INTRODUCTION
SARS-CoV-2 (acute respiratory syndrome coronavirus) is a systemic hyperinflammatory disease that can cause respiratory infections such as pneumonia. SARS-CoV-2 belongs to the family of Coronaviridae, a single ribonucleic acid (RNA) virus (+ ssRNA) that can spread between humans and can be fatal (Ahmed et al., 2020; Mousavizadeh and Ghasemi, 2021; Wu et al., 2020). Up until now, there have been 1,537,967 cases of COVID-19, with 41,815 deaths and 1,381,677 recovery cases in Indonesia (Task Force for Handling COVID-19, 2021). There is currently no definitive cure for COVID-19. Therefore, there is an urgent need to develop a much faster and more accurate SARS-CoV-2 detection method and to find and develop drugs for the treatment of COVID-19.
Anticoronavirus therapy can be divided into two categories depending on the target: the first targets the human immune system or human cells, while the second targets the coronavirus proteins. In the immune system pathway, the innate immune system response plays an important role in controlling the replication of and infection by the coronavirus, and interferon is expected to play a role in increasing the immune response. The blockage of human cellular pathways is necessary to inhibit viral replication and may exhibit antiviral effects. Therapy that is aimed at the coronavirus itself is carried out to prevent the synthesis of viral RNA through modification of the viral genetic material, inhibiting viral replication by inhibiting the enzymes needed by the virus and blocking the virus from binding to human cell receptors or inhibiting the viral assembly process through the inhibition of viral structural proteins (Wu et al., 2020; Zumla et al., 2016). Nonstructural protein (NSP) is a functional protein in SARS-CoV-2 that plays a role in the process of transcription, RNA translation, protein synthesis and modification, viral replication, and infection of humans. Several NSPs are important targets in the discovery and development of COVID-19 drugs, especially in the form of small molecules, namely, 3CLpro, PLpro, RdRp, and helicase. Also besides, structural proteins such as Spike-ACE2 and TMPRSS2 are important targets to inhibit the process of the virus binding to surface receptors and prevent the entry of genetic material into human cell membranes (Hoffmann et al., 2020; Wu et al., 2020).
Indonesia, a country with a high level of biodiversity, has great potential to develop herbal preparations that have implications for inhibiting the replication of the SARS-CoV-2 virus in the body. One of them is Melaleuca cajuputi, a member of the Melaleuca genus, which can be found throughout the territory of Indonesia. Melaleuca cajuputi is a plant that contains 1,8-cineol and α-terpineol, which is known to have antibacterial, anti-inflammatory, and antioxidant properties (Bosni? et al., 2006; Hendry et al., 2009). In traditional medicine, people use M. cajuputi as melaleuca cajuput oil to help increase disease resistance and to inhibit some bacteria and viruses (My et al., 2020; Sutrisno et al., 2018; Sebei et al., 2015). This study aims to evaluate the efficacy of cajuput oil as an adjuvant therapy complementing the standard treatment of patients with mild and moderate symptoms of COVID-19. The research was carried out by the Research Team at Bhayangkara Brimob Hospital, Cimanggis Depok, Indonesia.
METHODS
Study design and population
This study was an experimental clinical trial, conducted using good clinical practice and the ethical principles outlined in the Helsinki Declaration. All research procedures were approved by the Ethics Committee of the Bhayangkara Brimob Hospital (No. 01/EC/DR/I/2021). The goal was to determine the effectiveness and use of melaleuca cajuput oil as a complement to the standard treatment of COVID-19 patients of two groups, with measuring in terms of clinical, laboratory, polymerase chain reaction (PCR), and radiographic features of pneumonia during hospital admission and when the PCR result was negative and also measuring the length of hospital stay.
The subjects of the study were COVID-19 patients aged ≥18–60 years with mild and moderate symptoms defined as COVID-19 confirmed patients who develop symptoms of fever, nausea, anosmia, cough, dyspnea, limp, flu, ageusia, diarrhea, and bloating without evidence of viral pneumonia or without hypoxia and also patients with clinical signs of pneumonia (fever, cough, shortness of breath, and rapid breathing) but no signs of severe pneumonia including SpO2 > 93% with room air. All the patients were treated at the Bhayangkara Brimob Hospital from November 2020 to February 2021. In Indonesia, patients with mild degrees of COVID-19 were treated in hospitals because of infection regulations and the difficulty in implementing COVID-19 health protocols in the community. We collected some data from each patient: clinical improvement during treatment (including the symptoms of fever, nausea, anosmia, cough, dyspnea, limp, flu, ageusia, diarrhea, and bloating), laboratory results (including the measurements of leucocyte, thrombocyte, monocyte, lymphocyte, basophil, and eosinophil) at the beginning and end of treatment, radiological features of pneumonia at the beginning and end of treatment, and length of stay. The length of stay in the hospital was assessed by calculating the number of days between the patient’s admission to the hospital and the day of her/his first negative result for PCR test at the end of treatment.
The inclusion criteria in our study were age ≥ 18–60 years (male: 97 patients, female: 23 patients), positive pneumonia verified by radiology, PCR swab results showing positive COVID-19, and willingness to accept randomization results in either the standard therapy group or standard therapy plus melaleuca cajuput oil. The exclusion criteria were the doctor’s opinion that the patient was unable to follow the clinical trial protocol, patients under 18 years of age, pregnant, breastfeeding, patients who had an aspartate transaminase or alanine transaminase liver function more than five times the upper limit of normal, who had severe kidney abnormalities or were on hemodialysis or peritoneal dialysis, and patients who during randomization had been anticipated to be transferred to another hospital within next 72 hour or had received another experimental drug for COVID-19 therapy within the previous 30 days. Monitoring was carried out of early and late symptoms of treatment, laboratory results on day 8, nasopharyngeal swab on day 8 or after completion of the isolation period, and posteroanterior chest radiology on day 8. Clinical conditions, laboratory blood test results, preliminary and final radiology results, and PCR swabs were recorded for all research subjects. The outcomes assessed were clinical improvement or deterioration during observation, improvement or worsening of radiological images, improvement or worsening of laboratory blood test results, and length of stay in the hospital.
The eligible patients, 97 males and 23 females, were enrolled by the clinician and research team, and data collection was obtained. Subsequently, the patients were allocated to the melaleuca cajuput oil group and control group randomly. The control group received standard COVID-19 therapy with 500 mg azithromycin once daily, 200 mg N-acetyl cysteine three times daily, 100 mg vitamin B1 three times daily, 20 mg zinc twice daily, 500 mg selcom C twice daily, Vit D3 1000 IU twice daily, and 500 mg isoprinosine four times daily, while the cajuput oil group received standard COVID-19 therapy + 1.5 ml of 95% melaleuca cajuput oil every eight hours dripped onto a dense cotton ball attached to an internally modified inhalation mask for 7 days (Fig. 2). Monitoring was undertaken by assessing clinical symptoms at admission and the end of treatment, laboratory tests on day 1 and day 8, a nasopharyngeal swab on day 8 or when leaving the hospital, and radiological examination on day 1 and day 8. The main endpoints were the reduction in length of stay in the hospital after seven days of melaleuca cajuput oil therapy. Clinical symptoms during treatment were compared between the control group and the melaleuca cajuput oil group, based on laboratory measurements of leucocytes, platelets, monocytes, lymphocytes, basophils, and eosinophils, as well as on radiographic features of pneumonia. The level of safety was assessed during treatment by looking at allergic reactions, nausea and vomiting, and other clinical complaints.
Statistical analysis
Demographic data and baseline values are expressed as descriptive statistics in the form of the standard error of mean for continuous/numeric data or frequency (percentage) for categorical data (Table 1). The pre-post comparisons of outcomes in the form of categorical data were analyzed using McNemar’s test. Outcomes in the form of continuous data that were not normally distributed were analyzed using the nonparametric Mann–Whitney test. Significance was set at p < 0.05. SPSS software version 23 (IBM, USA) was used for data analysis.
RESULTS
A total of 127 patients with mild and moderate symptoms of COVID-19 were included in the study, comprising the melaleuca cajuput oil group (n = 67) and the control group (n = 60) (Fig. 1) and recruited between November 2020 and February 2021. During the clinical trial period, seven people were unable to continue because of irritation in the nose or allergies. Eventually, there were 57 males (95%) and three females (5%) in the cajuput oil group; 40 males (66.7%) and 20 females (33.3%) in the control group. The mean age of the subjects was 30.17 (±10.15) in the melaleuca cajuput oil group and 32.92 (±12.39) in the control group. Oxygen saturation was ≥ 93%, and there were no comorbidities such as hypertension and diabetes mellitus. Of all the observed symptoms, the most common symptom of COVID-19 in the melaleuca cajuput oil group was cough (47%), followed by anosmia (42%), while the control group predominantly reported fever (73%), followed by cough (70%). The percentage number of other COVID-19 symptoms such as fever, nausea, dyspnea, limp, flu, ageusia, diarrhea, bloating were less than 40%. All symptoms and complaints of COVID-19 improved when the PCR results were negative, the laboratory and radiology results improved, and the patient had finished undergoing treatment at the hospital or returned home. The baseline characteristics of the subjects are shown in Table 1.
 | Table 1. Baseline characteristics of the subjects. Numeric data are reported as mean (SD) and categorical data are reported as frequency (percentage). [Click here to view] |
 | Figure 1. Research methodology. [Click here to view] |
 | Figure 2. The modified mask used in this study. [Click here to view] |
In both groups of patients, the data showed significant improvements. Radiological examinations showed that seven (12%) COVID-19 patients in the melaleuca cajuput oil group had pneumonia, whereas nine (15%) COVID-19 patients in the control group had pneumonia at the time of initial admission and when there is clinical improvement, the radiological results of all patients showed improvement as well. Comparisons of the number of patients with normal laboratory results between the treatment and control groups are summarized in Table 2. It can be seen from Table 2 that, after treatment, there was an increase in the number of patients who had parameter values in the normal range. Leukocyte, lymphocyte, and eosinophil counts in the cajuput oil group at the beginning and the end of treatment were found to be significantly different. Monocyte counts in the cajuput oil group and the control group at the beginning and the end of treatment were significantly different. After 7 days of treatment, the melaleuca cajuput oil had a significantly shorter length of hospital stay ( 10.23 ± 5.44 days) than the control group (13.38 ± 4.62 days) as analyzed by the Mann–Whitney test (U = 1126.50; p < 0.01) with effect size (r = −0.32, 95% Confidence Interval 0.41–0.21).
None of the patients experienced severe side effects such as allergic reactions, nausea, or vomiting. However, seven patients experienced irritation around the nose, with the result that they could not continue the treatment.
DISCUSSION
Melaleuca cajuput essential oil is obtained from the distillation of melaleuca cajuput leaves and twigs of the melaleuca cajuput tree (M. cajuputi). The main components of M. cajuputi same with cajuput, of the distilled oil, are b-pinene, cineol, terpinolene, 4,11,11-tetramethyl-8-methylene, b-linalool, a-terpineol, caryophyllene, a-caryophyllene, iso-caryophyllene, p-cymene, 1,4 terpineol, α-terpineol, thymol, citral, and dehydro-1,1,4,7-tetramethyl elemol (Sutrisno et al., 2018). These compounds have antiviral activity against herpes simplex virus type 1 in vitro (Astani et al., 2010). Cajuput oil has been used as a traditional medicine in Asian countries such as Indonesia, Vietnam, and Thailand, for treating coughs, colds, bronchitis, cold symptoms, and inflammation of the mucous membranes of the upper respiratory tract (My et al., 2020).
The active components of cajuput oil also have an inhibitory effect on influenza A/PR/8 virus subtype H1 N1 replication at noncytotoxic concentrations (Garozzo et al., 2011). Cajuput oil is reported in the literature to be a potentially promising method of preventing infection by SARS-CoV-2. A preliminary study reported that the docking simulation predicts the capability of the molecular structure in cajuput oil for inhibiting angiotensin-converting enzyme 2 (ACE2) protein in the human body, causing SARS-CoV-2 to lose its host receptor and destroy its protein (PDB6LU7) at the same time. Notably, the ACE2 protein is the host receptor of SARS-CoV-2 and SARS-CoV. Therefore, if the ACE2 protein is inhibited, SARS-CoV-2 could potentially be prevented and treated (My et al., 2020).
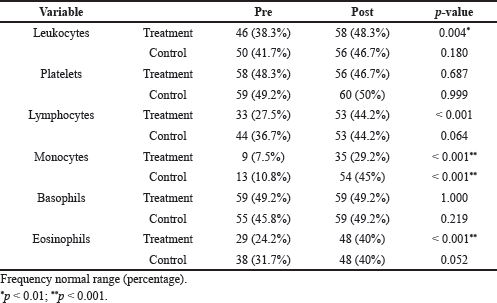 | Table 2. Comparison of laboratory results between the treatment and control groups. The data reported are the number and percentage of patients with blood parameters value within the normal range. All p-values were determined using McNemar’s test. [Click here to view] |
COVID-19 symptoms can include mild-to-severe symptoms. The pathogenic mechanism for SARS-CoV-2 to cause organs manifestation appears to be very complex. This viral infection is capable of producing an immune overreaction in the host that is known as a “cytokine storm” which can lead to multiorgan failure and death (Castelli et al., 2020). Although this study did not differentiate between mild and moderate COVID-19 patients, this is the first prospective and experimental comparative study examining the role of cajuput oil as an antiviral in patients with mild and moderate symptoms of COVID-19. All patients had no comorbidities and did not need to undergo daily clinical monitoring. Hence, we were able to draw on the baseline and final clinical data. We observed that cajuput oil yielded clinical improvement, accelerated the treatment, and shortened the length of stay in the hospital. Thus, cajuput oil may act as new potential adjuvant therapy.
Eligible patients from the result of inclusion and exclusion were more male than female patients in both groups. This was also according to the condition that more male patients are hospitalized than female patients. The symptom of COVID-19 that was mostly found in the melaleuca cajuput oil group was coughing, while the control group mostly reported fever. All complaints of COVID-19 improved when the PCR results were negative, laboratory and radiological conditions improved, and the patient had finished undergoing treatment at the hospital or had returned home.
In this study, the anti-inflammatory effect of melaleuca cajuput oil in the melaleuca cajuput oil group helped improve the symptoms of COVID-19, showing better progression on blood counts and radiographs than the control group. The active components, 1,8-cineol and α-terpineol, are found in melaleuca cajuput oil and eucalyptus oil. Eucalyptus oil has been used as traditional medicine by people in Europe, Australia, and various other countries, especially to treat coughs, colds, and bronchitis and to relieve symptoms of colds and inflammation of the mucous membranes of the upper respiratory tract. Retrospective studies show that eucalyptus preparations including eucalyptus oil have been used by approximately 12% of asthma patients in the United States and Mexican border populations (Council of Europe, 2004). The recommended dose for inhalation to treat coughing, coughing up mucus, and upper respiratory tract infections in adults is up to 3–8 drops per 250 ml of boiling water, three times a day, and in children between 4 and 12 years of age up to 2–4 drops per 250 ml of boiling water, three times a day.
Based on in silico docking analysis of compounds in M. cajuputi, the essential oil is capable of inhibiting ACE2 and Mpro (PDB6LU7) protein in SARS-CoV-2 (Dev and Kaur, 2020; My et al., 2020). Sharma and Kaur also found that 1,8-cineole from the eucalyptus oil family was a potential inhibitor candidate for COVID-19 treatment. Due to its vital role in polyprotein processing necessary for coronavirus reproduction, the main viral proteinase (Mpro/3CLpro) has recently been regarded as a suitable target for drug design against SARS infection (Dev and Kaur, 2020).
CONCLUSION
Our study had several limitations. First, symptoms and daily laboratory blood tests were not repeated, and the data used were only at hospital admission and the end when the PCR result was negative and the patient was declared cured. Also besides, the patients in the study had no comorbidities, and their smoking status was not considered. Giving cajuput oil is a simple and safe technique even though evaporation will affect the level of the oil. Our study also did not differentiate between the conditions of mild and moderate COVID-19 patients or examine inflammatory parameters, apart from a simple routine laboratory test. This work identified M. cajuputi, one of Indonesia’s traditional herb, as an effective adjuvant to the standard therapy for mild and moderate COVID-19 patients. Further research is necessary to explore this possibility.
ACKNOWLEDGMENTS
The authors express their gratitude to the staff of Bhayangkara Brimob Hospital, who provided medical data and records, as well as all the patients who were involved in this study. The authors would like to thank Scribendi (scribendi.com) for the English language review.
FUNDING
The authors have received no support or funding.
AUTHOR CONTRIBUTIONS
DD: study conception and design, data analysis, drafting/revision of the manuscript, and final approval of the manuscript.
FF: drafting/revision of the manuscript and final approval of the manuscript.
APS: data analysis and final approval of the manuscript.
Written informed consent was obtained from the patients. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses, 2020; 12(3):254. CrossRef
Astani A, Reichling J, Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res, 2010; 24(5):673–9. CrossRef
Bosni? T, Softi? D, Gruji?-Vasi? J. Antimicrobial activity of some essential oils and major constituents of essential oils. Acta Med Acad, 2006; 35:19–22.
Castelli V, Cimini A, Ferri, C. Cytokine storm in COVID-19: “When you come out of the storm, you won’t be the same person who walked in.” Front Immunol, 2020; 11:2132. CrossRef
Council of Europe. European pharmacopoeia. 5th edition, vol. 2. Directorate for the quality of medicines. Council of Europe, Strasbourg, France.
Dev S, Kaur I. Bioactive molecules from eucalyptus essential oil as potential inhibitors of COVID 19 corona virus infection by molecular docking studies. Kragujevac J Sci, 2020; 42:29–43. CrossRef
Garozzo A, Timpanaro R, Stivala A, Bisignano G, Castro A. Activity of Melaleuca alternifolia (tea tree) oil on influenza virus A/PR/8: study on the mechanism of action. Antiviral Res, 2011; 89(1):83–8. CrossRef
Hendry ER, Worthington T, Conway BR, Lambert PA. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J Antimicrob Chemother, 2009; 64(6):1219–25. CrossRef
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 2020; 181(2):271–80.e8. CrossRef
Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect, 2021; 54(2):159–63. CrossRef
My TTA, Loan HTP, Hai NTT, Hieu LT, Hoa TT, Thuy BTP, Quang DT, Triet NT, Van Anh TT, Dieu NTX, Trung NT, Van Hue N, Van Tat P, Tung VT, Nhung NTA. Evaluation of the inhibitory activities of COVID-19 of Melaleuca cajuputi oil using docking simulation. ChemistrySelect, 2020; 5(21):6312–20. CrossRef
Task Force for Handling COVID-19. Map of the distribution of COVID-19. [ONLINE]. Available via https://covid19.go.id/peta-sebaran (Accessed 20 March 2021)
Sebei K, Sakouhi F, Herchi W, Khouja M, Boukhchina S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol Res, 2015; 48(1):7. CrossRef
Sutrisno S, Retnosari R, Poerwandar Asmaningrum H. Profile of the Indonesian essential oil from Melaleuca cajuputi. Adv Eng Res, 2018; 171:14–9. CrossRef
Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B, 2020; 10(5):766–88. CrossRef
Zumla A, Chan JFW, Azhar EI, Hui DSC, Yuen KY. Coronaviruses-drug discovery and therapeutic options. Nat Rev Drug Discov, 2016; 15(5):327–47. CrossRef