rutin) possess the potential to downregulate UGT1A6 expression in Caco-2 cells.
NAT1 is responsible for the metabolism of nitrogenous compounds such as aromatic amines/amides, hydrazine, and hydrazides and is also involved in the biotransformation, clearance, and toxicity of numerous pharmacologic agents and environmental toxicants (Walker et al., 2009). Rif, C3G, and MA suppressed expression of NAT1, while Keto and rutin did not show any significant change (Fig. 4B). Information about the effect of MA, C3G, and rutin on NAT1 is limited, and this is the first report of the inhibitory effects of MA and C3G on NAT1 expression in Caco-2 cells.
SULT1A1 is the key enzyme responsible for the sulfation of xenobiotics, including paracetamol, estrogens, and iodothyronines (Gamage et al., 2005; Rasool et al., 2019). Only Rif significantly induced the expression of SULT1A1, while the other tested compounds did not affect SULT1A1 expression (Fig. 4C). Previously, the expression of SULT2A1 mRNA was shown to be up-regulated by rifampicin in human hepatocytes (Fang et al., 2007). This might correlate with the observation that Rif induced SULT1A1 expression in Caco-2 cells.
OATP1B1 has been reported to be associated with the uptake transport of clinical drugs, including statins, antibiotics, and anticancer drugs (Zhang et al., 2007). Keto, Rif, C3G, and rutin at 10 µM and all doses of MA significantly suppressed the expression of OATP1B1 (Fig. 4D). The downregulation of OATP1B1 expression by Keto and Rif in the present study confirms that these two compounds are inhibitors for OATPs (Choi et al., 2011; Pahwa et al., 2017). Similarly, flavonoids, quercetin, and rutin have previously been reported to inhibit the uptake of OATP1B1 substrates (Wang et al., 2005).
ABCB1, the efflux transporter, also known as P-glycoprotein, regulates the plasma and intracellular concentrations of numerous xenobiotics (Gow et al., 2008). Keto, Rif, and C3G all slightly lowered the expression of ABCB1 (although this was not statistically significant), and rutin did not affect ABCB1 expression. The two higher tested doses of MA (250 and 500 µg/ml) suppressed ABCB1 expression (Fig. 4E). This was consistent with a previous study that showed MA root extract suppressed ABCB1 expression in human breast cancer MCF7 cells (Choi et al., 2013).
In summary, MA downregulated the expression of UGT1A6, NAT1, OATP1B1, and ABCB1. Therefore, MA fruit might cause drug interactions by disrupting of phase II biotransformation and drug transport via those related genes.
Effect of combinations of MA fruit and common drugs on phase I CYP expression
People are exposed to many different substances in their daily life that can affect phase I and II drug-metabolizing enzymes and transporters, especially through the consumption of commonly prescribed and over-the-counter medications. Paracetamol (acetaminophen or APAP) is a commonly used analgesic and antipyretic drug that is available in drugstores as an over-the-counter medication. APAP is mainly metabolized by CYP2E1, CYP1A2, and CYP3A4, and a metabolite of APAP, N-acetyl-p-benzoquinoneimine, can cause hepatotoxicity (Toes et al., 2005). Caffeine (Caf) is a bioactive component found in coffee, tea, soft drinks, and energy drinks. Although the daily intake of a cup of coffee, which contains 400 mg of Caf, is safe for health, caffeine can cause significant clinical pharmacokinetic interactions with many drugs (Belayneh and Molla, 2020). Low-dose aspirin (ASA) is recommended for the prevention of atherothrombotic and cardiovascular events. However, the antiplatelet action of ASA-induced drug interactions has high clinical importance (Saxena et al., 2013). Simvastatin (Sim) is a first-line antilipidemic drug commonly prescribed to reduce the risk of cardiovascular events, but it can also cause a variety of serious adverse effects such as myalgia, myopathy, and hepatotoxicity. Several drugs and compounds have been reported to disturb the metabolism of Sim, leading to an increase in the risk of these adverse effects (Kellick et al., 2014). Therefore, we examined the effect of MA combined with four commonly encountered compounds, APAP, ASA, Caf, and Sim, on profiles of CYPs and related genes in Caco-2 cells. On their own, APAP, ASA, Caf, and Sim did not change the expression of CYP1A2 or CYP2C19 mRNA, but when combined with MA, each of ASA, Caf, and Sim synergistically increased the expression of CYP1A2 and CYP2C19 mRNA (Fig. 5A and B). Although MA also induced CYP1A2 on its own, it had the opposite effect on CYP2C19, inhibiting the expression (Fig. 3B). The expression of CYP2D6 mRNA was not altered by all treatments, either as single compounds or in combination with MA (Fig. 5C). APAP and ASA did not change the expression of CYP3A4 mRNA (Fig. 5D) either on their own or in combination with MA. The treatment of Caco-2 cells with Caf both alone and in combination with MA suppressed the expression of CYP3A4 mRNA to similar levels, while the combination of Sim with the highest dose of MA (500 µg/ml) suppressed the expression of CYP3A4 mRNA (Fig. 5D). In a previous study, the Chinese traditional medicine formulation BuChang NaoXinTong, which contains MA twig and some other herbs, was shown to induce the expression of CYP2C19 in human primary hepatocytes and HepG2 cells via activation of PXR (Sun et al., 2016). Similarly, an aqueous extract of mulberry leaves increased CYP3A4 activity, while an ethanol extract of mulberry leaves reduced CYP1A2, CYP2D6, and CYP2C19 activity in a rat model (Sheng et al., 2021). Therefore, there are several other factors such as the part of the MA plant being used, the extraction method employed, and the combination of MA with other compounds and plants that could affect the regulatory profiles of CYP expression.
 | Figure 4. Effects of MA fruit, C3G, and rutin on phase II metabolizing enzymes and transporters mRNA expression in Caco-2 cells. *p < 0.05, **p < 0.001 versus control (DMSO). Keto, 10 µM ketoconazole; Rif, 10 µM rifampicin; C3G, 1 and 10 µM C3G; Rutin, 1 and 10 µM rutin; MA, 125, 250, and 500 µg/ml MA fruit (n = 4–5). [Click here to view] |
Effect of combinations of MA fruit and common drugs on phase II conjugation enzymes and transporters
UGT1A6 is an important member of the human UGT1 family expressed in the liver and extrahepatic tissues, including the intestine, that biotransforms planar phenols and arylamines (Walter Bock and Köhle, 2005). It is responsible for APAP-glucuronide conjugation (Zhao and Pickering, 2011). When tested on their own, both APAP and ASA induced the expression of UGT1A6 mRNA (Fig. 6A) while MA suppressed UGT1A6 expression (Fig. 4A). In combination, MA and APAP showed increased induction of UGT1A6 expression (Fig. 6A). On the other hand, the combination of MA with ASA, Caf, and Sim resulted in the inhibition of the expression of UGT1A6. At present, there is limited information about the effect of MA on UGTs. One report found that morusin, a flavonoid isolated from the root bark of MA, inhibited UGTs in liver microsomes of humans, rats, dogs, monkeys, and minipigs (Shi et al., 2016).
 | Figure 5. Combinatorial effects of MA fruit and common drugs on CYPs in Caco-2 cells. *p < 0.05, **p < 0.001 versus control (DMSO); @p < 0.05 versus ASA; #p < 0.05 versus Caf; $p < 0.05 versus Sim. APAP, 5 mM paracetamol; ASA, 5 mM aspirin; Caf, 50 µM caffeine; Sim, 10 µM simvastatin; MA, 125, 250, and 500 µg/ml MA fruit (n = 4–5). [Click here to view] |
NAT1 is responsible for the metabolism of pharmacological agents and environmental toxicants (Walker et al., 2009). All treatments, including singly applied APAP, ASA, Caf, and Sim and their combinations with MA, extensively suppressed expression of NAT1 (Fig. 6B).
 | Figure 6. Combinatorial effects of MA fruit and common drugs on phase II metabolizing enzymes in Caco-2 cells. *p < 0.05, **p < 0.001 versus control (DMSO); @p < 0.05 versus APAP; #p < 0.05 versus ASA; $p < 0.05 versus Caf; &p < 0.05 versus Sim. APAP, 5 mM paracetamol; ASA, 5 mM aspirin; Caf, 50 µM caffeine; Sim, 10 µM simvastatin; MA, 125, 250, and 500 µg/ml MA fruit (n = 4–5). [Click here to view] |
SULT1A1 is responsible for the sulfation of APAP (Rasool et al., 2019). APAP significantly elevated the expression of SULT1A1 mRNA (Fig. 6C), while ASA, Caf, and Sim did not. Interestingly, ASA, Caf, and Sim in combination with MA significantly induced SULT1A1 expression.
OATP1B1 transports clinical drugs into cells (Zhang et al., 2007). Flavonoids have been reported to inhibit the uptake of OATP1B1 substrates (Wang et al., 2005). APAP, ASA, Caf, Sim, APAP+MA, and ASA+MA all markedly suppressed OATP1B1 expression, while Caf+MA and Sim+MA controversially showed synergistic induction of OATP1B1 expression (Fig. 7A). The ethanol extract of MA root was reported to downregulate ABCB1 expression in MCF7 cells (Choi et al., 2013).
ABCB1 is involved in the intestinal absorption of aspirin (Li et al., 2017). ASA significantly suppressed the expression of ABCB1 mRNA (Fig. 7B). However, ASA in combination with the highest dose of MA strongly induced the expression of ABCB1 mRNA in Caco-2 cells. To date, information about the effect of MA on drug transporters is very limited. It would be interesting to unravel the effect of MA and its constituents on the expression and activity of other drug transporters.
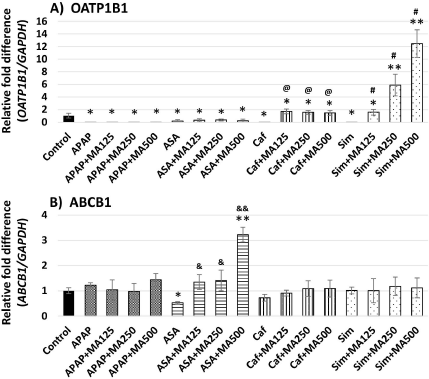 | Figure 7. Combinatorial effects of MA fruit and common drugs on drug transporters in Caco-2 cells. *p < 0.05, **p < 0.001 versus control (DMSO); &p < 0.05, &&p < 0.001 versus ASA; @p < 0.05 versus Caf; #p < 0.05 versus Sim. APAP, 5 mM paracetamol; ASA, 5 mM aspirin; Caf, 50 µM caffeine; Sim, 10 µM simvastatin; MA, 125, 250, and 500 µg/m MA fruit (n = 4–5). [Click here to view] |
CONCLUSION
MA fruit, C3G, and rutin did not alter the expression of CYP2D6 and SULT1A1 in Caco-2 cells. Nevertheless, they down-regulated the expression of CYP2C19, CYP3A4, UGT1A6, NAT1, and OATP1B1, and MA upregulated the expression of CYP1A2. The combination of MA with aspirin, caffeine, or simvastatin showed additional induction of CYP1A2 and CYP2C19, while the combination of MA and paracetamol synergistically upregulated the expression of UGT1A6 and SULT1A1. Therefore, the consumption of MA fruit or mulberry supplements poses a risk for drug interactions via the modulation of CYP and conjugation enzyme-associated metabolism and OATP1B1- or ABCB1-mediated drug transport in the human intestine.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit it to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdulla MA, Ali HM, Ahmed KAA, Noor SM, Ismail S. Evaluation of the anti-ulcer activities of Morus alba extracts in experimentally-induced gastric ulcer in rats. Biomed Res, 2009; 20:35–9.
Alvin G, Catambay N, Vrgara A, Jamora MJ. A comparative study of the safety and efficacy of 75% mulberry (Morus alba) extract oil versus placebo as a topical treatment for melasma: a randomized, single-blind, placebo-controlled trial. J Drugs Dermatology, 2011; 10:1025–31.
Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants, 2017; 6(4):42; http//doi.org/10.3390/plants6040042 CrossRef
Belayneh A, Molla F. The effect of coffee on pharmacokinetic properties of drugs: a review. BioMed Res Int, 2020; 2020;7909703; http//doi.org/10.1155/2020/7909703 CrossRef
Ben Sghaier M, Pagano A, Mousslim M, Ammari Y, Kovacic H, Luis J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed Pharmacother, 2016; 84:1972–8. CrossRef
Brück S, Strohmeier J, Busch D, Drozdzik M, Oswald S. Caco-2 cells - expression, regulation and function of drug transporters compared with human jejunal tissue. Biopharm Drug Dispos, 2017; 38:115–26. CrossRef
Chan EWC, Lye PY, Wong SK. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med, 2016; 14:17–30.
Chang BY, Koo BS, Kim SY. Pharmacological activities for Morus alba L., focusing on the immunostimulatory property from the fruit aqueous extract. Foods, 2021; 10:1966. http//doi.org/10.3390/foods10081966 CrossRef
Chen C, Mohamad Razali UH, Saikim FH, Mahyudin A, Mohd Noor NQ. Morus alba L. plant: bioactive compounds and potential as a functional food ingredient. Foods, 2021; 10(3):689; http//doi.org/10.3390/foods10030689 CrossRef
Chen L, Liu L, Chen Y, Liu M, Xiong Y, Zhang H, Huang S, Xia C. Modulation of transporter activity of OATP1B1 and OATP1B3 by the major active components of Radix Ophiopogonis. Xenobiotica, 2019; 49:1221–8. CrossRef
Choi MK, Jin QR, Choi YL, Ahn SH, Bae MA, Song IS. Inhibitory effects of ketoconazole and rifampin on OAT1 and OATP1B1 transport activities: considerations on drug-drug interactions. Biopharm Drug Dispos, 2011; 32:175–84. CrossRef
Choi YK, Cho SG, Choi HS, Woo SM, Yun YJ, Shin YC, Ko SG. JNK1/2 Activation by an extract from the roots of Morus alba L. reduces the viability of multidrug-resistant MCF-7/Dox cells by inhibiting YB-1-dependent MDR1 expression. Evid Based Complement Altern Med, 2013; 2013:741985; http//doi.org/10.1155/2013/741985 CrossRef
Chu Q, Lin M, Tian X, Ye J. Study on capillary electrophoresis-amperometric detection profiles of different parts of Morus alba L. J Chromatogr A, 2006; 1116(1-2):286–90. CrossRef
Darney K, Lautz LS, Béchaux C, Wiecek W, Testai E, Amzal B, Dorne JLCM. Human variability in polymorphic CYP2D6 metabolism: implications for the risk assessment of chemicals in food and emerging designer drugs. Environ Int, 2021; 156:106760; http//doi.org/10.1016/j.envint.2021.106760 CrossRef
Du QZ, Zheng J, Xu Y. Composition of anthocyanins in mulberry and their antioxidant activity. J Food Compos Anal, 2008; 21:390–5. CrossRef
Fang HL, Strom SC, Ellis E, Duanmu Z, Fu J, Duniec-Dmuchowski Z, Falany CN, Falany JL, Kocarek TA, Runge-Morris M. Positive and negative regulation of human hepatic hydroxysteroid sulfotransferase (SULT2A1) gene transcription by rifampicin: roles of hepatocyte nuclear factor 4α and pregnane X receptor. J Pharmacol Exp Ther, 2007; 323:586–98. CrossRef
Ferrari D, Speciale A, Cristani M, Fratantonio D, Molonia MS, Ranaldi G, Saija A, Cimino F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol Lett, 2016; 264:51–8. CrossRef
Fugh-Berman A. Herb-drug interactions. Lancet, 2000; 355:134–8. CrossRef
Gamage NU, Tsvetanov S, Duggleby RG, McManus ME, Martin JL. The structure of human SULT1A1 crystallized with estradiol: an insight into active site plasticity and substrate inhibition with multi-ring substrates. J Biol Chem, 2005; 280:41482–6. CrossRef
Glaeser H, Drescher S, Eichelbaum M, Fromm MF. Influence of rifampicin on the expression and function of human intestinal cytochrome P450 enzymes. Br J Clin Pharmacol, 2005; 59:199–206. CrossRef
Gow JM, Hodges LM, Chinn LW, Kroetz DL. Substrate-dependent effects of human ABCB; coding polymorphisms. J Pharmacol Exp Ther, 2008; 325:435–42. CrossRef
Gryn-Rynko A, Bazylak G, Olszewska-Slonina D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed Pharmacother, 2016; 84:628–36. CrossRef
Hansawasdi C, Kawabata J. α-Glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia, 2006; 77(7-8):568–73. CrossRef
Hunyadi A, Martins A, Hsieh TJ, Seres A, Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One, 2012; 7:e50619. CrossRef
Hussain F, Rana Z, Shafique H, Malik A, Hussain Z. Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: a review. Asian Pac J Trop Biomed, 2017; 7(10):950–6. CrossRef
Iftikhar M, Iftikhar A, Zhang H, Gong L, Wang J. Transport, metabolism and remedial potential of functional food extracts (FFEs) in Caco-2 cells monolayer: A review. Food Res Int, 2020; 136:109240. https://doi.org/10.1016/j.foodres.2020.109240. CrossRef
Kar A, Mukherjee PK, Saha S, Bahadur S, Ahmmed SK, Pandit S. Possible herb-drug interaction of Morus alba L.-A potential anti-diabetic plant from Indian Traditional medicine. Indian J Trad Knowl, 2015; 14(4):626–31.
Kayesh R, Farasyn T, Crowe A, Liu Q, Pahwa S, Alam K, Neuhoff S, Hatley O, Ding K, Yue W. Assessing OATP1B1- and OATP1B3-mediated drug-drug interaction potential of vemurafenib using R-value and physiologically-based pharmacokinetic models. J Pharm Sci, 2021; 110:314–24. CrossRef
Kellick KA, Bottorff M, Toth PP. A clinician’s guide to statin drug-drug interactions. J Clin Lipid, 2014; 8(3):30–46. CrossRef
van de Kerkhof EG, de Graaf IAM, Ungell ALB, Groothuis GMM. induction of metabolism and transport in human intestine: validation of precision-cut slices as a tool to study induction of drug metabolism in human intestine in vitro. Drug Metab Dispos, 2008; 36:604–13. CrossRef
Kim SB, Kim KS, Kim DD, Yoon IS. Metabolic interactions of rosmarinic acid with human cytochrome P450 monooxygenases and uridine diphosphate glucuronosyltransferases. Biomed Pharmacother, 2019; 110:111–7. CrossRef
Kükürt A, Gelen V, Ba?er ÖF, Deveci HA, Karapehlivan M. Thiols: role in oxidative stress-related disorders. In: Atukeren P (ed.). Accenting lipid peroxidation. IntechOpen, London, UK, 2021; http//doi.org/ 10.5772/intechopen.96682 (Accessed 23 February 2022) CrossRef
Kuncharoenwirat N, Chatuphonprasert W, Jarukamjorn K. Effects of phenol red on rifampicin-induced expression of cytochrome P450s enzymes. Pharmacophore, 2020; 11(3):13–20.
Li X, Zhao K, Ma N, Sun S, Miao Z, Zhao Z. Association of ABCB1 promoter methylation with aspirin exposure, platelet function, and clinical outcomes in Chinese intracranial artery stenosis patients. Eur J Clin Pharmacol, 2017; 73:1261–9. CrossRef
Mei M, Ruan JQ, Wu WJ, Zhou RN, Lei JP, Zhao HY, Yan R, Wang YT. In vitro pharmacokinetic characterization of mulberroside A, the main polyhydroxylated stilbene in mulberry (Morus alba L.), and its bacterial metabolite oxyresveratrol in traditional oral use. J Agric Food Chem, 2012; 60(9):2299–308. CrossRef
Meszaros P, Hummel I, Klappe K, Draghiciu O, Hoekstra D, Kok JW. The function of the ATP-binding cassette (ABC) transporter ABCB1 is not susceptible to actin disruption. Biochim Biophys Acta - Biomembr, 2013; 1828:340-51. CrossRef
Netsch MI, Gutmann H, Schmidlin CB, Aydogan C, Drewe J. Induction of CYP1A by green tea extract in human intestinal cell lines. Planta Med, 2006; 72:514–20. CrossRef
Nikulin SV, Tonevitsky EA, Poloznikov AA. Effect of ketoconazole on the transport and metabolism of drugs in the human liver cell model. Russ Chem Bull, 2017; 66:150–5. CrossRef
Novotna A, Korhonova M, Bartonkova I, Soshilov AA, Denison MS, Bogdanova K, Kolar M, Bedlar M, Dvorak Z. Enantiospecific effects of ketoconazole on aryl hydrocarbon receptor. PLoS One, 2014; 9:e101832. CrossRef
Pahwa S, Alam K, Crowe A, Farasyn T, Neuhoff S, Hatley O, Ding K, Yue W. Pretreatment with rifampicin and tyrosine kinase inhibitor dasatinib potentiates the inhibitory effects toward OATP1B1- and OATP1B3-Mediated Transport. J Pharm Sci, 2017; 106:2123–35. CrossRef
Pandurangan M, Kim DH. ZnO nanoparticles augment ALT, AST, ALP and LDH expressions in C2C12 cells. Saudi J Biol Sci, 2015; 22:679–84. CrossRef
Park KT, Kim JK, Hwang D, Yoo Y, Lim YH. Inhibitory effect of mulberroside A and its derivatives on melanogenesis induced by ultraviolet B irradiation. Food Chem Toxicol, 2011; 49(12):3038–45. CrossRef
Rana R, Chen Y, Ferguson SS, Kissling GE, Surapureddi S, Goldstein JA. Hepatocyte nuclear factor 4α regulates rifampicin-mediated induction of CYP2C genes in primary cultures of human hepatocytes. Drug Metab Dispos, 2010; 38:591–9. CrossRef
Rasool MI, Bairam AF, Gohal SA, El Daibani AA, Alherz FA, Abunnaja MS, Alatwi ES, Kurogi K, Liu M. Effects of the human SULT1A1 polymorphisms on the sulfation of acetaminophen, O-desmethylnaproxen, and tapentadol. Pharmacol Reports, 2019; 71:257–65. CrossRef
Saxena A, Balaramnavar VM, Hohlfeld T, Saxena AK. Drug/drug interaction of common NSAIDs with antiplatelet effect of aspirin in human platelets. Eur J Pharmacol, 2013; 721(1-3):215–24. CrossRef
Sepulveda JL. 2019. Chapter 10: Challenges in routine clinical chemistry analysis: proteins and enzymes. In: Dasgupta A, Sepulveda JL, ed. Accurate results in the clinical laboratory 2nd edition. New York: Elsevier 141–63. http//doi.org/10.1016/B978-0-12-813776-5.00010-8 CrossRef
Sheng C, Shi Xiaoyan, Ding Z, Chen Y, Shi Xiaoqian, Wu Y, Zhang W, Chen W. Effects of mulberry leaf extracts on activity and mRNA expression of five cytochrome P450 enzymes in rat. Brazilian J Pharm Sci, 2021; 57:e18059. CrossRef
Shi X, Yang S, Zhang G, Song Y, Su D, Liu Y, Guo F, Shan L, Cai J. The different metabolism of morusin in various species and its potent inhibition against UDP-glucuronosyltransferase (UGT) and cytochrome p450 (CYP450) enzymes. Xenobiotica, 2016; 46:467–76. CrossRef
Sorf A, Hofman J, Ku?era R, Staud F, Ceckova M. Ribociclib shows potential for pharmacokinetic drug-drug interactions being a substrate of ABCB1 and potent inhibitor of ABCB1, ABCG2 and CYP450 isoforms in vitro. Biochem Pharmacol, 2018; 154:10-7. CrossRef
Sousa MC, Braga RC, Cintra BAS, de Oliveira V, Andrade CH. In silico metabolism studies of dietary flavonoids by CYP1A2 and CYP2C9. Food Res Int, 2013; 50:102–10. CrossRef
Sriset Y, Chatuphonprasert W, Jarukamjorn K. Bergenin attenuates sodium selenite-induced hepatotoxicity via improvement of hepatic oxidant-antioxidant balance in HepG2 cells and ICR mice. J Biol Act Prod from Nat, 2021; 11:97–115. CrossRef
Srovnalova A, Svecarova M, Kopecna Zapletalova M, Anzenbacher P, Bachleda P, Anzenbacherova E, Dvorak Z. Effects of anthocyanidins and anthocyanins on the expression and catalytic activities of CYP2A6, CYP2B6, CYP2C9, and CYP3A4 in primary human hepatocytes and human liver microsomes. J Agric Food Chem, 2014; 62:789–97. CrossRef
Sun H, Lou XY, Wu XY, Wang H, Qu Q, Tan SL, Ruan JS, Qu J, Chen H. Up-regulation of CYP2C19 expression by BuChang NaoXinTong via PXR activation in HepG2 cells. PLoS One, 2016; 11:e0160285. CrossRef
Takano M, Hasegawa R, Fukuda T, Yumoto R, Nagai J, Murakami T. Interaction with P-glycoprotein and transport of erythromycin, midazolam and ketoconazole in Caco-2 cells. Eur J Pharmacol, 1998; 358:289–94. CrossRef
Toes MJ, Jones AL, Prescott L. Drug interactions with paracetamol. Am J Ther, 2005; 12(1):56–66. CrossRef
Udomsak W, Chatuphonprasert W, Jarukamjorn K. Dill shows potential for herb-drug interactions via up-regulation of CYP1A2, CYP2C19, SULT1A1, NAT2 and ABCB1 in Caco-2 Cells. Pakistan J Biol Sci, 2022; 25(1):56–66. CrossRef
Uppugunduri RS, Daali Y, Desmeules J, Dayer P, Krajinovic M, Ansari M. Transcriptional regulation of CYP2C19 and its role in altered enzyme activity. Curr Drug Metab, 2012; 13:1196–204. CrossRef
Vaessen SFC, van Lipzig MMH, Pieters RHH, Krul CAM, Wortelboer HM, van de Steeg E. Regional expression levels of drug transporters and metabolizing enzymes along the pig and human intestinal tract and comparison with Caco-2 cells. Drug Metab Dispos, 2017; 45:353–60. CrossRef
Walker K, Ginsberg G, Hattis D, Johns DO, Guyton KZ, Sonawane B. Genetic polymorphism in N-acetyltransferase (NAT): population distribution of NAT1 and NAT2 activity. J Toxicol Environ Heal Part B, 2009; 12:440–72. CrossRef
Walter Bock K, Köhle C. UDP-glucuronosyltransferase 1A6: structural, functional, and regulatory aspects-Phase II conjugation enzyme and transportataion system. Method Enzymol, 2005; 400:57–75. CrossRef
Wang X, Wolkoff AW, Morris ME. Flavonoids as a novel class of human organic anion-transporting polypeptide OATP1B1 (OATP-C) modulators. Drug Metab Dispos, 2005; 33:1666–72. CrossRef
Witham KL, Minchin RF, Butcher NJ. Role for human arylamine N-acetyltransferase 1 in the methionine salvage pathway. Biochem Pharmacol, 2017; 125:93–100. CrossRef
Zafar MS, Muhammad F, Javed I, Akhtar M, Khaliq T, Aslam B, Waheed A, Yasmin R, Zafar H. White mulberry (Morus alba): a brief phytochemical and pharmacological evaluations account. Int J Agric Biol, 2013; 15(3):612–20.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther, 2013; 138:103–41. CrossRef
Zhang H, Ma ZF, Luo X, Li X. Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: a mini-review. Antioxidants, 2018; 7:69; http//doi.org/10.3390/antiox7050069 CrossRef
Zhang W, He YJ, Gan Z, Fan L, Li Q, Wang A, Liu ZQ, Deng S, Huang YF, Xu LY, Zhou HH. OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin Exp Pharmacol Physiol, 2007; 34:1240–4. CrossRef
Zhang X, Song J, Shi X, Miao S, Li Y, Wen A. Absorption and metabolism characteristics of rutin in Caco-2 cells. Sci World J, 2013; 2013:382350; http//doi.org/10.1155/2013/382350 CrossRef
Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metab Rev, 2011; 43:41–52. CrossRef
Zhao S, Park CH, Li X, Kim YB, Yang J, Sung GB, Park NI, Kim S, Park SU. Accumulation of rutin and betulinic acid and expression of phenylpropanoid and triterpenoid biosynthetic genes in mulberry (Morus alba L.). J Agric Food Chem, 2015; 63:8622–30. CrossRef
Zhou SF, Wang B, Yang LP, Liu JP. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab Rev, 2010; 42:268–354. CrossRef