INTRODUCTION
Malaria remains one of the deadliest infectious diseases worldwide causing approximately 409,000 deaths in 2019, with higher mortality rates among children aged below 5 years and pregnant women (WHO, 2020). The disease is caused by protozoan parasites of the genus Plasmodium that is spread to humans during the blood meal of infected Anopheles mosquitoes. Malarial infection triggers robust host immune responses characterized by an inflammatory reaction, mediated by cytokines as well as parasite cytoadhesion in several organs, predominantly in the livers and spleens of infected hosts (Brugat et al., 2014). Rapid induction of proinflammatory mediators, including interleukin (IL)-1, IL-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, is critical for the resolution of malaria (Bakir et al., 2011; Junaid et al., 2017; Mubaraki et al., 2016). Nonetheless, the exuberant inflammatory response has been associated with the development of immunopathology and host tissue damage (Kumar et al., 2019). Accordingly, IL-10 has emerged as a potent immunoregulatory cytokine to dampen excessive inflammation and consequently reduce progressive tissue damage (Redpath et al., 2014). Therefore, an intricate balance between pro- and anti-inflammatory mediators is fundamental to the appropriate control of parasite growth and the prevention of excessive inflammation and tissue damage (Bakir et al., 2011).
The widespread prevalence of antimalarial drug resistance poses an enormous challenge to the global malaria control programs and elimination strategies. Artemisinin-based combination therapies (ACTs), which are the current first- and second-line malaria treatment in endemic countries, have shown signs of therapeutic failure in Southeast Asia primarily mediated by mutations in a parasite gene known as Kelch13 (Ippolito et al., 2021). The consequence of this treatment failure would be devastating since alternatives to ACTs are not readily available (Miguel-Blanco et al., 2021). Therefore, there is a pressing need for new antimalarial agents acting on novel molecular targets. Natural products especially plants used in folk medicine harbor a huge repertoire of yet untapped resources for novel, diverse, and affordable antimalarial drug lead compounds (Christensen, 2021). In fact, two of the most notable antimalarial drugs, quinine, and artemisinin, were originally derived from medicinal plants (Uzor, 2020).
Goniothalamus lanceolatus Miq. is found native to Borneo, including the rainforest jungles of Sarawak, Malaysia. It is a member of the Annonaceae family and has been used by the indigenous people of Sarawak as a remedy for fever, colds, and skin diseases. The leaf and stem infusions are taken orally to help reduce fever and relieve symptoms of colds while the roots are used in aromatic steam baths to treat skin diseases (Wiart, 2007). Compelling evidence from previous preliminary screenings of G. lanceolatus crude extracts demonstrated that the dichloromethane (DCM) stem bark extract exhibited the most active in vivo antiplasmodial activity, which was comparable to the standard antimalarial drug chloroquine (Zohdi et al., 2017). At a dose of 30 mg/kg bwt, the extract exhibited the highest chemosuppressive effect (66.3%) and was able to increase the survival rate of Plasmodium berghei-infected mice without causing acute toxicity (LD50 > 300 mg/kg). Nonetheless, the therapeutic implications of the extract in modulating the host immune response and malaria pathogenesis have not been established. Thus, this study was carried out to elucidate the therapeutic role of the DCM stem bark extract of G. lanceolatus during malarial infection using a P. berghei-infected mouse model for malaria. The levels of several proinflammatory cytokines such as IL-1α, IL-1β, IL-6, TNF-α, and IFN-γ and the anti-inflammatory cytokine IL-10 were measured as these cytokines are critically involved in cell communication and immune system activation (Bakir et al., 2011; Chin et al., 2019; Junaid et al., 2017). Additionally, the efficacy of the extract to ameliorate the pathogenesis of malaria-infected mice was evaluated using histopathological analysis.
MATERIALS AND METHODS
Plant material and extract preparation
The collection of the plant was carried out in June 2012 from the Malaysian State of Sarawak. The plant material was identified and authenticated by the late Professor Dr. Kamaruddin Mat Salleh formerly of Universiti Kebangsaan Malaysia. A voucher specimen (FBAUMS 108) was deposited at the Herbarium of Universiti Malaysia Sarawak for reference purposes. The DCM stem bark extract was prepared following the protocol described earlier (Kaharudin et al., 2020).
Experimental animals
Twenty-four male BALB/c mice (4–5 weeks old, weighing 25–30 g) were procured from the Laboratory Animal Facility and Management, Universiti Teknologi MARA (UiTM) Selangor Branch, Puncak Alam Campus. The animals were allowed to acclimatize for 1 week in the animal house and had ad libitum access to a standard pellet diet and drinking water. All animal procedures were conducted in conformity with the National and International Guidelines for Handling of Laboratory Animals with approval from the UiTM Animal Research and Ethics Committee (103/2015).
Parasite inoculation
Chloroquine-sensitive P. berghei (NK65) was obtained from the Malaria Research and Reference Reagent Resource Centre (MR4, Manassas, VA) and maintained by serial passage in mice on a weekly basis. The establishment of malarial infection in BALB/c mice was adopted from a method described by Peters et al. (1975). The mice were intraperitoneally injected with 0.2 ml of 1 × 107 parasitized erythrocytes. Parasitemia was monitored daily by microscopic analysis of Giemsa-stained thin blood smears.
Experimental design
The infected mice were randomly divided into three groups of six mice each. Group I received 30 mg/kg bwt of the DCM stem bark extract. The dose of the plant extract was selected based on a previous report (Zohdi et al., 2017). Group II received chloroquine at 1 mg/kg bwt while Group III was given 0.2 ml/kg bwt of the vehicle (Tween 60). All doses were dissolved in the vehicle and administered intraperitoneally starting 4 hours after infection and continued once daily for four consecutive days. Additionally, normal BALB/c mice receiving 0.2 ml normal saline were also included as the uninfected control group.
Cytokines measurement
On day 8 after infection, blood was collected by cardiac puncture while the animals were under general anesthesia produced via inhalation of diethyl ether. Whole blood was collected in a heparinized syringe, immediately transferred into a sterile, pyrogen-free test tube, and centrifuged for 10 minutes at 2,500 rpm. Proinflammatory cytokines IL-1α, IL-1β, IL-6, TNF-α, and IFN-γ and anti-inflammatory cytokine IL-10 were measured in the mouse plasma using single-analyte enzyme-linked immunosorbent assay kits as per manufacturer instructions. The animals were euthanized by cervical dislocation and the liver and spleen collected from each mouse for histopathological analysis.
Histopathological analysis
The liver and spleen were fixed in 10% buffered formalin and subjected to conventional histological processes for histopathological examination. Briefly, the tissue samples were processed overnight for dehydration, clearing, and impregnation using an automated tissue processor (Model TP1020, Leica, Germany). Then, the processed tissue samples were embedded in paraffin wax and sectioned at 3–5 μm thickness for routine hematoxylin and eosin (H&E) staining. The histopathological analyses were performed in a blinded manner by two observers at 400× magnification using a Leica DM2000 light microscope equipped with the ICC50 HD camera (Leica, Germany) and analysis system (Leica Application Suite 4.0).
Hepatic lesions were evaluated and scored based on the severity of four histological features as proposed by Viriyavejakul et al. (2014) with slight modifications. The four features include fatty change, inflammatory cell infiltration, hyperplastic Kupffer cells, necrosis, and hemozoin deposition. Accordingly, the degree of splenic injury was scored based on the expansion of white pulp areas, degree of leukocytes infiltration, and hemozoin depositions as previously described (Giamarellos-Bourboulis et al., 2006; Murshed et al., 2020). The severity level of each histopathological change was graded on a scale from 0 to 3, according to a semiquantitative scoring system, as shown in Table 1. The mean values for hepatic histopathological scores (HHS) and splenic histopathological scores (SHS) were determined, respectively.
Statistical analysis
Values are presented as mean ± standard error mean (SEM). The data were analyzed using one-way analysis of variance followed by Tukey’s test for variables with parametric distributions and the Kruskal–Wallis rank test for variables with nonparametric distributions. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) program for Windows, version 17.0 (SPSS, Chicago, IL). The difference between groups was considered significant when p < 0.05.
RESULTS
Cytokine levels in plasma
As shown in Figure 1, the levels of pro- and anti-inflammatory cytokines were elevated in the P. berghei-infected animals in comparison with the uninfected control mice. As anticipated, the vehicle-treated mice exhibited significantly (p < 0.05) increased levels of both pro- and anti-inflammatory cytokines compared to the uninfected control mice. Interestingly, the mice treated with 30 mg/kg bwt of the DCM stem bark extract resulted in significantly (p < 0.05) lower levels of IL-1α, IL-1β, IL-6, and IFN-γ albeit a persistent level of IL-10 when compared with the vehicle-treated group. Likewise, the chloroquine-treated group showed reduced levels of proinflammatory cytokines, with significant (p < 0.05) reduction of the IFN-γ level as compared to the vehicle-treated group. However, the level of IL-10 remained elevated in the chloroquine-treated mice.
Histopathological examination
Histopathological analysis revealed that malarial infection induced severe damage to the histoarchitecture of the livers and spleens of the experimental animals. The livers of vehicle-treated mice showed marked inflammatory infiltrates with swollen hepatocytes and large clusters of hemozoin (malarial pigment) within the hyperplastic Kupffer cells (Fig. 2B). In general, P. berghei infection induced significant (p < 0.05) increase in HHS as shown in Figure 4A. However, treatment with the DCM stem bark extract (30 mg/kg bwt) was effective in reducing this damage. The liver sections of the infected mice treated with the extract showed improvement in the hepatocellular structure with mild inflammatory infiltrates and less hemozoin deposition (Fig. 2C). These observations were further supported by a significantly (p < 0.05) reduced value of HHS in the extract-treated mice (Fig. 4A). Although the mice treated with chloroquine showed improved liver parenchyma with viable hepatocytes, moderate amounts of hemozoin pigment were still observed (Fig. 2D). Furthermore, the HHS was high in the chloroquine-treated animals with no significant (p > 0.05) difference when compared to the vehicle-treated group (Fig. 4A).
The spleens from the vehicle-treated mice showed evidence of tissue damage with poorly discernible regions of red and white pulp. There was extensive disorganization of the white pulp and loss of germinal center with hemozoin deposition (Fig. 3B). In contrast, there was less extensive lesion characterized by hyperplastic changes and enlargement of the white pulp in the DCM stem bark extract-treated mice (Fig. 3C). Moreover, the SHS was significantly (p < 0.05) reduced in the extract-treated mice when compared to the vehicle-treated group (Fig. 4B). The chloroquine-treated mice exhibited moderately disorganized splenic pulp with poorly defined white pulp regions (Fig. 3D). Moreover, the SHS was high with no significant (p > 0.05) difference when compared to the vehicle-treated group (Fig. 4B).
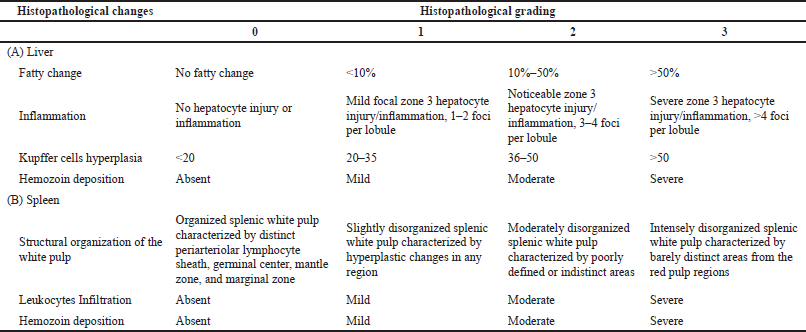 | Table 1. Histopathological changes and grading schemes used for the liver and spleen lesions. [Click here to view] |
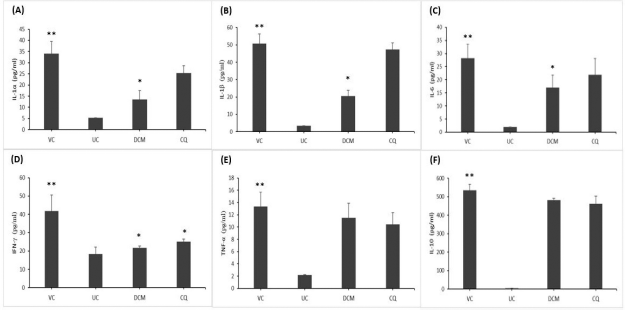 | Figure 1. The cytokine levels of (A) IL-1α, (B) IL-1β, (C) IL-6, (D) IFN-γ, (E) TNF-α, and (F) IL-10 in P. berghei-infected mice. The DCM stem bark extract of G. lanceolatus significantly reduced the proinflammatory cytokines of IL-1α, IL-1β, IL-6, and IFN-γ (A–D) when compared to the vehicle-treated group. VC and UC represent the vehicle-treated and uninfected control mice, respectively. DCM represents the infected mice treated with the DCM extract at 30 mg·kg−1 bwt. CQ represents the infected mice treated with chloroquine at 1 mg·kg−1 bwt. Data are presented as mean ± SEM (n = 6 mice per group). *Significant at p < 0.05 with reference to the vehicle-treated mice. **Significant at p < 0.05 with reference to the uninfected control mice. [Click here to view] |
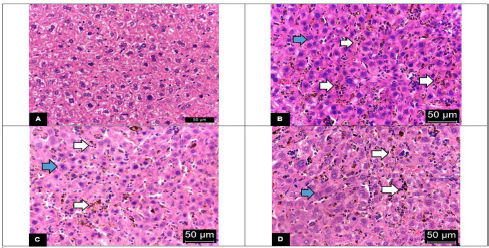 | Figure 2. Representative photomicrographs of hepatic tissues (H&E stain) with 400× magnifications of (A) the uninfected control mice showing polyhedral-shaped hepatocytes with rounded vesicular nuclei and acidophilic cytoplasm; (B) the vehicle-treated mice showing severe hepatic necrosis with massive clusters of hemozoin (white arrow), inflammatory infiltration, and enlargement of hepatocyte cytoplasm and nucleus (blue arrow); (C) the DCM stem bark extract-treated mice showing mild hepatic necrosis with improved tissue damage; (D) the chloroquine-treated mice showing inflammatory infiltration, clusters of hemozoin (white arrow), and enlargement of hepatocyte cytoplasm and nucleus (blue arrow). [Click here to view] |
DISCUSSION
Malarial infection triggers dynamic and complex immune responses in order to initiate host defense against parasite invasion (Xia et al., 2018). Cytokines are key elements in modulating the immune response toward protective immunity and play a role in the interplay between the innate and adaptive immune systems (Keustermans et al., 2013). Malarial infection is associated with the development of a T-helper 1- (Th1-) type response characterized by increased production of proinflammatory cytokines including IL-1, IL-6, IFN-γ, and TNF-α (Bakir et al., 2011; Chin et al., 2019; Junaid et al., 2017). Similar to earlier reports, the present study also recorded high levels of IL-1α, IL-1β, IL-6, IFN-γ, and TNF-α in the malaria-infected mice. These proinflammatory mediators are essential in inhibiting parasite replication through the activation of effector cells such as macrophages (Bakir et al., 2011; Chua et al., 2013). IFN-γ is the primary cytokine responsible for the activation of macrophages and monocytes to produce other inflammatory mediators and reactive oxygen intermediates which mediate parasite killing (Shibui et al., 2009). Furthermore, TNF-α increases the phagocytic uptake of parasites, by orchestrating the production of proinflammatory cytokine cascade (such as IL-1 and IL-6) and stimulating the function of several immune cells including dendritic cells, regulatory T cells, and T-helper cells (Parameswaran and Patial, 2010).
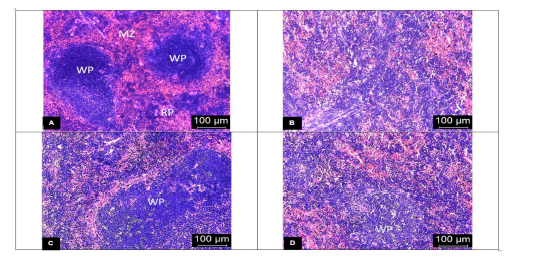 | Figure 3. Representative photomicrographs of the spleen (H&E stain) with 400× magnifications of (A) the uninfected control mice showing clear distinction between red and white pulp with discernible marginal zone; (B) the vehicle-treated mice exhibiting complete distortion of the marginal zone with the indistinct appearance of red and white pulp; (C) the DCM stem bark extract-treated mice showing slightly disorganized white pulp with hyperplastic changes; (D) the chloroquine-treated mice showing moderately disorganized white pulp with poorly discernible regions. WP: white pulp, RP: red pulp, and MZ: marginal zone. [Click here to view] |
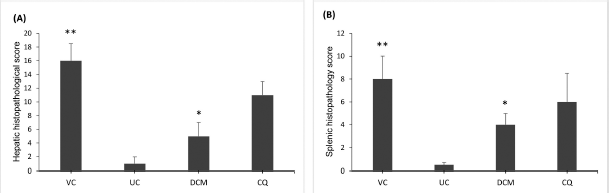 | Figure 4. Comparison of mean histopathological scores for the (A) liver and (B) spleen of P. berghei-infected mice showing that the DCM stem bark extract significantly reduced the HHS and SHS when compared to the vehicle-treated group. VC and UC represent the vehicle-treated and uninfected control mice, respectively. DCM represents the infected mice treated with the DCM extract at 30 mg·kg−1 bwt. CQ represents the infected mice treated with chloroquine at 1 mg·kg−1 bwt. Data are presented as mean ± SEM (n = 6 mice per group). *Significant at p < 0.05 with reference to the vehicle-treated mice. **Significant at p < 0.05 with reference to the uninfected control mice. [Click here to view] |
Although the generation of proinflammatory cytokines plays an important role in facilitating parasite clearance, their exacerbated production has been implicated in malaria pathogenesis (Drewry and Harty, 2020; Kumar et al., 2019). It is well established that much of the pathogenesis of malaria is caused by inappropriate or aggravated inflammatory responses resulting in host tissue damage (Fang and Zhu, 2020; Julius, 2013). In addition to detrimental inflammation, vascular sequestration of parasitized erythrocytes has been shown to contribute to the development of organ-specific syndromes including cerebral malaria and placental malaria (Autino et al., 2012; Clark et al., 2006; Martins and Daniel-Ribeiro, 2013; Nishanth and Schlüter, 2019). Therefore, a coordinated immune response is required to mediate the resolution of the infection without causing severe pathological changes (Stanley and Engwerda, 2007).
The present study demonstrated that the levels of the inflammatory cytokines, mainly IL-1α, IL-1β, IL-6, and IFN-γ, were significantly decreased following treatment with the DCM stem bark extract of G. lanceolatus. The results indicate that the extract is implicated in regulating the systemic levels of proinflammatory cytokines, mainly the Th1 response, by suppressing their production during malarial infection. Styryl lactones have been identified as the major constituents of G. lanceolatus with several new and known styryl lactones isolated from the roots and stem bark of the plant (Bihud et al., 2019; Rasol et al., 2018). A growing body of evidence indicates that styryl lactones are a group of secondary metabolites ubiquitous in the genus Goniothalamus with promising anti-inflammatory and antioxidant properties (Kim et al., 2013; Orlikova et al., 2013; Vendramini-Costa et al., 2017). Thus, it can be postulated that styryl lactones which are present in the plant might contribute to its anti-inflammatory activity during malarial infection.
The liver and spleen are two main organs known to be affected during malarial infection (Taghreed and Murad, 2016). Although parasite elimination occurs mostly in the spleen under normal circumstances, the liver has been shown to function as an alternative clearing site (Dockrell et al., 1980). In the liver, the Kupffer cells are resident macrophages that play an essential role in phagocytizing hemozoin and parasitized erythrocytes (Viriyavejakul et al., 2014). Common histopathological changes in the liver of the infected host include hepatocyte necrosis, Kupffer cells hyperplasia, hemozoin pigmentation, and portal inflammation (Kim et al., 2018; Viriyavejakul et al., 2014). Accordingly, the spleen plays a critical role in activating the host immune response, removing the infected red blood cells, and producing new red blood cells during malarial infection (Del-Portillo et al., 2012; Schofield and Grau, 2005). Splenic architecture is altered during malarial infection which includes expansion of white pulp, disorganized marginal zone, increased splenic vasculature, and activation of barrier cells, indicating congestion of splenic circulation (Murshed et al., 2020). These changes enable the spleen to trigger immune regulation and recruit responder cells while preventing the access of parasitized erythrocytes to splenic tissues (Angulo and Fresno, 2002).
The histopathological analysis carried out in the present study indicates host tissue damage which occurs as a result of the parasitic infection as evidenced by the extensive hepatic inflammation and expanded splenic white pulp with diffuse hypercellularity in the red pulp. Moreover, the livers and spleens of the malaria-infected mice were heavily laden with hemozoin depositions. Hemozoin or malarial pigment is formed in the digestive vacuole of Plasmodium as a product of hemoglobin catabolism and is sequestered in various organs predominantly in the liver and spleen of the infected host (Deroost et al., 2012). Hemozoin has been shown to suppress the immune response and cause significant inflammation through complex proinflammatory reactions that may progress to tissue injury (Jaramillo et al., 2004). However, treatment with the DCM stem bark extract of G. lanceolatus ameliorated hepatic and splenic injuries during malarial infection. These observations were supported by significantly reduced inflammatory infiltrates and decreased hemozoin depositions which were associated with minimal alterations of the liver and spleen histological structure. Taken together, these observations lead to the conclusion that the extract may potentially attenuate tissue damage by suppressing the release of proinflammatory cytokines.
Limitations of the study
The present study has some limitations, including the small sample size and the single-dose level employed. Hence, further studies investigating the dose-response effects with longer durations of treatment using larger sample sizes are required. This could provide a basis for future investigation to elucidate the molecular mechanism underlying the therapeutic actions of the plant.
CONCLUSION
In summary, the DCM stem bark extract of G. lanceolatus demonstrated therapeutic potential during malarial infection through inhibition of proinflammatory cytokines and alleviation of tissue damage caused by the infection. However, further studies involving dose-response should be carried out to identify the optimal dosage of the extract and determine its full potential as an adjunct therapy for current malaria treatment.
ACKNOWLEDGMENTS
This study received financial support under the Fundamental Research Grant Scheme [600-RMI/FRGS 5/3 (0021/2016)] awarded by the Malaysian Ministry of Higher Education. The authors thank the Malaria Research and Reference Reagent Resource Centre (MR4) for providing the P. berghei NK65 strain. Special thanks are due to Prof. Fasihuddin Badruddin Ahmad from Universiti Malaysia Sarawak and Dr. Nur Vicky Bihud from UiTM for providing the plant extract.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Angulo I, Fresno M. Cytokines in the pathogenesis of and protection against malaria. Clin Vaccine Immunol, 2002; 9(6):1145–52. CrossRef
Autino B, Corbett Y, Castelli F, Taramelli D. Pathogenesis of malaria in tissues and blood. Mediterr J Hematol Infect Dis, 2012; 4(1):e2012061. CrossRef
Bakir HY, Tomiyama C, Abo T. Cytokine profile of murine malaria: stage-related production of inflammatory and anti-inflammatory cytokines. Biomed Res, 2011; 32:203–8. CrossRef
Bihud NV, Rasol NE, Imran S, Awang K, Ahmad FB, Mai CW, Leong CO, Cordell GA, Ismail NH. Goniolanceolatins A–H, cytotoxic Bis-styryllactones from Goniothalamus lanceolatus. J Nat Prod, 2019; 82(9):2430–42. CrossRef
Brugat T, Cunningham D, Sodenkamp J, Coomes S, Wilson M, Spence PJ, Jarra W, Thompson J, Scudamore C, Langhorne J. Sequestration and histopathology in Plasmodium chabaudi malaria are influenced by the immune response in an organ-specific manner. Cell Microbiol, 2014; 16(5):687–700. CrossRef
Chin VK, Asyran AMY, Zakaria ZA, Abdullah WO, Chong PP, Nordin N, Ibraheem ZO, Basir R. TREM-1 modulation produces positive outcome on the histopathology and cytokines release profile of Plasmodium berghei-infected mice. J Parasitic Dis, 2019; 43(1):139–53. CrossRef
Christensen SB. Natural products that changed Society. Biomedicines, 2021; 9(5):472. CrossRef
Chua CL, Brown G, Hamilton JA, Rogerson S, Boeuf P. Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol, 2013; 29(1):26–34. CrossRef
Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malaria J, 2006; 5:85. CrossRef
Deroost K, Lays N, Noppen S, Martens E, Opdenakker G, Van den Steen PE. Improved methods for haemozoin quantification in tissues yield organ-and parasite-specific information in malaria-infected mice. Malaria J, 2012; 11:166. CrossRef
Del-Portillo HA, Ferrer M, Brugat T, Martin JL, Langhorne J, Lacerda MV. The role of the spleen in malaria. Cell Microbiol, 2012; 14(3):343–55. CrossRef
Dockrell HM, de Souza JB, Playfair JH. The role of the liver in immunity to blood-stage murine malaria. Immunology, 1980; 41(2):421–30.
Drewry LL, Harty JT. Balancing in a black box: potential immunomodulatory roles for TGF-β signaling during blood-stage malaria. Virulence, 2020; 11(1):159–69. CrossRef
Fang D, Zhu J. Molecular switches for regulating the differentiation of inflammatory and IL-10-producing anti-inflammatory T-helper cells. Cell Mol Life Sci, 2020; 77(2):289–303. CrossRef
Giamarellos-Bourboulis EJ, Tziortzioti V, Koutoukas P, Baziaka F, Raftogiannis M, Antonopoulou A, Adamis T, Sabracos L, Giamarellou H. Clarithromycin is an effective immunomodulator in experimental pyelonephritis caused by pan-resistant Klebsiella pneumoniae. J Antimicrob Chemother, 2006; 57(5):937–44. CrossRef
Ippolito MM, Moser KA, Kabuya JB, Cunningham C, Juliano JJ. Antimalarial drug resistance and implications for the WHO global technical strategy. Curr Epidemiol Rep, 2021; 8:46–62. CrossRef
Jaramillo M, Plante I, Ouellet N, Vandal K, Tessier PA, Olivier M. Hemozoin-inducible proinflammatory events in vivo: potential role in malaria infection. J Immunol, 2004; 172(5):3101–10. CrossRef
Julius M. Cytokine levels associated with experimental malaria pathology during Plasmodium berghei ANKA infection in a mouse model. J Clin Immunol Immunopathol Res, 2013; 5(1):1–8.
Junaid QO, Khaw LT, Mahmud R, Ong KC, Lau YL, Borade PU, Liew JWK, Sivanandam S, Wong KT, Vythilingam I. Pathogenesis of Plasmodium berghei ANKA infection in the gerbil (Meriones unguiculatus) as an experimental model for severe malaria. Parasite, 2017; 24:38. CrossRef
Kaharudin FA, Zohdi RM, Mukhtar SM, Sidek HM, Bihud NV, Rasol NE, Ahmad FB, Ismail NH. In vitro antiplasmodial and cytotoxicity activities of crude extracts and major compounds from Goniothalamus lanceolatus. J Ethnopharmacol, 2020; 254:112657. CrossRef
Keustermans GC, Hoeks SB, Meerding JM, Prakken BJ, de Jager W. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods, 2013; 61(1):10–7. CrossRef
Kim J, Wang S, Lee C, Sung S, Shin Y, Song KS, Cha HJ, Ock M, Jung Y. Blood-stage Plasmodium Berghei ANKA infection promotes hepatic fibrosis by enhancing hedgehog signaling in mice. Cell Physiol Biochem, 2018; 50(4):1414–28. CrossRef
Kim RPT, Bihud V, Bin Mohamad K, Leong KH, Bin Mohamad J, Bin Ahmad F, Hazni H, Kasim N, Halim SN, Awang K. Cytotoxic and antioxidant compounds from the stem bark of Goniothalamus tapisoides Mat Salleh. Molecules, 2013; 18(1):128–39. CrossRef
Kumar R, Ng S, Engwerda C. The role of IL-10 in malaria: a double edged sword. Front Immunol, 2019; 10:229. CrossRef
Martins YC, Daniel-Ribeiro CT. A new hypothesis on the manifestation of cerebral malaria: the secret is in the liver. Med Hypotheses, 2013; 81(5):777–83. CrossRef
Miguel-Blanco C, Murithi JM, Benavente ED, Angrisano F, Sala KA, van Schalkwyk DA, Vanaerschot M, Schwach F, Fuchter MJ, Billker O, Sutherland CJ. The antimalarial efficacy and mechanism of resistance of the novel chemotype. Sci Rep, 2021; 11(1):1–9. CrossRef
Mubaraki MA, Hafiz TA, Dkhil MA, Saleh AQ. Beneficial effect of Punica granatum peel extract on murine malaria-induced spleen injury. BMC Complement Alternat Med, 2016; 16:221. CrossRef
Murshed M, Dkhil MA, Al-Shaebi EM, Mahmood AAQ, Mohammed MM, Hossam MAA, Ghada A, Rewaida AG, Saleh AQ. Biosynthesized silver nanoparticles regulate the iron status in the spleen of Plasmodium chabaudi–infected mice. Environ Sci Pollut Res, 2020; 27:40054–60. CrossRef
Nishanth G, Schlüter D. Blood-brain barrier in cerebral malaria: pathogenesis and therapeutic intervention. Trends Parasitol, 2019; 35(7):516–28. CrossRef
Orlikova B, Schumacher M, Juncker T, Yan CC, Inayat-Hussain SH, Hajjouli S, Cerella C, Dicato M, Diederich M. Styryl-lactone goniothalamin inhibits TNF-α-induced NF-κB activation. Food Chem Toxicol, 2013; 59:572–8. CrossRef
Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr, 2010; 20(2):87–103. CrossRef
Peters W, Portus JH, Robinson BL. The chemotherapy of rodent malaria, XXII: the value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol, 1975; 69(2):155–71. CrossRef
Rasol NE, Ahmad FB, Mai CW, Bihud NV, Abdullah F, Awang K, Ismail NH. Styryl lactones from roots and barks Goniothalamus lanceolatus. Nat Prod Commun, 2018; 13(12):1575–8. CrossRef
Redpath SA, Fonseca NM, Perona-Wright G. Protection and pathology during parasite infection: IL-10 strikes the balance. Parasite Immunol, 2014; 36(6):233–52. CrossRef
Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol, 2005; 5(9):722–35. CrossRef
Shibui A, Hozumi N, Shiraishi C, Sato Y, Iida H, Sugano S, Watanabe J. CD4(+) T cell response in early erythrocytic stage malaria: Plasmodium berghei infection in BALB/c and C57BL/6 mice. Parasitol Res, 2009; 105(1):281–6. CrossRef
Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol, 2007; 85(2):138–47. CrossRef
Taghreed AH, Murad AM. The potential role of Ziziphus spina-christi leaf extracts against Plasmodium berghei-induced liver and spleen injury. Biomed Res, 2016; 27(4):1027–32.
Uzor PF. Alkaloids from plants with antimalarial activity: a review of recent studies. Evid Based Complement Alternat Med, 2020; 3:1–17. CrossRef
Vendramini-Costa DB, Francescone R, Posocco D, Hou V, Dmitrieva O, Hensley H, de Carvalho JE, Pilli RA, Grivennikov SI. Anti-inflammatory natural product goniothalamin reduces colitis-associated and sporadic colorectal tumorigenesis. Carcinogenesis, 2017; 38(1):51–63. CrossRef
Viriyavejakul P, Khachonsaksumet V, Punsawad, C. Liver changes in severe Plasmodium falciparum malaria: histopathology, apoptosis and nuclear factor kappa B expression. Malaria J, 2014; 13:106. CrossRef
Wiart C. Goniothalamus species: a source of drugs for the treatment of cancers and bacterial infections? Evid Based Complement Alternat Med, 2007; 4(3):299–311. CrossRef
WHO. World malaria report. 2020. Available via https://www.who.int/publications/i/item/9789240015791 (Accessed 24 August 2021).
Xia L, Wu J, Pattaradilokrat S, Tumas K, He X, Peng YC, Huang R, Myers TG, Long CA, Wang R, Su XZ. Detection of host pathways universally inhibited after Plasmodium yoelii infection for immune intervention. Sci Rep, 2018; 8(1):1–4. CrossRef
Zohdi RM, Mukhtar SM, Bihud NV, Rasol NE, Ahmad FB, Awang K, Ismail NH. In vivo antiplasmodial and toxicological effects of Goniothalamus lanceolatus crude extracts. Nat Prod Commun, 2017; 12(8):1251–4. CrossRef