INTRODUCTION
Atherosclerosis is the accumulation of lipids and ?brous elements in large arteries. It is an important contributor to the growing burden of cardiovascular diseases. The three major processes involved in the development of atherosclerosis include in?ammation, oxidation, and hypercholesterolemia/hyperlipidemia (Katselou et al., 2014). The elevated levels of total cholesterol (TC) along with high and low-density lipoprotein concentrations in the plasma are the major risk factors for ischemic heart disease by developing atherosclerosis, whereas an increased level of high-density lipoprotein in the plasma has a protective role against atherosclerosis (Lei et al., 2014). Hyperlipidemia is treated with therapeutic agents like statins, fibrates, niacin, and resins. However, these are also associated with several adverse effects. Therefore, alternative therapy serves as a better approach than conventional therapy in terms of safe and effective use. (Hu et al., 2006).
The management of hyperlipidemia focuses on reducing the elevated plasma concentration of lipids by altering the biosynthesis and elimination of cholesterol. The various experimental studies have demonstrated the role of ginger (Zingiber officinale) as an antihyperlipidemic agent in high-fat diet (HFD) animal models. Ginger is the most commonly used species worldwide. [6]-Gingerol is the major bioactive, pungent, and pharmacological active component of ginger.
The active constituents of ginger are divided into volatile and nonvolatile components. The volatile constituents of ginger are different types of terpenoids, while the nonvolatile constituents include gingerols, shogaols, paradols, and zingerone (Ahmad et al., 2015). Gingerol has a series of chemical analogs due to the presence of the hydroxyl group at different positions. The ginger analogs are [4]-gingerol, [6]-gingerol, [8]-gingerol, [10]-gingerol, and [12]-gingerol (Bhattarai et al., 2007). The presence of aliphatic chain moiety with the hydroxyl group is responsible for the biological activity.
[6]-Gingerol-loaded nanoparticles were prepared with the combination of Eudragit RS100, polyvinyl alcohol, and organic phase. The Box–Behnken design was used to evaluate the role of independent variables on responses, including particle size, % entrapment efficiency (%EE), and zeta potential. The physicochemical properties of the optimized formulation were stable with an acceptable spherical nanoparticle. The particle size, %EE, and zeta potential of the optimized formulation were 323 ± 2.36 nm, 82 ± 0.89, and +24 ± 1.11 mV, respectively. The present study investigates the hypolipidemic potential of [6]-gingerol-loaded Eudragit nanoparticles in the HFD-induced hyperlipidemic animal model.
MATERIALS AND METHODS
Materials
[6]-Gingerol was purchased from Shaanxi Pioneer Biotech Co. Ltd., and polymer, solvents, and reagents were purchased from Sigma-Aldrich, India. All the other solvents and chemicals used in the experiment were purchased from Fisher Scientific, Mumbai, India.
Male Wistar albino rats (160–200 g) were used. Animals were procured from Central Drug Research Institute (CDRI), Lucknow, and approved by the Institutional Animal Ethics Committee (IAEC/SHUATS/PA/2017III/SVKS02, dated: April 10, 2017). The animals were kept under standard housing conditions in a polyacrylic cage (humidity = 60%–65% and room temperature = 24°C–27°C, with 12:12 light:dark cycle). A normal animal diet with purified water was provided. The experiment was conducted as per the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals.
Methods
Formulation of [6]-Gingerol-loaded Eudragit nanoparticles
We prepared [6]-gingerol-loaded Eudragit nanoparticles using the emulsification solvent evaporation method (Vardhan et al., 2017). [6]-Gingerol (100 mg) and Eudragit (446 mg) were dissolved in acetone to form an organic phase that was added dropwise in an aqueous phase consisting of an aqueous solution of polyvinyl alcohol (4% w/v) and was stirred continuously to form a uniform mixture.” The mixture was homogenized by high shear homogenizer (IKA) at 15,000 rpm for 10 minutes. The homogenized mixture was sonicated via an Ultra Probe Sonicator (UP50H, Hielscher) for 12.5 minutes. The obtained product was left overnight for evaporation of the organic solvent. The residue was centrifuged, lyophilized, and stored in an airtight container for further use.
In-vitro drug release profile
The dialysis membrane of 12,000–14,000 Da was used for the in-vitro drug release profile. The optimized formulation was kept in a dialysis membrane (bag) and immersed in a beaker containing phosphate buffer at pH 7.4. The beaker was placed on a magnetic stirrer for uniform mixing and temperature was maintained at 37°C ± 0.5°C. The samples were collected at 1, 2, 4, 8, 12, 16, 20, and 24 hours. The collected samples were analyzed using UV-visible spectroscopy at a wavelength of 430 nm.
Induction of hyperlipidemia
The efficacy of the prepared compound was tested against hyperlipidemia using a HFD animal model.
Hyperlipidemia was induced in animals by feeding cholesterol (500 mg/kg) and cholic acid (250 mg/kg) suspension in groundnut oil (10 ml/kg) once daily for 28 days (Bidkar et al., 2012; Yan et al., 2006).
The animals were divided into five different groups, with six animals in each group. Animals in Group I (non-hyperlipidemic control) were fed a normal pellet diet and treated with distilled water (vehicle); animals in Group II (hyperlipidemic control) were fed a HFD and treated with vehicle; and animals in Group III, IV, and V (treated groups) were fed a HFD and treated with [6]-gingerol of 25 and 50 mg/kg body weight and atorvastatin of 20 mg/kg body weight, respectively. Treatment was given orally, once daily for 28 days. On day 0, 7, 14, and 28, the blood samples were collected via retro-orbital sinus and triglycerides (TG), TC, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL-C) levels were analyzed using an automated analyzer (Beckman Coulter AU480).
Concluding the experiment, the animals were sacrificed. The heart, liver, and kidney organs were isolated for histopathological examination.
Cardiac risk index and Atherogenic index (AI)
AI = (TC − HDL-C)/HDL-C (Dobiasova and Frohlich, 2001; Lemieux et al., 2001).
Coronary risk index (CRI) = TC / HDL-C (Lemieux et al., 2001).
Histopathology
For histopathological analysis, the transverse sections of the organ were prepared with a thickness of 5 µm. The hematoxylin and eosin-dyed sections were mounted with Canada Balson. The organ section was fixed in 10% v/v formalin and placed in paraffin. The slides were viewed by an optical microscope at 200× magnification (Donates, 2014).
Radiolabeling and scintigraphy evaluation
Gamma scintigraphy is a noninvasive technique to determine the release characteristics of the radiolabeled formulation and is a measure of gastrointestinal (GI) performance (Davis et al., 1992; Wilding et al., 2001). The optimized formulation was labeled with the radioactive substance 99mTechnetium (99mTc) using a stannous-based reduction method. 99mTc forms a covalent complex with the optimized formulation (Sharma et al., 2017). The radiolabeling binding was determined by thin-layer chromatography using acetone and chloroform in a 7:3 ratio as mobile phase. The quantification of radiolabeling was done by gamma counter (Captintec, USA). The formulation–99mTc complex was administered orally to the rat for investigating the gastric-retention time of the radiolabeled formulation.
Statistical analysis
The results are presented in mean ± SEM. The data was analyzed using one-way ANOVA, followed by Dunnett’s test (GraphPad Prism 5). p-value ≤ 0.005 was considered significant.
RESULT AND DISCUSSION
The drug release kinetics were studied by the Higuchi model and Peppas–Korsmayer’s model by comparing the regression coefficient value for zero-order and first-order (Fazil et al., 2012; Masood et al., 2013; Zhang et al., 2013). The values closer to 1 are considered standard drug release kinetic data values. The release of [6]-gingerol from Eudragit-loaded nanoparticles was calculated using Peppas–Korsmeyer’s kinetic model (Fig. 1). The regression coefficient of 0.918 was found to be significant for pharmacological action.
The plasma TC, TG, HDL-C, LDL-C, and VLDL-C levels of the non-hyperlipidemic control, hyperlipidemic control, [6]-gingerol (25 and 50 mg/kg), and atorvastatin-treated groups on day 0, 7, 14, 21, and 28 are shown in Table 1.
The plasma TC of Group (GP)-I (non-hyperlipidemic control) was found to be uniform and the average mean value was 67.42 ± 0.33 mg/dl from day 0 to 28, whereas the plasma TC of GP-II (hyperlipidemic control) was found to be 67 ± 2.63 mg/dl on day 0, which gradually increased to 119.83 ± 5.06, 178.33 ± 4.08 and 210.33 ± 4.55 mg/dl on day 7, 14, and 28, respectively. However, the plasma TC of GP-III (the hyperlipidemic-treated group with 25 mg/kg of [6]-gingerol) was found to be varied from 65.33 ± 4.409 to 92.83 ± 3.32 mg/dl and the plasma TC of GP-IV (the hyperlipidemic-treated group with 50 mg/kg of [6]-gingerol) varied from 67.5 ± 2.14 to 99.17 ± 2.104 mg/dl. The plasma TC of GP-V (the hyperlipidemic-treated group with 20 mg/kg of atorvastatin) was in the range of 65.17 ± 3.68 to 97.17 ± 4.29 mg/dl. Both the groups treated with [6]-gingerol, (GP-III and GP-IV) and the group treated with standard atorvastatin (GP-V) showed a marked increase in TC levels on day 7 and a significant reduction on day 14 and 28 of treatment.
The plasma TG levels of GP-I (non-hyperlipidemic control) was found to be uniform and the average mean value was 81.38 ± 0.20 mg/dl from day 0 to 28, whereas the plasma TG of GP-II (hyperlipidemic control) was found to be 83.67 ± 2.2 mg/dl on day 0, which gradually increased to 131.83 ± 2.57, 169 ± 3.58, and 198.33 ± 4.39 mg/dl on day 7, 14, and 28, respectively. However, the plasma TG of GP-III (the hyperlipidemic-treated group with 25 mg/kg of [6]-gingerol) was found to be varied from 81.33 ± 1.58 to 116.17 ± 2.72 mg/dl and the plasma TG of GP-IV (the hyperlipidemic-treated group with 50 mg/kg of [6]-gingerol) varied from 82.33 ± 2.08 to 124 ± 2.34 mg/dl. The plasma TG of GP-V (the hyperlipidemic-treated group with 20 mg/kg of standard atorvastatin) was in the range of 78.5 ± 1.89 to 106.33 ± 2.25 mg/dl. Both the groups treated with [6]-gingerol, (GP-III and GP-IV) and the group treated with standard atorvastatin (GP-V) showed a marked increase in TG levels on day 7 and a significant reduction on day 14 and 28 of treatment.
The plasma HDL-C levels of GP-I (non-hyperlipidemic control) was found to be uniform and the average mean value was 33.79 ± 0.54 mg/dl from day 0 to 28, whereas the plasma HDL-C of GP-II (hyperlipidemic control) was found to be 32.17 ± 1.11 mg/dl on day 0, which gradually decreased to 29 ± 1.06, 27.17 ± 1.14, and 25.33 ± 1.14 mg/dl on day 7, 14, and 28, respectively. However, the plasma HDL-C of GP-III (the hyperlipidemic-treated group with 25 mg/kg of [6]-gingerol) was found to be varied from 31.83 ± 1.05 to 27 ± 0.63 mg/dl and the plasma HDL-C of GP-IV (the hyperlipidemic-treated group with 50 mg/kg of [6]-gingerol) varied from 36 ± 1.39 to 32.83 ± 0.17 mg/dl. The plasma HDL-C of GP-V (the hyperlipidemic-treated group with 20 mg/kg of standard atorvastatin) was in the range of 34.17 ± 1.25 to 32.33 ± 1.5 mg/dl. In the GP-III (the hyperlipidemic-treated group with [6]-gingerol 25 mg/kg), no significant changes were observed in the plasma HDL-C levels as compared to the hyperlipidemic non-treated control group; in both the non-treated GP-II group and treated GP-III group the plasma (HDL-C) levels showed a significant decline on day 7, 14, and 28, respectively. However, in the GP-IV (the hyperlipidemic-treated group with [6]-gingerol 50 mg/kg), the serum HDL-C levels significantly reduced on day 7 and 14 and showed a slight increase on day 28 of treatment. The GP-V (the hyperlipidemic-treated group with 20 mg/kg of standard atorvastatin) showed equal value reduction of HDL-C levels on day 7 and 14 with an increase on day 28, which was nearly similar to day 0 (HDL-C) levels. Hence, the plasma HDL-C levels in the [6]-gingerol-treated groups, GP-IV group, was found to be more promising than the GP-III group.
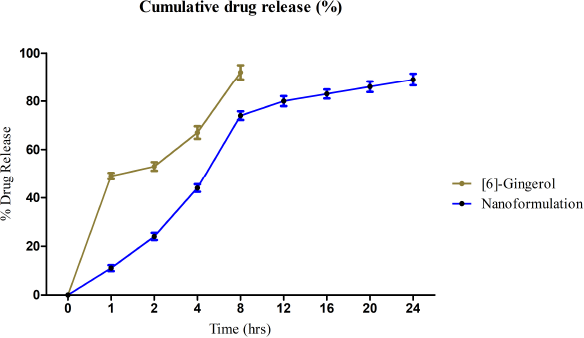 | Figure 1. In-vitro release profile of [6]-gingerol from nanoformulation in phosphate buffer (pH 7.4). [Click here to view] |
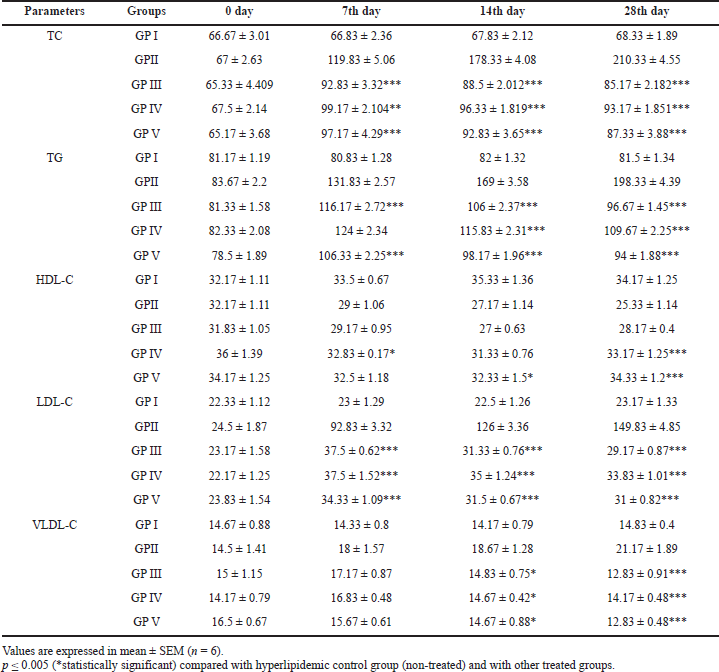 | Table 1. Effect of [6]-gingerol on serum TC, TG, HDL-C, LDL-C, and VLDL-C levels of treated animals on day 0, 7, 14, and 28 of the HFD-induced animal (rat) model. [Click here to view] |
The plasma LDL-C levels of GP-I (non-hyperlipidemic control) were found to be uniform and the average mean value was 67.42 ± 0.33 mg/dl from day 0 to 28, whereas the plasma LDL-C of GP-II (hyperlipidemic control) was found to be 24.5 ± 1.87 mg/dl on day 0, which gradually increased to 92.83 ± 3.32, 126 ± 3.36, and 149.83 ± 4.85 mg/dl on day 7, 14, and 28, respectively. However, the plasma LDL-C of GP-III (the hyperlipidemic-treated group with 25 mg/kg of [6]-gingerol) was found to be varied from 23.17 ± 1.58 to 37.5 ± 0.62 mg/dl and the plasma LDL-C of GP-IV (the hyperlipidemic-treated group with 50 mg/kg of [6]-gingerol) varied from 22.17 ± 1.25 to 37.5 ± 1.52 mg/dl. The plasma LDL-C of GP-V (the hyperlipidemic-treated group with 20 mg/kg of standard atorvastatin) was in the range of 23.83 ± 1.54 to 34.33 ± 1.09 mg/dl. Both the groups treated with [6]-gingerol, GP-III and GP-IV, and the group treated with standard atorvastatin, GP-V, showed a marked increase in LDL-C levels on day 7 and a significant reduction on day 14 and 28 of treatment.
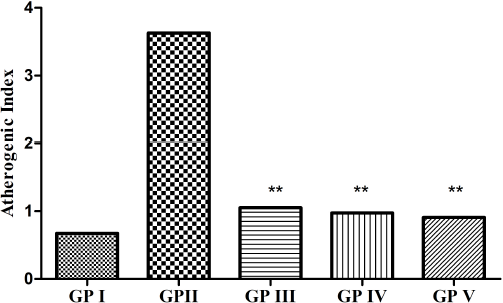 | Figure 2. AI of GP-I (non-hyperlipidemic control), GP-II (hyperlipidemic control), GP-III (treated with [6]-gingerol 25 mg/kg), GP-IV (treated with [6]-gingerol 50 mg/kg), and GP-V (treated with atorvastatin 20 mg/kg). Values expressed as mean (n = 6). **p ≤ 0.005 (statistically significant) when compared with GP II. [Click here to view] |
The plasma VLDL-C levels of GP-I (non-hyperlipidemic control) were found to be uniform and the average mean value was 14.50 ± 0.12 mg/dl from day 0 to 28, whereas the plasma VLDL-C of GP-II (hyperlipidemic control) was found to be 14.5 ± 1.41 mg/dl on day 0, which gradually increased to 18 ± 1.57, 18.67 ± 1.28, and 21.17 ± 1.89 mg/dl on day 7, 14, and 28, respectively. However, the plasma VLDL-C of GP-III (the hyperlipidemic-treated group with 25 mg/kg of [6]-gingerol) was found to be varied from 12.83 ± 0.91 to 17.17 ± 0.87 mg/dl and the plasma VLDL-C of GP-IV (the hyperlipidemic-treated group with 50 mg/kg of [6]-gingerol) varied from 14.17 ± 0.48 to 16.83 ± 0.48 mg/dl. The plasma VLDL-C of GP-V (the hyperlipidemic-treated group with 20 mg/kg of standard atorvastatin) was in the range of 12.83 ± 0.48 to 16.5 ± 0.67 mg/dl. Both the groups treated with [6]-gingerol, GP-III and GP-IV, showed a marked increase in VLDL-C levels on day 7, whereas the GP-IV group treated with standard atorvastatin showed a slight increase. However, VLDL-C levels were found to be gradually decreasing on day 14 and 28 in the [6]-gingerol-treated groups, GP-III and GP-IV, and standard atorvastatin group, GP-V, with a marked decrease was observed on day 28 of treatment.
AI and CRI are the predictive markers for evaluating the risk of atherosclerosis and coronary heart disease. The AI of the experimental groups is shown in Figure 2. In our study, it was found that the AI of the treated groups (GP-III and GP-IV) was significantly decreased as compared to the non-treated group (GP-II) and no significant difference was seen between the treated groups (GP-III and GP-IV) with the standard group (GP-V). The CRI of the experimental groups is shown in Figure 3. In the present study, it was found that the CRI of the treated groups (GP-III and GP-IV) showed a marked decrease as compared to the non-treated group (GP-II) and no significant difference was observed between the treated groups (GP-III and GP-IV) with the standard group (GP-V). However, among the treated groups, GP-IV exhibited greater significance as compared to GP-III. We found that AI and CRI had an affirmative correlation with the lipid surrogate markers.
Histopathology was performed on the group that had received the highest dose (50 mg/kg) of [6]-gingerol. The transverse sections of kidney, heart, and liver were prepared and observed. Figure 4 shows the observational findings of the kidney section. The slide showed normal glomeruli, focal hemorrhage, dilated blood vessels, tubules with minimal cloudy change, vessels normal, interstitial tissue minimal, and no inflammation/abnormality. Figure 5 shows the observational findings of the heart section. The slide showed congested blood vessels, focal myocardial inflammation, pericardium, and endocardium within normal limits. Figure 6 shows the observational findings of the liver section. The slide showed lobular architecture, portal triad normal, mild lymphocytic infiltrate in periportal area, and trabeculae show normal thickness. We found that the histopathological findings were nonsignificant.
Evaluation of gastric-retention time of formulation using scintigraphy technique
The radiolabeling efficiency of covalent complex formulation–99mTc was 93% at 4 hours, 91.7% at 8 hours, and 89% after 24 hours. The GI performance was determined by gamma scintigraphy (Siemens, Germany). The real-time serial scanning was done at 4, 8, 12, and 24 hours after the oral administration of the complex. The changing intensity of impressions was recorded with time. The captured real-time image (Fig. 7) showed the 24-hour gastric-retention time.
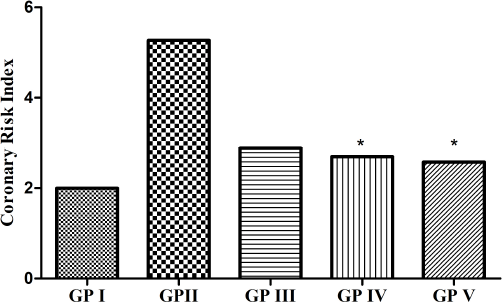 | Figure 3. CRI of GP-I (non-hyperlipidemic control), GP-II (hyperlipidemic control), GP-III (treated with [6]-gingerol 25 mg/kg), GP-IV (treated with [6]-gingerol 50 mg/kg), and GP-V (treated with atorvastatin 20 mg/kg). Values expressed as mean (n = 6). *p ≤ 0.005 (statistically significant) when compared with GP II. [Click here to view] |
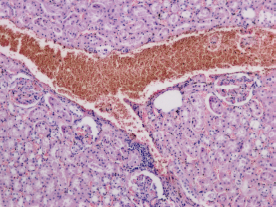 | Figure 4. Transverse section of the kidney of animal treated with [6]-gingerol 50 mg/kg. [Click here to view] |
The results of our study are substantiated by the study conducted by Nammi et al. (2009), in which the ethanolic extract of Zingiber officinale showed a protective effect against the HFD animal model on rats at doses of 100, 200, and 400 mg/kg body weight. The elevated levels of glucose, TG, TC, LDL-C phospholipids, and free fatty acids after HFD treatment were decreased significantly when treated with ethanolic extract of Zingiber officinale, but there was no significant change in the HDL-C level. The study results are also corroborated by a study conducted by Kadnur and Goyal (2005), in which they observed a significant decrease in fructose-induced elevation in lipid levels when treated with methanolic extract of Zingiber officinale. The study outcomes also bear resemblance with the study conducted by Bhandari et al. (1998), in which they investigated the hypolipidemic potential of ethanolic extract of Zingiber officinale (200 mg/kg, p.o.) in cholesterol-fed rabbits. The increased levels of TC, TG, phospholipids, and lipoproteins in serum and tissue after cholesterol feeding was significantly decreased by ethanolic extract of ginger in comparison with gemfibrozil as standard.
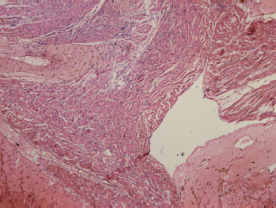 | Figure 5. Transverse section of the heart of animal treated with [6]-gingerol 50 mg/kg. [Click here to view] |
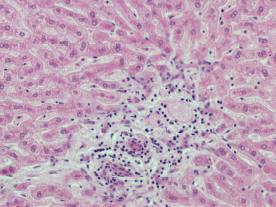 | Figure 6. Transverse section of the liver of animal treated with [6]-gingerol 50 mg/kg. [Click here to view] |
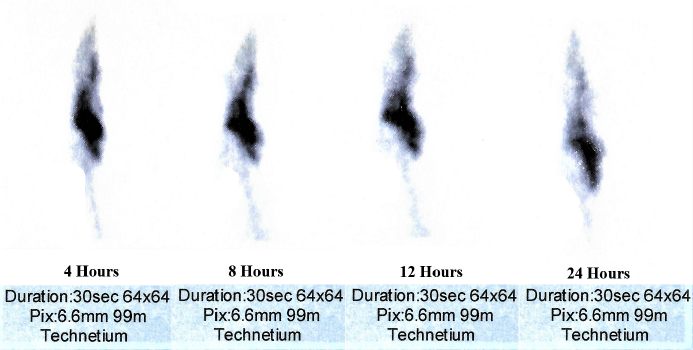 | Figure 7. Gamma scintigraphy showing the serial scanning at 4, 8, 12, and 24 hours after oral administration of radiolabeled nanoformulation. [Click here to view] |
CONCLUSION
The present study demonstrated a significant antihyperlipidemic action of [6]-gingerol containing nanoformulation in HFD-induced rats. The [6]-gingerol-loaded Eudragit polymeric nanoparticles showed significant lipid-lowering potential with reduced TC, TG, LDL-C, VLDL-C, and increased HDL-C levels. After 28 days of treatment with nanoformulation, the lipid profile of both nanoformulation-treated groups (p ≤ 0.005) significantly altered the plasma lipid levels to the normal range. No significant differences were observed in the antihyperlipidemic profile between the nanoformulation-treated groups (GP-III and GP-IV). However, GP-IV with a dose of 50 mg/kg was found to give more encouraging results as compared to GP-III with a dose of 25 mg/kg. Further studies are suggested for investigating the possible mechanism(s) of action and to develop a new sustained release dosage form. However, the findings are of great interest and clinical significance.
ACKNOWLEDGMENT
The authors express their gratitude to Prof. Avinash Chandra Pandey, Nano-Application Centre (NAC), Allahabad University, for his continued guidance and support.
CONFLICT OF INTEREST
The authors declare that the research work is novel and they have no conflict of interest.
FUNDING
The study was not sponsored by any government or industrial organization.
ETHICAL APPROVAL
The study was approved by the Institutional Animal Ethics Committee (IAEC) with Reg No.: IAEC/SHUATS/PA/2017III/SVKS02.
AUTHORS’ CONTRIBUTION
All authors have significantly contributed to the concept, design, acquisition, analysis, and interpretation of data; draft and critical revision of the manuscript for relevant intellectual content; and gave final approval for publication in the current issue of the journal. All authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICJME) requirement/guidelines and are accountable for all aspects of the work.
REFERENCES
Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, Afzal I, Hussain I, Bilal S. A review on pharmacological properties of zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci World J, 2015; 2015:816364. CrossRef
Bhandari U, Sharma JN, Zafar R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J Ethnopharmacol, 1998; 61(2):167–71. CrossRef
Bhattarai S, Tran VH, Duke CC. Stability of [6]-gingerol and [6]-shogaol in simulated gastric and intestinal fluids. J Pharm Biomed Anal, 2007; 45(4):648–53. CrossRef
Bidkar JS, Ghanwat DD, Bhujbal MD, Dama GY. Anti-hyperlipidemic activity of Cucumis melo fruit peel extracts in high cholesterol diet induced hyperlipidemia in rats. J Complement Integr Med, 2012; 9(1):Article 22. CrossRef
Davis SS, Hardy JG, Newman SP, Wilding IR. Gamma scintigraphy in the evaluation of pharmaceutical dosage forms. Eur J Nucl Med, 1992; 19(11):971–86. CrossRef
Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin Biochem, 2001; 34(7):583–8. CrossRef
Donates YC. Pathogenic role of IL-15 in non-alcoholic fatty liver disease. Doctoral dissertation, Université de Sherbrooke, Sherbrooke, Quebec, 2014.
Fazil M, Md S, Haque S, Kumar M, Baboota S, Kaur Sahni J, Ali J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci, 2012; 47(1):6–15. CrossRef
Hu SH, Liang ZC, Chia YC, Lien JL, Chen KS, Lee MY, Wang JC. Antihyperlipidemic and antioxidant effects of extracts from Pleurotuscitrinopileatus. J Agric Food Chem, 2006; 54(6):2103–10. CrossRef
Kadnur SV, Goyal RK. Beneficial effects of Zingiber officinale Roscoe on fructose induced hyperlipidemia and hyperinsulinemia in rats. Indian J Exp Biol, 2005; 43:1161–4.
Katselou MG, Matralis AN, Kourounakis AP. Multi-target drug design approaches for multifactorial diseases: from neurodegenerative to cardiovascular applications. Curr Med Chem, 2014; 21(24):2743–87. CrossRef
Lei L, Liu Y, Wang X, Jiao R, Ma KY, Li YM, Wang L, Man SW, Sang S, Huang Y, Chen ZY. Plasma cholesterol-lowering activity of gingerol-and shogaol-enriched extract is mediated by increasing sterol excretion. J Agric Food Chem, 2014; 62(43):10515–21. CrossRef
Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, Dagenais GR, Després JP. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med, 2001; 161(22):2685–92. CrossRef
Masood F, Chen P, Yasin T, Hasan F, Ahmad B, Hameed A. Synthesis of poly-(3-hydroxybutyrate-co-12 mol% 3-hydroxyvalerate) by Bacillus cereus FB11: its characterization and application as a drug carrier. J Mater Sci Mater Med, 2013; 24(8):1927–37. CrossRef
Nammi S, Sreemantula S, Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol, 2009; 104(5):366–73. CrossRef
Sharma BG, Khanna K, Kumar N, Nishad DK, Basu M, Bhatnagar A. Development and gamma scintigraphy evaluation of gastro retentive calcium ion-based oral formulation: an innovative approach for the management of gastro-esophageal reflux disease (GERD). Drug Dev Ind Pharm, 2017; 43(11):1759–69. CrossRef
Vardhan H, Mittal P, Adena SK, Mishra B. Long-circulating polyhydroxybutyrate-co-hydroxyvalerate nanoparticles for tumor targeted docetaxel delivery: formulation, optimization and in vitro characterization. Eur J Pharm Sci, 2017; 99:85–94. CrossRef
Wilding IR, Coupe AJ, Davis SS. The role of γ-scintigraphy in oral drug delivery. Adv Drug Deliv Rev, 2001; 46(1–3):103–24. CrossRef
Yan LP, Chan SW, Chan AS, Chen SL, Ma XJ, Xu HX. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci, 2006; 79(4):324–30. CrossRef
Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliv Rev, 2013; 65(1):104–20. CrossRef