INTRODUCTION
Pain presents a major threat to survival and requires humans to perceive their environment effectively, identify hazards, act to prevent damage to one’s body, and promote effective recovery in the event of damage (Tabor et al., 2017). Pain is a fundamental experience associated with the perception of actual or potential harm to oneself. It can result in an infection, inflammation, peripheral tissue damage, or neural damage. In general, it is recognized that neurotransmitters, sodium channels, and neuromodulators mediate painful responses in the clinic, and in each of the different experimental models of nociception they may act differently (Bannon, 2001; Kopach et al., 2012). The evidence follows that a large proportion of patients with chronic pain can develop major depressive disorder compared to patients with other chronic medical conditions (Tenti et al., 2021).
Inflammation is a response of the organism against a harmful stimulus and is always associated with pain (da Silva et al., 2011). It takes place in vascularized connective tissue and involves vascular changes, cellular events, and the production of chemical mediators of inflammation. All these components of the system are closely linked and involve a complicated series of events including arteriolar dilation, increased vascular permeability in venules and capillaries, fluid exudate (including plasma proteins), and migration of leukocytes to the inflamed area (de Morais Lima et al., 2011; Trivellatograssi et al., 2013).
The existence of a relationship between chronic pain and depression is now widely accepted among researchers (Rojas-Corrales et al., 2003). Chronic pain and depression are two very frequent and debilitating concurrent conditions. They present a bidirectional longitudinal relationship due to the high probability of developing depression secondary to chronic pain and vice versa (Maallo et al., 2021). Various antidepressant drugs used to treat depression are effective for treating pain associated with damage to the nerves (neuropathic pain). At least a third of patients with neuropathic pain that received traditional antidepressants (like amitriptyline) had relief of moderate pain. There is also evidence that venlafaxine, a newer antidepressant, has similar effectiveness to traditional antidepressants. Nevertheless, approximately a fifth of the patients that receive these drugs for pain must stop treatment due to adverse effects. Neuropathic pain can be treated with antidepressants, and its effect is independent of any effect on depression (Gregory et al., 2014).
Aloysia polystachya (Griseb.) Moldenke (Verbenaceae), known commonly as “burrito,” is a perennial shrub that grows in Paraguay, northwestern Argentina, Bolivia, and Brazil. The leaves infusion of A. polystachya is used as a tonic, digestive, and carminative agent, for stomach pains and slow digestionz, against liver disorders, for the treatment of “empacho,” and as a tonic of the nerves (Consolini et al., 2011). In previous studies, we have described, among other effects, the sedative, anxiolytic, and antidepressant effects of the hydroalcoholic extract of A. polystachya leaves in mice and rats (Hellión-Ibarrola et al., 2006; Hellión-Ibarrola et al., 2008; Mora et al., 2005).
The presence of glycosides phenylethanoids, triterpenes/steroids, phenols/tannins, flavonoids, and traces of saponins and lactones has been reported as chemical components of the hydroalcoholic extract (Carmona et al., 2019; Pereira et al., 2019). Additionally, compounds such as terpenes, monoterpenes (carvacrol, carvone, eucarvone, limonene (−), limonene, α-pinene, sabinene (+), sabinene (−), thujone, α-thujone, α- (−) thujone, β-thujone, isothujone, y(+) isothujone, and sesquiterpenes are presents in the essential oil (Carmona et al., 2019). Data not published by our lab accounts for the presence of zinc, copper, calcium, and vitamins B1 and B2 in significant amounts.
Considering that the preclinical studies with A. polystachya demonstrated its anxiolytic and antidepressant activity (Hellión-Ibarrola et al., 2006; Hellión-Ibarrola et al., 2008; Mora et al., 2005) as well as its clinical validation as an anxiolytic in the treatment of anxiety (Carmona et al., 2019) and that antidepressant drugs such as amitriptyline are effective in the treatment of chronic pain, it is reasonable to evaluate the influence of A. polystachya on experimental pain as a potential alternative or complementary therapy with the use of medicinal plants. Therefore, this work aims to determine the influence of the oral administration of the crude extract of A. polystachya (CEAp) on different preclinical models of acute pain induced by harmful stimuli (mechanical pressure, 0.8% acetic acid, and thermal) and its influence on carrageenan-induced inflammation in mice.
MATERIALS AND METHODS
Plant material and extraction procedure
Cultivated samples of A. polystachya were collected in Colonia Potrero Sur y Mafussi de Pedro Juan Caballero, Paraguay. A voucher sample was authenticated and deposited at the Herbarium of the Faculty of Chemical Sciences UNA (Code C. Céspedes 953). The collected samples were air-dried, protected by sunlight, and conditioned in appropriated labeled containers. In traditional Paraguayan medicine, all medicinal plants are milled with a hardwood stick inside a wooden container and later placed in a container filled with tap water and used, unfiltered, as a beverage or for drinking a traditional “terere” (Martínez and Barboza, 2010). Consequently, in addition to the supernatant liquid, small particles are swallowed, whose components can potentially be absorbed from the gastrointestinal tract. Based on the latter, for this study, we selected a more powerful extraction system and closer to the popular procedure, using the ethanol: water mixture (60:40). The dried material was subjected to grinding to obtain a fine powder, which was extracted with ethanol: water (60:40) using the method of conventional reflux for 1 hour in a bath and at 50ºC. The procedure of extraction was repeated two times, and the filtered hydroalcoholic extracts were mixed, homogenized, and evaporated at low pressure. The concentrated CEAp was frozen and finally lyophilized for its use in all biological experiments.
Drugs
All the drugs used were of analytical quality. Carrageenan, indomethacin, and acetic acid were obtained from Sigma (USA), and sodium chloride was acquired from Wako (Japan). Verbascoside (purity > 98%) was purchased from ABCAM Inc. (Discovery Drive, Cambridge Biomedical Campus, Cambridge, CB2 0AX, UK) Morphine, ethanol, and propylene glycol for pharmaceutical use were obtained locally.
Animals
Swiss Albino female mice (20–30 g, b.w.) obtained from the Animal Facility of the Department of Pharmacology at the Faculty of Chemical Sciences (National University of Asunción) were used. All animals were maintained in a controlled environment (19°C–23°C temperature and 55% ± 5% relative humidity) with 12 hours of light/dark cycle. The animals received commercial foods and were fasted overnight before corresponding experiments, with free access to water. The experimental procedures, handling, and treatment of animals were conducted in accordance with international standards of animal welfare established by the Ethics Committee of the European Community (Real Decreto, 2005). The experimental protocol was submitted and approved by the institutional Ethics Research Committee of the Faculty of Chemical Sciences on October 6, 2016 (Code 238/16).
UPLC-ESI-MS (Ultra Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry) analysis
The analyses were carried out using a Waters (Milford, MA) Acquity ultra-performance liquid chromatograph coupled with a Xevo TQD triple quadrupole mass spectrometer used as the detector. A Phenomenex KINETEX core-shell EVO-C18 (2.1 × 100 mm, 1.7 μm) column was used for separation purposes. The temperature of the column was kept at 40°C. The mobile phase used was methanol (phase A) and water (phase B) (LC-MS grade, Merck KGaA, Darmstadt, Germany), with 0.1% formic acid and 10 mM ammonium formate for both. The flow rate was 0.3 ml/minute in gradient mode, as follows: 0–0.7 minutes, 80%–80% A; 0.7–3.2 minutes, 80%–60% A; 3.2–7.6 minutes, 60%–20% A; 7.6–8.3 minutes, 20%–0% A; 8.3–10.4 minutes, 0%–80% A, 10.4–13 minutes, 80%–80% A. The samples were dissolved in LC-MS MeOH, filtered through 0.22 µm nylon syringe filters, and injected at 5 mg/ml.
The MS spectra were acquired in full scan mode (mz 80–800, at a speed of 2,000 m/z per second) with ESI as the ionization source in negative and positive modes. The mass spectrometer conditions were as follows: electrospray capillary voltage 2.5 kV, source temperature 150°C, desolvation temperature 350°C, cone gas flow 80 l/hour, and desolvation gas flow 900 l/hour. The cone voltage was set at 30 V. The system was controlled using the Waters MassLynx V4.1 software.
Evaluation of the analgesic activity of the CEAp
Mechanical pressure-induced painful stimulus in mice (Randall-Selitto Test)
Swiss Albino female mice (20–30 g b.w.) were used and distributed randomly in five groups with six mice per group. One group was treated orally with the vehicle (0.1 ml for each 10 g, b.w.), and a second group was treated with indomethacin (10 mg/kg, p.o.). The other three groups received CEAp (100, 200, and 500 mg/kg, p.o.) dissolved in a 0.9% saline solution. After 1 hour of treatments, the tail pressure threshold was registered (Randall-Selitto test) with the tail/paw pressure analgesy meter (Singh et al., 2017). Increasing pressure was applied to the midpoint of the tail, previously marked with ink, at a linear rate of 10 g/second, with maximum applied force limited to 250 g to avoid damage to the skin.
Chemically induced painful stimulus in mice (Writhing test)
Swiss Albino female mice (20–30 g b.w.) were used in this experiment and distributed randomly into five groups of six mice per group. One group was treated orally with the vehicle (0.1 ml/10 g of b.w.) and a second group with indomethacin (10 mg/kg, p.o.). The other three groups received CEAp (100, 200, and 500 mg/kg, p.o.). After 1 hour of treatments, 0.8% diluted acetic acid in a saline solution was injected intraperitoneally (0.1 ml/10 g of b.w.) to all the animals in each group to their corresponding temporal sequences (Ma et al., 2011). The number of contortions was counted every 5 minutes during a period of 30 minutes and tabulated for suitable analysis.
Thermally induced painful stimulus in mice (Hot Plate test)
Female mice (25–30 g) were randomly distributed in five groups of six mice per group. One group was treated orally with the vehicle (0.1 ml/10 g of b.w.) and a second group with morphine (6 mg/kg, i.p.). The other three groups received CEAp (100, 200, and 500 mg/kg, p.o.). After 60 minutes, the animals were placed individually on a hot plate (56oC) apparatus to their corresponding temporal sequences (Qnais et al., 2010). The reaction time of the animal to the thermal stimulus (measured in seconds), characterized by the behavior of lifting or licking the feet, was considered an indicator of the nociceptive effect and the time to be removed from the plate (Gregory et al., 2014). The maximum amount of contact of the animal with the hot plate was considered 30 seconds. If there was no reaction, the animal was removed after this amount of time to avoid damage to the feet.
Evaluation of the anti-inflammatory activity of the CEAp
Carrageenan-induced paw edema in mice
Female mice (25–30 g) were distributed randomly in five groups of six mice per group. One group was treated orally with the vehicle (0.1 ml/10 g of b.w.) and a second group with indomethacin (10 mg/kg, a positive anti-inflammatory agent). The other three groups received CEAp (100, 200, and 500 mg/kg, p.o.). One hour after receiving treatment, the animals were injected in the subplantar (s.p.) region of the right hind legs with 40 μl of carrageenan (1%) as described by Kim et al. (2013). A similar volume of a normal saline solution was injected into the contralateral foot. Paw volume measurements were recorded immediately before, every 30 minutes, and up to 3 hours after carrageenan injection. Briefly, the procedure consisted of immersing the rear legs up to the lateral malleolus inside the digital plethysmograph vessel (LE 7500 Panlab, Harvard Apparatus, Spain). The displaced volume is automatically recorded and tabulated as an individual value (Muhammad et al., 2012). The difference in volumes between the legs was considered as the edema value for each animal.
Statistical analysis
The results were expressed as mean ± standard deviation. Analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was performed as statistical analysis using the GraphPad Prism 5.0 software. The level p < 0.05 was considered to be statistically significant.
RESULTS
Verbascoside identification
Verbascoside was identified in the crude extract by LC-MS (Fig. 1). The chromatogram of the standard showed a peak at 7.37 minutes with a deprotonated molecular ion of m/z 623.89 [M-H]-. The extract showed a peak at 7.46 minutes, with the same molecular ion of m/z 623.89 [M-H]- as the standard (Fig. 2). This fact confirmed the presence of the compound in the extract.
Effect of CEAp on mechanical pressure-induced pain behavior in mice (Randall-Selitto test)
As depicted in Figure 3, groups of animals treated with doses of 100 (241.4 ± 47.41 g; p ? 0.001) and 200 mg/kg (210.0 ± 81.20 g; p ? 0.01) of CEAp revealed a statistically significant analgesic effect in comparison to the group treated with the vehicle (104.0 ± 25.80 g). The pain threshold increased by 233%, 136%, and 102% in groups treated with indomethacin, 100 and 200 mg/kg of CEAp in comparison to the control group, respectively. Nevertheless, the dose of 500 mg/kg (147 ± 35.80 g) did not induce a statistically significant response compared to the control group. As was indicated above, indomethacin at 10 mg/kg (346.0 ± 105.5 g; p ? 0.001) demonstrated a statistically significant effect which validated the method used.
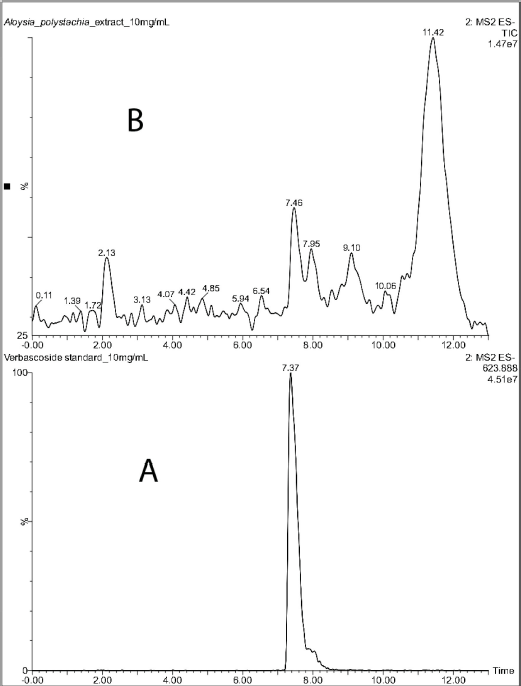 | Figure 1. LCMS chromatograms of verbascoside standard (A) and Aloysia polystachia crude extract (B). [Click here to view] |
Effect of CEAp on chemically induced (acetic acid 0.8%) pain behavior in mice (Writhing test)
The groups of animals treated orally with the doses of 100 (30.90 ± 20.66; 51%), 200 (30.80 ± 11.55; 51%), and 500 mg/kg (19.88 ± 11.51; 68%) of CEAp caused a very significant reduction (p < 0.001) in the number of abdominal contortions compared to the group treated with the vehicle (62.78 ± 14.85), compatible with an analgesic activity of the sample (Fig. 4). In the same sense, administration of indomethacin denoted a significant reduction of 51% in the number of contortions (30.78 ± 6.92) elicited, in comparison to the group treated with the vehicle, validating the utilized method. These results indicated that the dose of 500 mg/kg is more potent than the positive control indomethacin 20 mg/kg in the reduction of pain behavior induced chemically with acetic acid. The observed effects in this test are not dose dependent in nature.
The analysis of variation in the number of chemically induced writhing behavior as a function of time denotes that the speed of reduction of the painful behavior in groups of animals treated with indomethacin (10 mg/kg) and CEAp (500 mg/kg) begins at 10 minutes after the injection of 0.8% acetic acid, respectively. Undoubtedly, the reduction in the number of writhing behavior is more intense, as a function of time, with all doses of CEAp than the vehicle-treated group. Furthermore, the indomethacin curve shows a lower number of writhing behavior than the negative control group, demonstrating its effectiveness as an analgesic and thus validating the method used (Fig. 5). Linear regression analysis between indomethacin-treated and CEAp groups (100, 200, and 500 mg/kg) differs significantly when compared to the vehicle-treated group.
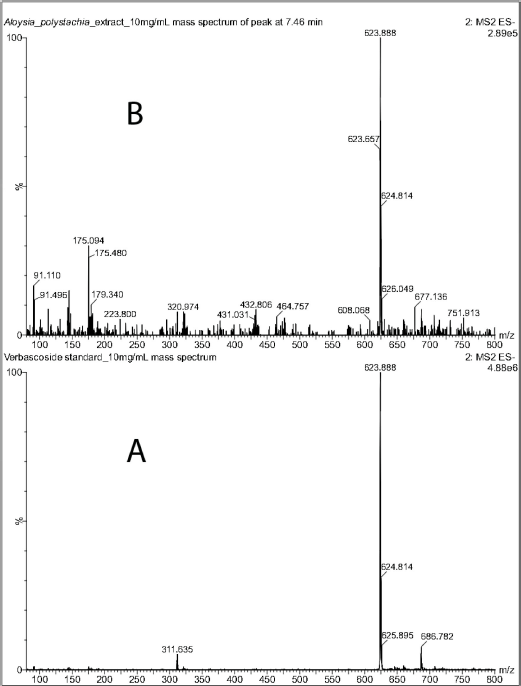 | Figure 2. Mass spectra (ESI) in negative mode of verbascoside standard (A) and of the peak at 7.46 minutes in the chromatogram of the CEAp. [Click here to view] |
Effect of CEAp on thermally induced pain behavior in mice (Hot Plate Test)
Figure 6 shows the influence of oral administration of CEAp on reaction time (latency) to heat-induced pain behavior in mice. There is a significant increase in the reaction time of the group treated with 100 mg/kg of CEAp (20.52 ± 4.16 seconds; p < 0.01; 171%) in comparison to the group treated with the vehicle (7.57 ± 2.56 seconds). Nevertheless, it should be noted that doses of 200 (13.50 ± 3.96 seconds) and 500 (13.73 ± 7.70 seconds) mg/kg of CEAp do not raise the latency time compared to the group treated with the vehicle. Additionally, a statistically significant increase in morphine-induced latency (28.04 ± 3.46; p < 0.001; 270%) was observed compared to the negative control group, which validates the method used.
Anti-inflammatory effect of the CEAp on carrageenan-induced acute paw edema in mice
Figure 7 shows the influence of the oral administration of CEAp on carrageenan-induced acute paw edema in mice. There is a significant decrease of 56% in the paw edema provoked by carrageenan (10.63 ± 2.93; µl) by treatment with 100 mg/kg de CEAp (6.00 ± 1.55; µl; p < 0.05). In the same sense, 10 mg/kg of indomethacin (5.56 ± 2.88; µl; p < 0.01) provoked a statistically significant decrease of 52% in the inflammatory response elicited by carrageenan, thus validating the phlogistic method used. Nevertheless, it should be noted that groups treated with doses of 200 (7.20 ± 1.92; µl) and 500 (7.00 ± 2.53; µl) mg/kg of CEAp did not significantly reduce the level of edema. Concurrently, the statistically significant increase in the volume of edema induced by carrageenan (10.63 ± 2.93; µl; p < 0.001) in comparison with the untreated basal group (2.75 ± 2.50; µl) was observed. Finally, in the group treated with 10 mg/kg of indomethacin (5.56 ± 2.88; p < 0.01), an efficient reduction of the volume of edema was observed and globally validates the method used.
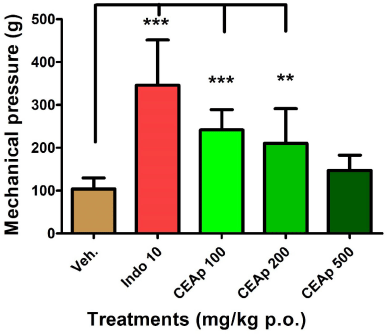 | Figure 3. Influence of the oral administration of CEAp to mice subjected to the caudal mechanical pressure test (Randall-Selitto). The bars represent the means ± standard deviation. The statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test. N = 6, ***p ? 0.001; **p ? 0.01 significantly differentiates the treatment group with the vehicle. [Click here to view] |
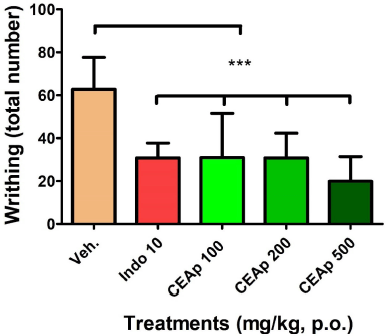 | Figure 4. Influence of the oral administration of CEAp to mice subjected to the test of reaction to pain induced by the administration of acetic acid (i.p.) (contortions). The bars represent the means ± standard deviation. The statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparison test. N = 6, ***p ? 0.001 significantly differentiates the group treated with the vehicle. [Click here to view] |
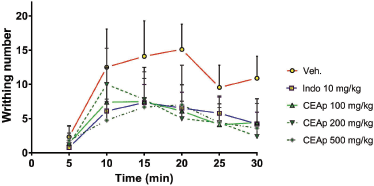 | Figure 5. Curves of variation in the number of contortions as a function of time obtained following treatment. [Click here to view] |
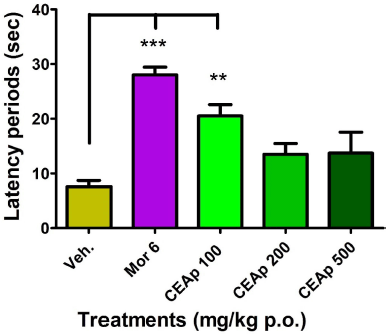 | Figure 6. Influence of the oral administration of CEAp to mice subjected to the painful thermal stimulus reaction test (hot plate). The bars represent the means ± standard deviation. The statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparison test. N = 6, ***p ? 0.001; **p ? 0.01 significantly differentiates the treatment groups with the vehicle. [Click here to view] |
DISCUSSION
This work evaluated the antinociceptive and anti-inflammatory activity of CEAp, with previously demonstrated antidepressant activity in our lab (Hellión-Ibarrola et al., 2006; Hellión-Ibarrola et al., 2008; Mora et al., 2005). Usually, in the validation of plants used for medicinal purposes in Paraguay, the first step is to select plants with a long history of use, the procedure for obtaining the extract, followed by the selection of the preclinical method to be used (induced pain and inflammation in rodents), consistent with popular use. The next step is the oral treatment of the groups of animals with different doses of the sample to determine their safety and efficacy. Then, if the result deserves efficacy, it is considered to carry out refined studies to determine the components, molecular mechanisms of action of the effects caused by the extract.
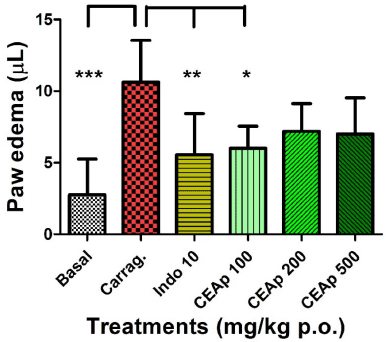 | Figure 7. Influence of the oral administration of CEAp to mice subjected to the carrageenan-induced plantar edema assay. The bars represent the means ± standard deviation 3 hours after the administration of the carrageenan. The statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparison test. N = 6, ***p ? 0.001; **p ? 0.01; *p ? 0.05 significantly different from the vehicle-treated group. [Click here to view] |
Based on the International Association for the Study of Pain, Maallo et al. (2021) explain pain as “an unpleasant sensory or emotional experience associated with actual or potential tissue damage.” These pain components have their own pathways, centers, and regulatory mechanisms. In general, pain can be classified as nociceptive pain provoked by the normal activation of peripheral nociceptors (“alarm” and protection of the organism against harmful stimuli, with high threshold pain and often accompanied by a withdrawal reflex), inflammatory pain provoked by tissue damage and activated by invasion of inflammation-mediating cells (macrophages, neutrophil mast cells, and granulocytes) with low pain threshold, and pathological pain (neuropathic and dysfunctional) that appears as a consequence of a lesion to the nervous system (central or peripheral) or abnormal central processing (Woolf, 2010).
In the same context, the pain-depression or depression-pain couple is accepted as very common comorbidities in the general adult population with chronic pain. 19% of European adults and 20.4% of American adults have chronic pain, and depression is the most frequently associated comorbidity. The prevalence of depression in the European and American societies is 7% and 7.5%, respectively (Tenti et al., 2021). Severe chronic pain is still poorly managed due to the continuous imbalance between pharmacological analgesia and tolerability, which leads to a poor treatment outcome (Barroso et al., 2021). The experimental models of pain are separated as acute, persistent, and neuropathic. Experimental acute pain is induced by mechanical, chemical, and thermal stimuli. Persistent pain is represented by the formalin test and neuropathic pain by nerve ligation assays (Bannon and Malmberg, 2007). Therefore, A. polystachya has sufficient merits to be evaluated for its potential analgesic and anti-inflammatory activity in preclinical models.
The oral administration of CEAp in mice denotes an efficient analgesic and anti-inflammatory capacity in the models used. In the mechanical pressure-induced pain assay (Randall-Selitto test), an effective non-dose-dependent effect was observed. The pain threshold was increased by 136% and 102% in the groups treated with 100 and 200 mg/kg of CEAp, respectively, compared to the control. This indicates a major analgesic potential with the lowest dose of CEAp, while a decreasing degree in the intensity of the analgesic effect was found with increasing dosage until it resulted in nonsignificant values with the dose of 500 mg/kg of CEAp. Additionally, the analgesia induced with the extract was moderate in intensity and 30% lower than that obtained with indomethacin (analgesic reference drug) which increased the pain threshold by 233% compared to the control. It should be noted that the behavioral measures of mechanical sensitivity are commonly used to measure allodynia (a painful response to nonharmful stimuli) and hyperalgesia. Systems of application of mechanical pressure (caudal, plantar, etc.) have been developed to measure pain responses provoked by mechanical stimuli. Tail withdrawal or vocalization of the animal (fastened) is used as the endpoint of the test (Gregory et al., 2014). Assessing withdrawal response to pressure applied to tissue using the Randall-Selitto model reproduces similar results in the reduction of pressure pain threshold, commonly observed in clinical pain conditions (fibromyalgia, myofascial pain, or osteoarthritis). Considering the decreasing pain sensitivity (analgesia) that was observed with all doses when pain was inflicted by chemical methods, CEAp may have clinical resemblance and utility for acute pressure pain such as in fibromyalgia (Gregory et al., 2014). Additionally, the intensity of the observed effects was similar to that of the indomethacin. This model was characterized by the induction of acute pain of longer duration (compared to caudal pressure) due to peritoneal irritation, involving tissue damage in which inflammatory signaling mechanisms of higher intensity and sensitivity operate. In agreement with Carvalho et al. (2006), the principal mediators are eicosanoids and sympathomimetic amines preceded by the release of TNF-α (nociceptive cytokine). Nevertheless, the obtained results show the efficacy of CEAp as an analgesic and its probable anti-inflammatory effect.
In addition, in the heat-induced pain assay, an efficient increase in the latency time of the hot plate jump or paw lick compatible with the analgesic effect of CEAp (100 mg/kg) was observed. The act of jumping or licking involves supraspinal structures indicating that it goes beyond nociceptive reflexes because it involves information processing in higher structures (Gregory et al., 2014). Accordingly, previous work with CEAp has shown antidepressant and anxiolytic activity in mice (Hellión-Ibarrola et al., 2006; Hellión-Ibarrola et al., 2008; Mora et al., 2005) treated via the oral route. There is a possibility that analgesic activity is mediated by regulatory pathways at higher levels because it increases the latency time in the hot plate assay. Efforts to carry out studies to elucidate the regulatory mechanism of the analgesic effect must be reinforced to advance this work.
Likewise, CEAp was active against the acute inflammation caused by subplantar injection of carrageenan. Carrageenan-induced paw edema in the mouse is also associated with nociceptive changes in the paw and migration of inflammatory cells to the injection site (Gregory et al., 2014; McCarson, 2015) and is sensitive to nonsteroidal anti-inflammatory drugs (Lapa et al., 2002). Recently, it has been demonstrated that the phase of the inflammatory response after the injection of carrageenan in the paw of the mice is associated with the production of cyclooxygenase 2 (COX-2), high prostaglandin production (by the action of COX-1 and COX-2), free oxygenated radicals (Nitric Oxide derivatives from endothelial nitric oxide synthase, and inducible nitric oxide synthase), and neutrophil infiltration (Duarte et al., 2016; Fehrenbacher et al., 2012; McCarson, 2015). As demonstrated, CEAp shows anti-inflammatory activity by reducing the edema by an unknown mechanism. However, the presence of acteoside or verbascoside, among other components of CEAp, is highly probably accountable for analgesic/anti-inflammatory activity. According to Gutiérrez-Rebolledo et al. (2016), the verbascoside is a well-known COX-2 inhibitor with antioxidant capacity in addition to its MAO-A (Monoamine oxidase A) inhibitory effects (Pereira et al., 2019), related at least partially to antidepressant/anxiolytic properties (Carmona et al., 2019). Interestingly, drugs able to block inflammatory cytokines or associated inflammatory pathways (COX-2) are effective in reducing depressive symptoms in patients with chronic inflammation (rheumatoid arthritis, autoimmune disease, cancer, etc.) and those patients suffering from major psychiatric disorders (Pereira et al., 2019). Indeed, we have no knowledge about the molecular mechanism of action of CEAp, and with the available results, it is not possible to make a complete hypothesis to address which of the pathways mentioned above and if verbascoside or which other component(s) are involved in analgesia and inflammation. Consequently, this study is not yet focused on determining molecular mechanisms involved in increasing pain threshold or correcting tissue damage caused by inflammation. The presence of verbascoside in CEAp accounts for the huge potential to be responsible for these analgesic and anti-inflammatory activities. Really, the aim of this study is to determine the influence of the oral administration of the CEAp on different preclinical models of acute pain induced by harmful stimuli (mechanical pressure, 0.8% acetic acid, and thermal) and its influence on carrageenan-induced inflammation in mice. Certainly, the pharmacological study of the specific molecular mechanism is the prospective core to be examined experimentally.
It should also be mentioned that components obtained from the essential oil (carvone and α-thujone) or from CEAp (phenolic compounds, glycosides phenylethanoids, and terpenes) are potentially responsible for the wide profile of pharmacological effects observed in animals and ultimately clinical trials of this noble and appreciated natural product (Carmona et al., 2019; Consolini et al., 2011; Hellión-Ibarrola et al., 2008; Pereira et al., 2019). Among the pharmacological effects studied are described low acute toxicity and anxiolytic and antidepressant activity in mice and rats. Additionally, antispasmodic effects were depicted from the aqueous extract and dyes of A. polystachya (Griseb.) Moldenke probably mediated by blocking Ca+2 channels (Consolini et al., 2011). The most remarkable result is that CEAp proved to be clinically effective in moderate anxiety (Carmona et al., 2019) and its principle component, acteoside (verbascoside), also present in other species, proved to be effective as an anti-inflammatory treatment (Gutiérrez-Rebolledo et al., 2016). Therefore, the results of this work are correlated with available literature and validate its popular use as an analgesic and anti-inflammatory treatment. Consequently, in addition to the popular procedure, these outcomes reinforce the real possibility of developing innovative products such as the formulation of standardized phytomedicine from semipurified or isolated components from A. polystachya for a more precise and effective treatment of pain and inflammation associated with depression or vice versa.
Limitations of this study include the restricted knowledge about the profile of components and antioxidant properties of the crude extract. Also, the pain or inflammation mechanism affected is unknown, and molecular/docking studies to shed light on these questions are required. Therefore, the next steps with A. polystachya include solving the chemical components, antioxidant properties, and the deep examination of the molecular/docking mechanism involved in analgesic, anti-inflammatory, and antidepressant activities.
CONCLUSION
The present work demonstrated that the oral administration of the CEAp produces moderate analgesic activity using the models of induced pain in mice through three harmful stimuli: a) caudal mechanical pressure, b) chemical pain of the abdominal contortions, and c) thermal pain with indomethacin as an analgesic and anti-inflammatory positive control. The presence of verbascoside was determined by chromatographical methods.
Based on these results, it was shown in the Randall-Selitto test that the doses of 100 and 200 mg/kg of CEAp increase the pain threshold by 136% and 102% compared to the group of animals treated with the vehicle, respectively. Also, it was determined that 100, 200, and 500 mg/kg of CEAp have the capacity to reduce considerably the contortions induced by 0.8% acetic acid in a dose-dependent manner by 51%, 51%, and 68% compared to the vehicle. These results are compatible with the analgesic activity of the CEAp. The potency of 100 and 200 mg/kg CEAp was similar to indomethacin, while that of 500 mg/kg was much better than the positive control in the decrease of contortions induced by intraperitoneal administration of 0.8% acetic acid solution. Likewise, the pain threshold was increased with 100 mg/kg of CEAp by 171% using the hot plate, reinforcing the potential effectiveness of this natural product as an alternative analgesic treatment.
Likewise, the experimental edema induced by carrageenan was reduced in over 50% with 100 mg/kg of CEAp whose potency was very similar to that caused by indomethacin (10 mg/kg). We conclude that the results are correlated with the popular use of CEAp and open a variety of possibilities for pharmacotoxicological investigation and the potential development of innovative phytopharmaceuticals with relevant health-commercial importance. Complementary pharmacological and chemical works are underway to determine the possible mechanism of action and a full component, in addition to verbascoside, involved in the analgesic-anti-inflammatory effect.
ACKNOWLEDGMENTS
This research work was performed under the facilities provided by the Facultad de Ciencias Químicas de la Universidad Nacional de Asunción. The authors would like to thank Departamento de Botánica and Departamento de Fitoquimica from Facultad de Ciencias Químicas for supporting them with identification of plant material and obtention of the extract, respectively.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimental protocol was submitted and approved by the institutional Ethics Research Committee of the Faculty of Chemical Sciences on October 6, 2016 (Code 238/16).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Bannon AW. 2001. Models of pain: hot-plate and formalin test in rodents. Curr Protoc Pharmacol, 2001; 5.7.1–11.
Bannon AW, Malmberg AB. 2007. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci, 2007; 8.9.1–16. CrossRef
Barroso J, Branco P, Apkarian AV. Brain mechanisms of chronic pain: critical role of translational approach. Transl Res, 2021; 238:1–14. CrossRef
Carmona F, Coneglian FS, Batista PA, Aragon DC, Angelucci MA, Martinez EZ, Pereira AMS. Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) powdered leaves are effective in treating anxiety symptoms: a phase-2, randomized, placebo-controlled clinical trial. J Ethnopharmacol, 2019; 242:112060. CrossRef
Carvalho, A.P., Bezerra, M.M., Girão, V.C.C., Cunha, F.Q., Rocha, F.A.C. ANTI-INFLAMMATORY AND ANTI-NOCICEPTIVE ACTIVITY OF RISEDRONATE IN EXPERIMENTAL PAIN MODELS IN RATS AND MICE. Clin. Exp. Pharmacol. Physiol. 2006; 33, 601–606. CrossRef
Consolini AE, Berardi A, Rosella MA, Volonté MG. Antispasmodic effects of Aloysia polystachya and A. gratissima tinctures and extracts are due to non-competitive inhibition of intestinal contractility induced by acethylcholine and calcium. Rev Bras Farmacogn, 2011; 21:889–900. CrossRef
da Silva JB, Temponi Vdos S, Fernandes FV, de Assis Dias Alves G, de Matos DM, Gasparetto CM, Ribeiro A, de Pinho Jde J, Alves MS, de Sousa OV. New approaches to clarify antinociceptive and anti-inflammatory effects of the ethanol extract from Vernonia condensata leaves. Int J Mol Sci, 2011; 12:8993–9008. CrossRef
de Morais Lima GR, de Albuquerque Montenegro C, de Almeida CL, de Athayde-Filho PF, Barbosa-Filho JM, Batista LM. Database survey of anti-inflammatory plants in South America: a review. Int J Mol Sci, 2011; 12:2692–749. CrossRef
Duarte DB, Vasko MR, Fehrenbacher JC. Models of inflammation: carrageenan air pouch. Curr Protoc Pharmacol, 2016; 5.6.1–9. CrossRef
Fehrenbacher, J.C., Vasko, M.R., Duarte, D.B., Models of inflammation: Carrageenan- or complete freund’s adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr. Protoc. Pharmacol. 2012; 56, 5.4.1-5.4.7. CrossRef
Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain, 2014; 14:1–26. CrossRef
Gutiérrez-Rebolledo GA, Garduño-Siciliano L, García-Rodríguez RV, Pérez-González MZ, Chávez MI, Bah M, Siordia-Reyes GA, Chamorro-Cevallos GA, Jiménez-Arellanes MA. Anti-inflammatory and toxicological evaluation of Moussonia deppeana (Schldl. & Cham) Hanst and Verbascoside as a main active metabolite. J Ethnopharmacol, 2016; 187:269–80. CrossRef
Hellión-Ibarrola MC, Ibarrola DA, Montalbetti Y, Kennedy ML, Heinichen O, Campuzano M, Ferro EA, Alvarenga N, Tortoriello J, De Lima TC, Mora S. The antidepressant-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. Phytomedicine, 2008; 15:478–83. CrossRef
Hellión-Ibarrola MC, Ibarrola DA, Montalbetti Y, Kennedy ML, Heinichen O, Campuzano M, Tortoriello J, Fernández S, Wasowski C, Marder M, De Lima TC, Mora S. The anxiolytic-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. J Ethnopharmacol, 2006; 105:400–8. CrossRef
Kim, H.S., Kwon, O.K., Park, J.W., Jeong, H.G., Oh, S.R., Lee, H.K., Bach, T.T., Hai, D. Van, Ahn, K.S., Anti-inflammatory activities of methanol extract of Mastixia arborea C.B. Clarke as to mouse macrophage and paw edema. Biosci. Biotechnol. Biochem. 2013; 77, 2356–2361. CrossRef
Kopach O, Viatchenko-Karpinski V, Belan P, Voitenko N. Development of inflammation-induced hyperalgesia and allodynia is associated with the upregulation of extrasynaptic AMPA receptors in tonically firing lamina II dorsal horn neurons. Front Physiol, 2012; 3:1–8. CrossRef
Lapa AJ, Souccar C, Lima-Landman MT, De Lima TC. Métodos de avaliação da atividade farmacológica de plantas medicinais. Sociedade Brasileira de Plantas Medicinais, Sao Paulo, Brazil, 2002.
Ma KJ, Zhu ZZ, Yu CH, Zhang H, Liu J, Qin LP. Analgesic, anti-inflammatory, and antipyretic activities of the ethanol extract from Desmodium caudatum. Pharm Biol, 2011; 49:403–7. CrossRef
Maallo AMS, Moulton EA, Sieberg CB, Giddon DB, Borsook D, Holmes SA. A lateralized model of the pain-depression dyad. Neurosci Biobehav Rev, 2021; 127:876–83. CrossRef
Martínez GJ, Barboza GE. Natural pharmacopoeia used in traditional Toba medicine for the treatment of parasitosis and skin disorders (Central Chaco, Argentina). J Ethnopharmacol, 2010; 132:86–100. CrossRef
McCarson KE. Models of inflammation: carrageenan- or complete freund’s adjuvant (CFA)–induced edema and hypersensitivity in the rat. Curr Protoc Pharmacol, 2015; 70:5.4.1–9. CrossRef
Mora S, Díaz-Véliz G, Millán R, Lungenstrass H, Quirós S, Coto-Morales T, Hellión-Ibarrola MC. Anxiolytic and antidepressant-like effects of the hydroalcoholic extract from Aloysia polystachya in rats. Pharmacol Biochem Behav, 2005; 82:373–8. CrossRef
Muhammad N, Saeed M, Gilani SN, Haq I, Khan H. Analgesic and anti-inflammatory profile of n-hexane fraction of Viola betonicifolia. Trop J Pharm Res, 2012; 11:963–9. CrossRef
Pereira A, Guimarães C, Pereira S, Crevelin E, Pinto G, Morel L, Bertoni B, França S, Taleb-Contini S. Isolation and identification of phenylethanoid gycosides from Aloysia polystachya and its activity as inhibitors of monoamine oxidase-A. Planta Medica Int Open, 2019; 6:e1–6. CrossRef
Qnais EY, Abu-Dieyeh M, Abdulla FA, Abdalla SS. The antinociceptive and anti-inflammatory effects of Salvia officinalis leaf aqueous and butanol extracts. Pharm Biol, 2010; 48:1149–56. CrossRef
Real Decreto, M. de A.P. y A., Presidencia, C.E.E., 2005. Real decreto 1201/2005. Boe 252, 34367–34391.
Rojas-Corrales MO, Casas J, Moreno-Brea MR, Gibert-Rahola J, Micó JA. Antinociceptive effects of tricyclic antidepressants and their noradrenergic metabolites. Eur Neuropsychopharmacol, 2003; 13:355–63. CrossRef
Singh N, Bansal Y, Bhandari R, Marwaha L, Singh R, Chopra K, Kuhad A. Resveratrol protects against ICV collagenase-induced neurobehavioral and biochemical deficits. J Inflamm (United Kingdom), 2017; 14:1–15. CrossRef
Tabor A, Thacker MA, Moseley GL, Kording KP. Pain?: a statistical account. Plos Comput Biol, 2017; 13:1–13. CrossRef
Tenti, M., Raffaeli, W., Gremigni, P., A Narrative Review of the Assessment of Depression in Chronic Pain. Pain Manag. Nurs. 2022; 23, 158–167. CrossRef
TrivellatoGrassi L, Malheiros A, Meyre-Silva C, Buss Zda S, Monguilhott ED, Fröde TS, da Silva KA, de Souza MM. From popular use to pharmacological validation: a study of the anti-inflammatory, anti-nociceptive and healing effects of Chenopodium ambrosioides extract. J Ethnopharmacol, 2013; 145:127–38. CrossRef
Woolf, C.J., What is this thing called pain? J. Clin. Invest. 2010; 120, 3742–3744. CrossRef