INTRODUCTION
Hemophilia A is a congenital hemorrhagic disorder caused by factor VIII (FVIII) genetic abnormality (Castaman and Matino, 2019). It is a disorder that is complex to treat. Hemophilia A accounts for about 80% of approximately 400,000 hemophilia cases worldwide (Machin et al., 2018). Meanwhile, only 25% of hemophilia A cases receive adequate therapy (Kadhim et al., 2019). Arthropathy and cerebrovascular complications remain barriers to normal daily activities performance among hemophilia A patients (Baek et al., 2020). The current routine therapy to a treat bleeding event depends on replacement therapy of clotting factor concentrates since childhood. Meanwhile, chronic factor concentrates therapy causes the development of FVIII inhibitors (Santagostino et al., 2018). Studies have showed that 3%–13% of mild and most cases of moderate-to-severe hemophilia A patients developed FVIII inhibitors (Yamanouchi et al., 2018). This neutralizing antibody certainly causes inadequate replacement therapy and poor medical outcomes (Walsh et al., 2016).
Treatment of patients with FVIII inhibitors including porcine FVIII activated prothrombin complex concentrate and activated recombinant factor VII, desensitization, immune tolerance induction, and a monoclonal antibody that bridge factor IXa and factor X (Meeks and Batsuli, 2016). However, the therapies are difficult to obtain and expensive in the limited healthcare resources of low-income countries. Some studies recommend that steroid drugs may be useful as adjuvant therapy for hemophilia A patients with a higher titer of FVIII inhibitors (Cacciotti et al., 2021).
Methylprednisolone (MP) is a prednisone-derived drug with potent anti-inflammatory and immunosuppressive effects even in low doses. MP also has minimal side effects of water retention, sodium retention, weight gain, and gastric irritation in long-term therapy compared to other steroid derivatives (Permana et al., 2019). It is a cost-effective drug and is widely available in every healthcare setting. Hence, this study aims to investigate the effect of low-dose MP on hemophilia A patients with FVIII inhibitors.
MATERIALS AND METHODS
Hemophilia A patients and study setting
This 6-week randomized pretest–posttest clinical trial with a control group design was conducted from September 2020 to March 2021 at the Hematology Polyclinic of Dr. Moewardi General Hospital, Surakarta, Indonesia. The participant inclusion criteria included male patients older than 18 years with a history of FVIII concentrates therapy for more than 9 exposure days (Witmer and Young, 2013). The exclusion criteria are other bleeding disorders comorbidities, other system organs’ comorbidities, and being currently on steroid drug therapy. During the 3 months of the simple random sampling period, only 11 hemophilia A patients met the study criteria. Then, the participants were randomly divided into a intervention group (n = 6) and a control group (n = 5).
Variables and interventions
FVIII inhibitors were analyzed using the enzyme-linked immunosorbent assay (ELISA) method. The data were measured in ng/ml. In the pretest period, the baseline or pretest clinical parameter data were also measured. They included body weight, fasting blood glucose (FBS), hemoglobin (Hb), white blood cells (WBC), platelet, prothrombin time (PT), and activated partial thromboplastin time (APTT). The FVIII inhibitors and clinical parameter data were tested in both pretest and posttest studies.
The interventions included 1 mg/body weight/day MP for the intervention group and a 100 mg/day sugar pill placebo for the control group. The intervention duration was 6 weeks. The patients were educated not to carry out high-risk activities during the intervention period according to the Hemophilia Activities List. The patients were not allowed to consume vitamin E and certain herbal supplements to prevent the platelets from clumping. Aspirin and nonsteroidal anti-inflammatory drugs were prohibited because they may cause bleeding risk.
Data collection
The FVIII inhibitors and laboratory parameters of FBS, HB, WBC, platelet, PT, and APTT were obtained from participants’ vein blood. The BIOENZY Human Coagulation FVIII ELISA kits were used to analyze the FVIII inhibitors’ titers. It has a sensitivity of 0.28 ng/ml and a standard curve range of 0.5–150 ng/ml. Based on the participant group, either 1 mg/body weight MP or a 100 mg sugar pill was prescribed for as many as 42 drugs for 6 weeks.
Statistical analysis
The paired t-test was used to analyze the pretest–posttest FVIII inhibitors mean comparison of the intervention group and control group. The independent t-test was used to analyze FVIII inhibitors mean comparison of intergroups’ pretest and posttest (Fig. 1). The statistical analyses were conducted using Statistical Package for the Social Sciences 22 statistical software for Windows.
Ethical approval
This study was approved by the Health Research Ethics Committee of Dr. Moewardi General Hospital, No. 1.069/XI/HREC/2019. The study was also conducted following the Helsinki Declaration. All the participants received a study protocol explanation and gave written informed consent. The ethical approval is available for review by the Editor-in-Chief of this journal.
Results and Discussion
The clinical parameters’ baseline or pretest and posttest data were checked to ensure that the MP or placebo administration did not significantly (p > 0.05) affect the clinical and laboratory conditions of the participants (Table 1). MP has a wide range of physiologic effects, including endocrine, immunologic, and hematologic disorders (Yang et al., 2020). The only significant difference between the intervention and control groups in the posttest APTT parameters (p = 0.039) indicated that MP affected the intrinsic pathway of blood coagulation. FVIII inhibitors or autoantibodies synthesis in hemophilia A patients is a multifactorial process and involves cytokines and immune regulatory molecules (Tieu et al., 2020). This T-cell-mediated hypersensitivity is mediated by inflammatory cytokines like interleukin-1, interleukin-6, and tumor necrosis factor-alpha. The administration of MP can inhibit these inflammatory cascades, which significantly shortened the APTT duration in the intervention group (Ebrahimi et al., 2016).
The intervention group continued to undergo a significant increase in FVIII inhibitors levels (p = 0.001) within 6 weeks, as did the control group (Table 2). These indicated that administration of MP neither inhibited nor stopped the synthesis of FVIII inhibitors. The FVIII antibodies’ formation requires the FVIII endocytosis process through the role of antigen-presenting cells (Astermark, 2015). Human leukocyte antigen class II molecules then present the endocytosis results to the surface of the cluster of differentiation 4+ (CD4+) T cells. Moreover, T-regulatory cells (Tregs) have an inhibition role in the FVIII antibody formation process (Khalilian et al., 2020). Tregs inhibit the antigen-specific B-cell differentiation into memory B-cells and CD4+ activation by interleukin-10 (Delignat et al., 2019). Thus, there will be a potentiation effect to inhibit FVIII antibodies synthesis by an immunosuppressive drug. However, the relationship between FVIII inhibitors formation in hemophilia and Tregs function is still inconclusive. It is believed to be due to HMOX-1 gene polymorphism in hemophilia patients (Repessé et al., 2013).
Six weeks after MP and placebo administration, there was a significant difference (p = 0.034) in the mean levels of FVIII inhibitors between the intervention and control groups. The APPT posttest laboratory parameters between the intervention and the control groups are also significant (p = 0.039) (Table 1). A study also showed that prednisone adjuvant therapy alone could control bleeding episodes in hemophilia patients with FVIII inhibitors. Moreover, the study recommended the use of prednisone–cyclophosphamide as second-line therapy for FVIII inhibitors in patients who failed the prednisone adjuvant therapy (Sarah et al., 2016).
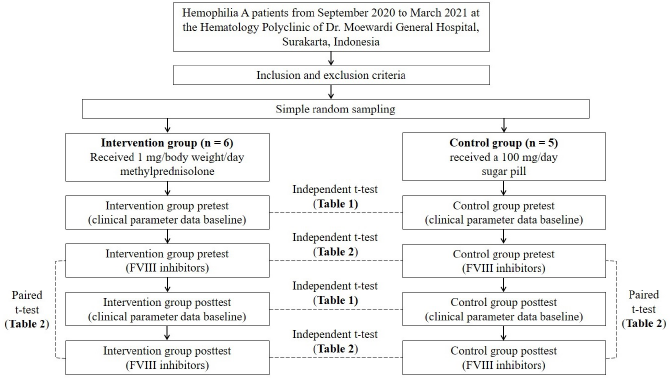 | Figure 1. Graphical flow chart diagram of the study. [Click here to view] |
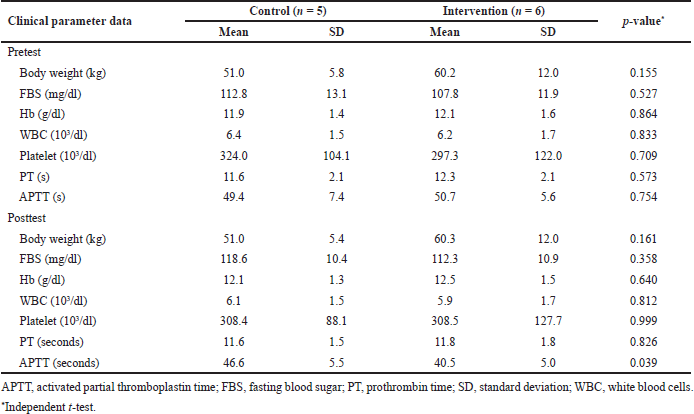 | Table 1. Clinical parameter data baseline or pretest and posttest of both control and intervention groups. [Click here to view] |
CONCLUSION
The low dose of 1 mg/body weight/day MP as adjuvant therapy in hemophilia A patients with FVIII inhibitors can suppress the synthesis pace of FVIII inhibitors within 6 weeks. Its long-term administration has minimal side effects. Furthermore, it can be a cost-effective adjuvant therapy in limited healthcare settings. Although low-dose MP did not inhibit the FVIII inhibitors synthesis, this adjuvant therapy is recommended for hemophilia A patients who received long-term FVIII concentrates therapy. Further research is needed to investigate the other effective therapeutic dose of MP. Similar interventional clinical research with more than 30 hemophilia A patients or different steroidal anti-inflammatory drugs is also needed to support this study result.
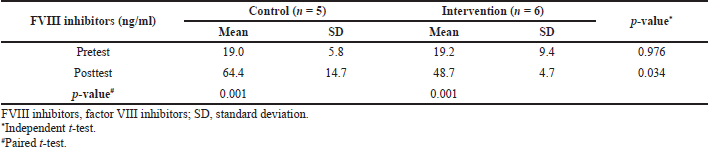 | Table 2. FVIII inhibitors’ pretest–posttest mean comparison of the intervention group and control group, FVIII inhibitors’ intergroups pretest and posttest mean comparison. [Click here to view] |
ACKNOWLEDGMENT
The authors are grateful to the internal medicine specialists of Dr. Moewardi General Hospital for providing feedback and technical support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Astermark J. FVIII inhibitors: pathogenesis and avoidance. Blood, 2015; 125:2045–51. CrossRef
Baek HJ, Park YS, Yoo KY, Cha JH, Kim YJ, Lee KS. Health-related quality of life of moderate and severe haemophilia patients: results of the haemophilia-specific quality of life index in Korea. PLoS One, 2020; 15(9):e0238686. CrossRef
Cacciotti C, Chan A, Bhatt M. Oral corticosteroid therapy as an adjuvant treatment for acute bleeding in hemophilia patients with high titer inhibitors. J Pediatr Hematol Oncol, 2021; 43(2):e237–9. CrossRef
Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica, 2019; 104(9):1702–9. CrossRef
Delignat S, Russick J, Gangadharan B, Rayes J, Ing M, Voorberg J, Kaveri SV, Lacroix-Desmazes S. Prevention of the anti-factor VIII memory B-cell response by inhibition of bruton tyrosine kinase in experimental hemophilia A. Haematologica, 2019; 104(5):1046–54. CrossRef
Ebrahimi L, Kheirandish M, Foroughi M. The effect of methylprednisolone treatment on fibrinolysis, the coagulation system, and blood loss in cardiac surgery. Turk J Med Sci, 2016; 46(6):1645–4. CrossRef
Kadhim KAR, Al-Lami FH, Baldawi KH. Epidemiological profile of hemophilia in Baghdad-Iraq. Inquiry, 2019; 56(20):1–8. CrossRef
Khalilian S, Motovali-Bashi M, Rezaie H. Factor VIII: perspectives on immunogenicity and tolerogenic strategies for hemophilia a patients. Int J Mol Cell Med, 2020; 9:33–49.
Machin N, Ragni M V, Smith KJ. Gene therapy in hemophilia A: a cost-effectiveness analysis. Blood Adv, 2018; 2(14):1792–8. CrossRef
Meeks SL, Batsuli G. Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. Hematol Am Soc Hematol Educ Program, 2016; 2016(1):657–62. CrossRef
Permana D, Barliana M, Hamijoyo L. Dosage and duration of methylprednisolone therapy affect the occurrence of cushing habitus in patients with systemic lupus erythematosus. J Pharm Bioallied Sci, 2019; 11(8):S628–34. CrossRef
Repessé Y, Peyron I, Dimitrov JD, Dasgupta S, Moshai EF, Costa C, Borel-Derlon A, Guillet B, D’Oiron R, Aouba A, Rothschild C, Oldenburg J, Pavlova A, Kaveri SV, Lacroix-Desmazes S. Development of inhibitory antibodies to therapeutic factor VIII in severe hemophilia A is associated with microsatellite polymorphisms in the HMOX1 promoter. Haematologica, 2013; 98(10):1650–5. CrossRef
Santagostino E, Young G, Carcao M, Mannucci PM, Halimeh S, Austin S. A contemporary look at FVIII inhibitor development: still a great influence on the evolution of hemophilia therapies. Expert Rev Hematol, 2018; 11:87–97. CrossRef
Sarah L, Prantik D, Gary B. Systemic therapy in acquired haemophilia—a single institute experience. Ulster Med J, 2016; 85(3):187–92.
Tieu P, Chan A, Matino D. Molecular mechanisms of inhibitor development in hemophilia. Mediterr J Hematol Infect Dis, 2020; 12:e2020001. CrossRef
Walsh CE, Jiménez-Yuste V, Auerswald G, Grancha S. The burden of inhibitors in haemophilia patients. Thromb Haemost, 2016; 116(S 01):S10–7. CrossRef
Witmer C, Young G. Factor VIII inhibitors in hemophilia A: rationale and latest evidence. Ther Adv Hematol, 2013; 4(1):59–72. CrossRef
Yamanouchi J, Tokumoto D, Ikeda Y, Maruta M, Kaneko M, Hato T, Yasukawa M. Development of an fviii inhibitor in a mild hemophilia patient with a phe595cys mutation. Intern Med, 2018; 57(21):3179–82. CrossRef
Yang R, Xiong Y, Ke H, Chen T, Gao S. The role of methylprednisolone on preventing disease progression for hospitalized patients with severe COVID-19. Eur J Clin Invest, 2020; 50(11):e13412. CrossRef