INTRODUCTION
An elderly person with a robust or healthy condition was an expectation for everyone. Data showed that the number of elderly people in the world was 10% in 2019 and the prediction is 15% in 2040 (National Institute on Aging, 2019). In Indonesia, according to the surveillance in the year 2010, people aged over 60 years were about 7.6%; the prediction in the year 2020 was 12%, and in the year 2050 it is expected to be 25% (Piesse, 2015). Therefore, the prediction of elderly people’s growth in Indonesia is faster than the rest of the world.
In developing countries, there is an increase in chronic noncommunicable diseases in people caused by lifestyle changes, such as diabetes mellitus, heart diseases, and cancer, and these also include aging (National Institute on Aging, 2019). Biological defects, such as enzyme defects, could accumulate damaged products. However, recently, the most known cause of aging is damage due to oxidative stresses (Engwa, 2018). On the other hand, increased free radicals also lead to cancer growth (Althubiti et al., 2014).
There are some aging theories known; among them is the mitochondrial theory (Neyrinck et al., 2017). The aging process proofed can be treated by calorie restriction and inhibition of target of rapamycin. The background mechanisms are different (Althubiti et al., 2014). The growth increase of elderly people is considered to increase the elderly frail people who are becoming an unproductive population.
Recently, it has been known that Spirulina has so many healing functions to overcome oxidative stresses correlated with the aging process (Gad and Aly, 2010; Neyrinck et al., 2017). Spirulina platensis (SP) is abundant in the Indonesian seas and is widely cultivated and harvested. There are many commercial products of Spirulina due to its benefits, but their roles in cellular development and degeneration are yet to be proven experimentally. Here, an attempt has been made to reveal the effect of Spirulina on viability in cells and used a mesenchymal stem cells (MSCs) culture as a model treated with hydroperoxide together with a SP ethanol extract.
MATERIALS AND METHODS
This experimental study consisted of two steps: firstly, extraction of SP from the Java Sea and phytochemical analysis and, secondly, treatment of MSC culture with H2O2 with and without SP (negative control) and culture with SP and without H2O2 (positive control). Identification of surface markers, proliferation, and aging were examined to understand the protective effect of SP on MSC. Markers measured were MSC surface markers (CD73, CD90, and CD105), cellular viability, and also the docking of SP on HIF-1 alpha.
Preparation of the extract and phytochemical analysis
SP powder (100 g) was macerated in 95% ethanol (1 l) for 48 hours at room temperature. The solution was filtered, and the precipitate was macerated again at 95% with ethanol (1 l), and after the last maceration, the solution was dried by a rotary evaporator. Phytochemical examinations were carried out for the extracts according to the standard methods (Idakiev and Baecker, 2018).
Phytochemistry test and thin-layer chromatography (TLC)
Phytochemistry analysis was conducted for these compounds: a flavonoid test with the Willstatter method, a saponin test with a standard test from the Ministry of Health, an alkaloid test with the Culvenor-Fitzgerald method, a steroid test based on the Liebermann-Burchard method, a terpenoid assay with the Robinson method, and a total flavonoid with the Chang test.
Molecular docking of Spirulina
In this research, SP compounds were screened based on their interaction with HIF-1, using a computer software application (in silico method) to determine the best compound. Analysis and screening were based on the Gibbs free energy (?G) values, affinity, conformation of the structure, and hydrogen bonding interaction between the compounds and the target protein. HIF-1 alpha (PDB ID 5LAS) was used as the target protein and downloaded from the Protein Data Bank (PDB). Its coordinates for the X-ray crystal structure of the HIF-1 alpha were retrieved from the Research Collaboratory for Structural Bioinformatics PDB, and hydrogen ions were added after download. The ligand molecules were constructed using the Build Fragment tool and minimizing the energy for 1,000 iterations until reaching a convergence equal to or lower than 0.01 kcal/mol Å. The docking experiment on HIF1 alpha (PDB ID 5LAS) was carried out by suitably positioning the energy-minimized ligand in the active site while carefully monitoring nonbonded interactions of the ligand–enzyme assembly. The docking experiment on HIF-1 alpha was carried out using the AutoDock 4.2 software.
MSCs maintenance culture
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) were thawed at 37°C for 2 minutes and subsequently transferred into a 5 ml culture medium [Dulbecco’s Modified Eagle Medium (DMEM) + 10% fetal bovine serum + 1% antibiotic antimycotic] (Life Technologies, USA) to remove the cryoprotectant (dimethyl sulfoxide). The cell mixture was centrifuged at 650 g for 5 minutes (low break). The supernatant was discarded, and the pellet was resuspended using a 1 ml culture medium. The cells were then cultured in a T-75 flask in a CO2 incubator at 37°C (seeding density 5,000 cells/cm2) (Lee et al., 2016; Wang et al., 2016).
MSCs harvesting
MSCs were harvested after 80%–90% confluence. After removing the spent medium, the cells were washed twice using PBS. Then, it was added with 0.5% Trypsin-ethylenediaminetetraacetic acid (Life Technologies) and incubated at 37°C for 5 minutes to detach the cells. The culture medium was then added to stop the enzymatic process. After that, the cell suspension was centrifuged at 650 g for 5 minutes (low break). The liquid supernatant was discarded and the pellet of cells resuspended using 1 ml of culture medium (Facchin et al., 2018).
H2O2 and Spirulina treatments
The cells were seeded in 6-well plates with an initial density of 15,000 cells/ cm2. Then, the plates were incubated in a CO2 incubator at 37°C for 3 days. After full confluence, the media were removed and then replaced with fresh media containing Spirulina (final concentration 125 ng/ml). After 24 hours of Spirulina treatment, the media were removed and then replaced with serum-free culture media (DMEM + 1% antibiotic antimycotic) containing H2O2 (final concentration 100, 300 μM). The cells were then incubated for the next 24 hours before being harvested for further analyses.
Cellular viability measurement
The cells and viability were assessed in a hemocytometer under an inverted microscope (Nikon, Japan). Trypan blue (Life Technologies) staining was used to distinguish viable cells (transparent) and dead cells (blue-colored).
Surface markers analysis
An amount of 105 cells was stained with the Human MSC Analysis Kit (BD Biosciences, USA) according to the manufacturer’s protocol. The cells were stained for 30 minutes in the dark and then washed with PBS. Surface markers (CD73, CD90, CD105, and Lin) were analyzed using a flow cytometer (FACSAria III, BD Biosciences).
RESULTS
Phytochemistry test
The phytochemistry test for the concentrated ethanol extract of SP showed a positive result for the metabolites of saponin, alkaloids, phenols, flavonoids, and glycosides (Table 1). This result is consistent with high-resolution mass spectrometry analysis and confirms that cyanobacterium microalga SP contains phycocyanin. Phytochemical analysis results (Table 1) show that the phytochemical compounds of the ethanol extract of SP contain terpenes, flavonoids, and phenolic compounds.
Thin-layer chromatography (TLC)
The SP ethanol extract was run through TLC and showed a result of six types of ingredients (Table 2), and also the phytochemistry test showed positive results for polyphenol, alkaloid, and polysaccharides.
Molecular docking
Bioinformatic examination of SP docking to HIF-1 alpha showed firstly the active site of HIF-1 in the green circle and DNA binding in the red circle (Fig. 1).
The phenolic profile of SP using high-performance liquid chromatography showed the presence of several phenolic acids, especially caffeic, o-coumaric, gallic, and other bioactive molecules. This molecule was docked with HIF-1 properties. The results of docking are shown in Table 3 and Figure 1. Lipinski’s rule based on the measurement of the Gibbs energy (?G value), affinity (pKi), and number of H bond was utilized for in silico molecular docking of 3,4-OH benzoic acid. The results are summarized in Table 3. Validation with structure using AutoDock 4.2 shows that the phenolic benzoate with an OH hydroxyl group in 3,4 has complex energy with HIF-1 with ΔG values of −8.4899 and −7.3452 mol/kcal, respectively. This value is lower than others and has a ΔG value of −7.9898 mol/kcal. The value of ΔG indicates that benzoic acid has complex energy that is more stable than gallic acid.
 | Table 1. Phytochemical tsest results of ethyl acetate and ethanol extract of SP. [Click here to view] |
 | Table 2. Extraction of SP. [Click here to view] |
 | Figure 1. The active site of HIF-1 in the green circle and DNA binding in the red circle. [Click here to view] |
The presence of phenolic and other bioactive molecules like phycocyanin is detected in SP. Those molecules were docked with HIF-1 properties. The results of docking are shown in Figure 2.
The docked ligand into the cavity and the binding interactions are shown in Figure 2. Thus, the phycocyanobilin compound is a promising candidate for inhibiting the DNA binding of HIF-1 agents and should be considered as the lead compound in the extract of SP. The stability and affinity of the ligand complex with the active side were influenced by the bond distance and complex score. In the figures, the phycocyanobilin could change the conformation of all receptor targets in the DNA binding site of HIF-1, or, in other words, it is able to enter the binding site of the receptor-binding target of HIF-1
The docked ligand into the cavity and the binding interactions are shown in Figure 3. Thus, canthaxanthin compounds are promising candidates for treatment and should be considered the lead compounds in the extract of SP. The stability and affinity of the ligand complex with the active side were influenced by the bond distance and complex score. In the figures, the phenolic compound of canthaxanthin could change the conformation of all receptor target cavities or, in other words, it is able to enter the binding site of the receptor-binding target of HIF-1 alpha.
Effect of SP on MSCs culture exposed to oxidative stress
Treatments for oxidative stress were run using hydroperoxide 100 and 300 μM in the culture of UC MSCs. Before hydroperoxide treatment, the thawed MSCs were viability-tested before and after culturing.
 | Table 3. The compounds of the SP ethanol extract. [Click here to view] |
 | Figure 2. Complex interaction of HIF-1 molecule phycocyanobilin in DNA binding. [Click here to view] |
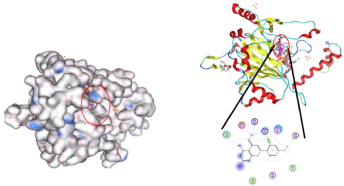 | Figure 3. Complex interaction of HIF-1 molecule canthaxanthin in the active site. [Click here to view] |
Viability of MSCs after thawing and harvesting
Figure 5 shows the formation of MSCs after 3 days in culture. After thawing, MSCs were measured for their viability before culturing; this showed that MSC viability was 84.13% after thawing (Table 4). These thawed MSCs were then cultured for 3 days and followed by a viability test using trypan blue and showed increased viability, 97.8%.
Amount and viability of MSCs after culturing in T-flask 75
MSCs viability is shown in Table 4.
Flow cytometry analysis
The stemness of MSCs was confirmed from positive cells expressing CD73, CD90, and CD105 positive cells as much as 99.9%, 99.9%, and 96,8%, respectively. The cells did not express hematopoietic markers (CD45, CD34, CD11b, CD19, and HLA-DR).
 | Figure 4. MSCs morphology NP 2 in T75 (day 3). [Click here to view] |
 | Figure 5. Histogram data of CD73 positive cells after treatment with SP (125 ng/ml) and H2O2 (300 μM). [Click here to view] |
H2O2 and Spirulina treatment
CD73 examination
Using antibody anti-CD73 to prove MSCs, the result of flow cytometry showed reduction or improvement in MSC viability. In this test, treated MSCs were compared to the negative control, positive control, and control and unstained MSCs (Fig. 5). The result showed that CD73 after treatment of H2O2 was decreased in amount (0.6%) but not significantly (Table 5). Treatment of only SP without H2O2 increased only 0.1%, same as SP + H2O2 treatment, 0.1%, but not significantly, Table 5.
 | Table 4. MSCs viability cultured in T-flask 75. [Click here to view] |
CD90 examination
Examination of CD90 used antibody anti-CD90 to figure and detect this MSC surface marker. The result shows that exposure to H2O2 300 μM only reduced it 0.4% to become 99.6% of CD90. In addition, treatment with SP only increased from 0.1% became 99.7% (Fig. 6 and Table 5).
CD105 examination
Examination of the CD105 surface marker showed a decrease in the treatment of H2O2, 0.83%, compared to control. This CD105 treatment with H2O2 and SP increased marker CD105, 0.9%, but not significantly (Fig. 7 and Table 5).
Rest of flow cytometry
The rest of the flow cytometry is shown in Table 5.
Viability
Measurement of cell viability using trypan blue showed variation in the result. Treatment of 300 μM H2O2 leads to a decrease in MSC viability markedly (40%). Conversely, 125 ng/ml SP addition could only slightly increase (25%) MSC viability (Figs. 8 and 9).
 | Table 5. Flow cytometry after treatments of MSCs. [Click here to view] |
 | Figure 6. Histogram data of CD90 positive cells after treatment with SP (125 ng/ml) and H2O2 > (300 μM). [Click here to view] |
 | Figure 7. Histogram data of CD105 positive cells after treatment with SP (125 ng/ml) and H2O2 (300 μM). [Click here to view] |
DISCUSSION
In this research, SP was extracted by ethanol, followed by treatment of SP in H2O2-treated UC MSCs. Some researchers have studied the effect of SP as superfood such as nutrient contents: repressed pathogenic microorganisms, and increased good microorganisms, repairing tissue, hematopoietic fertility anti-cancer, also antioxidant. However, the mechanisms were still uncovered (Seyidoglu et al., 2017). This research showed that there are two compounds of Spirulina which interact with HIF-1 based on docking analysis. The HIF-1 protein is the master of the transcription factor in hypoxia and oxidative stress condition, which regulates almost 200 functional proteins. One of these proteins is CD73 (Tan et al., 2019). This research also revealed that phycocyanobilin and canthaxanthin dock with HIF-1. Therefore, further exploration of this substance is needed. HIF-1 alpha was chosen to be investigated related to SP because some researchers correlated HIF-1 alpha to several diseases and oxidative stresses that have become the basis of many diseases, especially degenerative diseases. There was also a comparative study between SP and Spirulina fusiformis, but this research used the 2,2-diphenyl-1-picrylhydrazyl method; the Spirulina extraction used Aquadest and phosphate-buffered saline (Margiati et al., 2019).
In our studies, an extract with water could overcome oxidative stress in an aqueous medium such as carbonyl substances in cytoplasm or plasma or serum but not for malondialdehyde. Therefore, only a part of the cells could be healed.
The SP extract was used to overcome oxidative stress by H2O2. This result was not significant but tended to increase MSC viability based on the trypan blue test or CD73, CD90, and CD105 by flow cytometry. It is considered that the dose of H2O2 was not enough to create oxidative stress, although it was already referred to by some researchers. Some studies gave positive results in overcoming oxidative stress using the 125 ng/ml SP extract. However, in this study, it did not work but it might give have potential for further studies because increased viability was observed although it was not significant.
 | Figure 8. MSC viability measurement using trypan blue (S: 125 ng/ml Spirulina treatment). [Click here to view] |
 | Figure 9. Comparisons of MSC viability using trypan blue. [Click here to view] |
CONCLUSION
The contents of SP: phycocyanobilin and canthaxanthin were considered candidates to influence HIF-1 protein regulation. The effect of SP on H2O2 exposure tends to increase MSC viability using either trypan blue or CD73, CD90, and CD105 but not significantly. It needs further observation and exploration.
ACKNOWLEDGMENTS
The authors appreciate and thank the PDUPT Research Grant 2020 for funding this research. They also thank the Department of Medical Chemistry and IMERI-UI for facilitating the tools and place for this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Althubiti M, Lezina L, Carrera S, Jukes-Jones R, Giblett SM, Antonov A, Barlev N, Saldanha GS, Pritchard CA, Cain K, Macip S. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis, 2014; 5(11):e1528; doi:10.1038/cddis.2014.489 CrossRef
Engwa GA. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In: Phytochemicals - source of antioxidants and role in disease prevention, InTech, pp 50–73, 2018; doi:10.5772/intechopen.76719 CrossRef
Facchin F, Bianconi E, Romano M, Impellizzeri A, Alviano F, Maioli M, Canaider S, Ventura C. Comparison of oxidative stress effects on senescence patterning of human adult and perinatal tissue-derived stem cells in short and long-term cultures. Int J Med Sci, 2018; 15(13):1486–501; doi:10.7150/ijms.27181 CrossRef
Gad A, Aly M. Chemical composition and potential application of Spirulina platensis biomass. J Am Sci, 2010; 6(10):1238–91.
Idakiev H, Baecker S. Extraction of proteins and active substances from microalgae. INFORM, 2018; 29:22–5. CrossRef
Lee JH, Jung HK, Han YS, Yoon YM, Yun CW, Sun HY, Cho HW, Lee SH. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol Med Rep, 2016; 14(4):3777–84; doi:10.3892/mmr.2016.5706 CrossRef
Margiati D, Ramdani D, Wulandari AP. Comparative study of antioxidant phycocyanin extracts activity between S. platensis with S. fusiformis using DPPH method. Indo J Pharm Sci Technol, 2019; 6(2):52; doi:10.24198/ijpst.v6i2.11883 CrossRef
National Institute on Aging. Global health and ageing, 2019.
Neyrinck AM, Taminiau B, Walgrave H, Daube G, Cani PD, Bindels LB, Delzenne NM. Spirulina protects against hepatic inflammation in aging: an effect related to the modulation of the gut microbiota? Nutrients, 2017; 9(6)633; doi:10.3390/nu9060633 CrossRef
Piesse M. Social and demographic issues in Indonesia, 2015.
Seyidoglu N, Inan S, Aydin C. A prominent superfood: Spirulina platensis. In: Superfood and functional food - the development of superfoods and their roles as medicine, InTech, 2017; doi:10.5772/66118 CrossRef
Tan K, Zhu H, Zhang J, Ouyang W, Tang J, Zhang Y, Qiu L, Liu X, Ding Z, Deng X. CD73 expression on mesenchymal stem cells dictates the reparative properties via its anti-inflammatory activity. Stem Cells Int, 2019; 2019:8717694; doi:10.1155/2019/8717694 CrossRef
Wang N, Wang F, Gao Y, Yin P, Pan C, Liu W, Zhou Z, Wang J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J Pharmacol Sci, 2016; 132(3):192–200; doi:10.1016/j.jphs.2016.10.005 CrossRef