INTRODUCTION
Ovarian cancer is known to be one of the most aggressive gynecological cancers causing death in women. An estimated 240,000 new cases have been recorded in 2018, leaving ovarian cancer at ranking number seven as the most common cancer among women worldwide (Henderson et al., 2018). Ovarian cancer cases have been mostly identified in the advanced stages due to their asymptomatic characteristics (Jayson et al., 2014). Nonetheless, some patients may experience bloating, pelvic pain, frequent urination, and changes in bowel movements (Jayson et al., 2014). Cisplatin is a standard drug used for ovarian cancer treatment; however, the cell resistance has been shown to cause relapse in some patients following initial treatment (Noviyani et al., 2019). This substantially reduced the effectiveness and outcome of chemotherapy and resulted in less than 30% for a 5-year survival rate (Cornelison et al., 2017). Hence, it is extremely crucial to discover alternative approaches to treat ovarian cancer, especially in overcoming cisplatin resistance with minimal side effects on patients.
Goniothalamus is one of the largest paleotropical genera of a plant in the Annonaceae family, comprising over 130 species and is mainly distributed in the Malesian floristic region, comprising Malaysia, Borneo, New Guinea, Sumatra, and Philippines (Saunders, 2003; Yang et al., 2020). Goniothalamin (GTN) as the main bioactive compounds has been reported to exert a cytotoxic effect and anticancer properties in human promyelocytic leukemia (HL-60), leukemia monocytic (U937) (Petsophonsakul et al., 2013), squamous cell carcinoma (H400 cells) (Li et al., 2016), and cervical cancer (HeLa) cell lines (Sophonnithiprasert et al., 2017). The compound is capable of limiting the development of cancer cells by increasing the number of dead cancer cells without producing inflammatory reactions on the healthy cells (Seyed et al., 2014; Umar-Tsafe et al., 2004).
Goniothalamus lanceolatus (GL) is native to the rainforest in Sarawak, Malaysia, locally known as “Getimang,” and is believed by the Iban people to repel mosquitoes due to the pungent scent and thick smoke when burned. It has also been used to treat cold, fever, and skin diseases by the indigenous people as alternative medicine (Wiart, 2007). The isolation of the pure compounds and the cytotoxicity chemical classification of the active compounds are currently ongoing. The newly discovered alkaloids of goniolanceolactam and 2-acetyl-3-amino-1,4-naphthoquinone isolated from the dichloromethane (DCM) root extract were reported to have a cytotoxic effect on human colon and lung cancer cells (Rasol et al., 2018a). Moreover, eight new bis-styryllactones and goniolanceolatins A−H, and four known styryllactones with a rare (6S)-styrylpyrone and (1S)-pyranopyrone moieties were also isolated from the GL (Bihud et al., 2019). These compounds have been reported to be associated with antimalarial properties against chloroquine-sensitive (3D7) and chloroquine-resistant (K1) strains of P. falciparum (Kaharudin et al., 2020). Apart from these findings, antiproliferative and anticancer activities of GL on ovarian cancer cells have not yet been reported. Therefore, this study aims to investigate the cell viability, migration, and apoptotic effects of GL extracts in three different solvents on chemosensitive and chemoresistant ovarian cancer cells.
MATERIALS AND METHODS
Preparation of plant extracts
GL leaves and roots were collected from Sematan Sarawak, Malaysia, in June 2012, described as FBAUMS 108 by a botanist Professor Dr. Kamaruddin Mat Salleh (Universiti Kebangsaan Malaysia). The leaves and the roots of the plants were oven-dried before being subjected to extraction using DCM, hexane, and methanol solvents. The solvents were removed under a vacuum condition using a rotary evaporator resulting in a total of six extracts. The extracts were purified using high-performance liquid chromatography (HPLC) and recycling HPLC leaving the isolation of a series of compounds (Rasol et al., 2018b). The extracts were reconstituted in dimethyl sulfoxide (DMSO) to form a 20 mg/ml stock solution and preserved for further use at –20°C.
Cell culture
Two ovarian cancer cell lines, namely, PEO1 (chemosensitive) and PEO4 (chemoresistant), were provided by Dr. Normala Abd. Latip of the Atta-ur-Rahman Institute for Natural Product Discovery, which was previously purchased from the European Cell Culture Collection (ECACC, UK). The ovarian cancer cell lines were derived from ascites of the same patient who received cisplatin, 5-fluorouracil, and chlorambucil treatment. PEO1 was sensitive to cisplatin taken when the patient was able to respond to the treatments. Meanwhile, PEO4 was taken 10 months later after the patients developed resistance toward cisplatin chemotherapy. RPMI-1640 complete media containing 10% fetal bovine serum and 1% penicillin-streptomycin were used for cell culture and incubated in a humidified atmosphere containing 5% CO2 at 37°C. Seeding of monolayer cell cultures was performed in a 75 cm2 tissue culture flask and maintained up to 70% confluency prior to treatment.
MTT assay
Cell viability and inhibition were determined using the MTT assay. Briefly, each cell line was plated in a 96-well plate seeded with 2.0 × 104 cells/well followed by 24 hours cell treatment with different extract concentrations at two-fold serial dilutions (0–1,000 μg/ml). Cell viability was determined by adding 5 mg/ml MTT solution to each well. After 4 hours of incubation, 50 μl of DMSO was added to each well as a stop solution and incubated for 10 minutes at room temperature on a shaker. The absorbance was measured at 570 nm wavelength using a microplate reader (BMG Labtech, Offenburg, Germany). A graph of cell viability percentage versus extract concentration was plotted and the inhibitory concentration (IC50) was determined from the concentration-response inhibition curves. Cells without treatment (untreated cells) were used as negative control (NC) while cells treated with cisplatin were used as a positive control. The extracts with the lowest IC50 value were selected for subsequent testing.
Scratch assay
The migration rate of both cell lines was determined using a scratch assay. Briefly, the cells were cultured in a six-well plate until it reached 70% confluency. The cells were then treated with the extracts and cisplatin (positive control). A scratch was introduced using a sterile pipette tip followed by 16, 24, 48, and 72 hours incubation period. The closure of the scratched wounds was considered as the completion of the migration process. The migration rate of both cells was determined by comparing the migration rate of the treated cells with the untreated cells (negative control). The following formula was used to calculate the migration rate (Warden et al., 2020):
Rate of migration (%) =
where n = time of incubation.
Cell apoptosis assay by flow cytometry
Cell apoptosis was assessed by the BD PharmigenTM FITC Annexin V (AV) Apoptosis Detection Kit as per the manufacturer’s protocol. A cell density of 2 × 106 cells/well was seeded in a six-well plate, followed by cell treatment and incubation for 24 and 48 hours. The cells were harvested by trypsinization, washed three times with cold phosphate-buffered saline (PBS), pelleted, and resuspend pellet in a 1× binding buffer. The suspension was then stained with 5 µl FITC-conjugated AV and 5 µl propidium iodide (PI) and incubated for 15 minutes at room temperature in a dark space, followed by the addition of 400 µl binding buffer. Finally, the samples were analyzed by a flow cytometer (FACSCalibur, Becton-Dickinson, San Jose, CA). The cell distribution was analyzed using CellQuest™ software (Becton-Dickinson) within 1 hour of staining in which 10,000 cells were collected for each sample. Samples with different cell groups were separated in either early or late apoptosis, marked by AV while necrosis was marked by PI and presented in a separate plot group.
Statistical analysis
The statistical analysis test was carried out using GraphPad Prism Software version 6.0. Data are presented as mean ± standard mean error (SEM). The statistical comparative analysis was performed using one-way analysis of variance, followed by Tukey’s post-hoc test. A p-value of less than 0.05 was considered to be significant.
RESULTS
Effect of GL extracts on cell viability and cell inhibition
Figure 1 shows the cell viability in PEO1 after 24 hours of treatment with different concentrations of the extracts. DCM and methanol leaves extract significantly reduced the cell viability (p < 0.05) at the highest concentration (1,000 μg/ml) after 24 hours of treatment. A significantly decreased cell viability to below 20% was observed in DCM roots extract at 250 μg/ml and above concentration while methanol extract decreased the cell viability at around 50% at a concentration of 62.5–1,000 μg/ml.
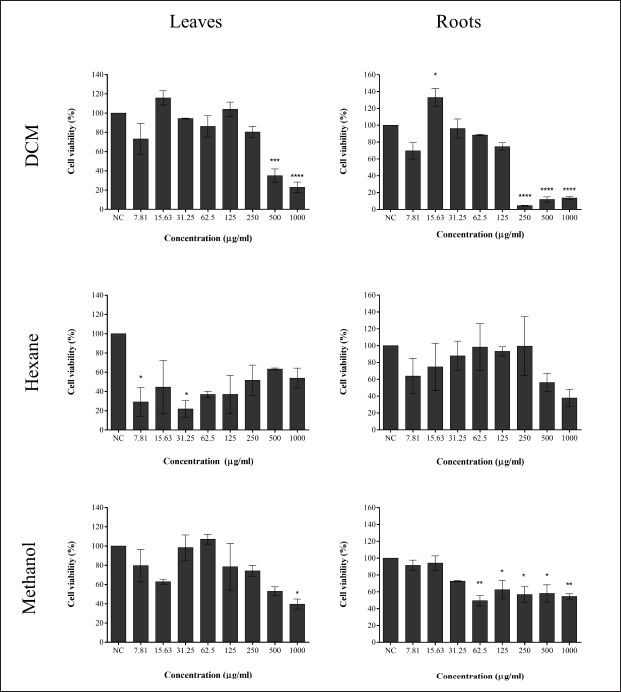 | Figure 1. Cell viability of the PEO1 cell line treated with GL leaf and root extracts. Each leaf and root extract was dissolved in DCM, hexane, and methanol and treated at different concentrations for 24 hours in triplicate. All data are expressed as mean ± SEM. *p < 0.05; **p < 0.01,; ****p < 0.0001 versus negative control (NC). [Click here to view] |
Figure 2 shows the cell viability in PEO4 after 24 hours of treatment with different concentrations of leaf and root extracts of GL. The viability of the cells decreased significantly by the DCM leaves extract at a concentration of 1,000 μg/ml compared to the hexane leaves extract at 500 μg/ml. In addition, the DCM root extracts significantly decreased the cell viability (p < 0.0001) to less than 20% at 125–1,000 μg/ml concentration. The methanol root extract showed a significant reduction of less than 50% cell viability with a concentration of 7.81–1,000 μg/ml compared to the control group.
Figure 3 shows the cell viability in both cell lines after 24 hours of cisplatin treatment. In the PEO1 cell line, the viability decreased significantly at 1.56 μM and almost 90% inhibition was observed at 6.25 μM as shown in Figure 3a. A nonsignificant cell viability decrease was seen in PEO4 after cisplatin treatment except at 3.125 μM as shown in Figure 3b.
Table 1 summarizes the IC50 of the leaf and root extracts in both cell lines. The lowest IC50 values for PEO1 were methanolic root extracts (IC50: 31.40 ± 0.77 μg/ml). DCM leaf extract (IC50: 22.02 ± 0.52 μg/ml) showed a moderate IC50 value in PEO4. Cisplatin inhibited PEO4 almost twice the concentration as seen in PEO1 (IC50: 4.07 ± 0.21 μM vs. 2.76 ± 0.05 μM).
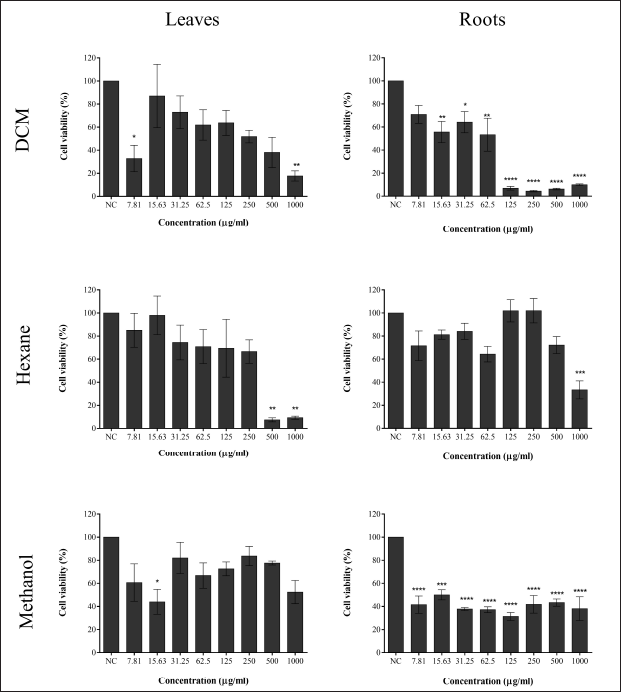 | Figure 2. Cell viability of the PEO4 cell line treated with GL leaf and root extracts at different concentrations for 24 hours in triplicate. All data are expressed as mean ± SEM. *p < 0.05; **p < 0.01,; ****p < 0.0001 versus negative control (NC). [Click here to view] |
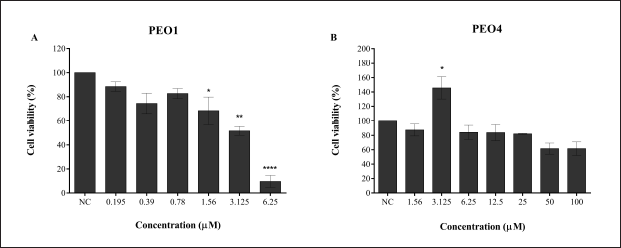 | Figure 3. Effect of cisplatin on cell viability of (A) PEO1 and (B) PEO4 cell lines. Cisplatin was used as a standard drug for ovarian cancer cell lines and served as a positive control. Both cell lines were treated with cisplatin in increasing concentrations in triplicate for 24 hours and determined by MTT assay. All data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ****p < 0.0001 versus negative control (NC). [Click here to view] |
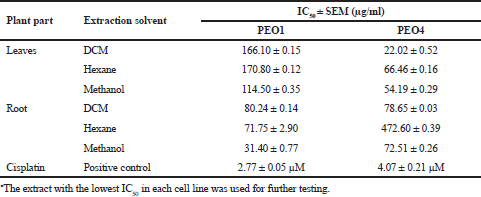 | Table 1. The IC50 values of GL leaf and root extracts for PEO1 and PEO4 cell lines. [Click here to view] |
Effect of GL on cell migration
The extract with the lowest IC50 value from the cell viability analysis for each cell line was used to assess cell migration. PEO1 cells were treated with methanolic roots extract at IC20, IC50, and IC80 concentrations at 16, 24, 48, and 72 hours time points, respectively (Fig. 4). At 72 hours, cell migration decreased from 21.37% at IC20 to 18.65% at IC80. In comparison, untreated cells showed a 76.63% cell migration. Cisplatin was able to fully inhibit PEO1 cell migration (–2.49%).
PEO4 cells were treated with DCM leaf extracts at IC20, IC50, and IC80 concentrations and incubated at four different time points (Fig. 5). At 72 hours, cell migration was suppressed to 16.73% at IC80 compared to 31.99% in the untreated cells. Meanwhile, the migration was almost the same in the untreated cells when treated with cisplatin (30.3%).
Effects of GL in inducing apoptosis
The effects of the extracts on inducing apoptosis using flow cytometry were examined at 24 and 48 hours. In PEO1, cells treated with methanol root extracts at IC20 and IC80 showed increased early apoptosis to 6.76% and 13.52%, respectively, after 24 hours. The cells at IC20 and IC80 shifted to late apoptosis at 4.48% and 6.64%, respectively. Early apoptosis significantly decreased while the cells shift toward late apoptosis after 48 hours of treatment as shown in Figure 6A–C.
In PEO4 cells, DCM leaves extract significantly increased early apoptosis at IC20 to 42.94% but dropped after 24 hours to 24.63% at IC80. However, early apoptosis at IC20 and IC80 increased to 16.28% and 26.5%, respectively, after 48 hours. Late apoptosis increased significantly at both dose-dependent times as shown in Figure 7A–C.
DISCUSSION
Cell viability assay following the administration of GL methanol root extract gave the lowest IC50 value at 31.40 ± 0.77 µg/ml in PEO1. However, a higher concentration at 72.51 ± 0.27 µg/ml was needed to inhibit PEO4. The results showed that methanol root extract exhibited a higher cytotoxic effect against chemosensitive cells compared to the chemoresistant cells. However, DCM leaves extract was found to be the most cytotoxic to the chemoresistant cells, PEO4 (IC50: 22.02 ± 0.52 µg/ml) compared to the chemosensitive cells and PEO1 (IC50: 166.10 ± 0.15 µg/ml). The selection of chemosensitive and chemoresistant cells was done to compare the effects of the plant extracts on cells toward different chemosensitivity profiles.
Chemoresistance in the ovarian cancer cells was initiated by the cell adaptation using different energy pathways such as glycolysis or oxidative phosphorylation (Dar et al., 2017). Chemosensitive ovarian cancer cell lines (A2780 and PEO1) appeared to exhibit a glycolytic phenotype and could not tolerate glucose deprivation. However, the chemoresistant counterparts (C200 and PEO4) showed a high metabolically active phenotype and the ability to switch between oxidative phosphorylation and glycolysis (Dar et al., 2017). Cell proliferation can be suppressed by apoptosis through inhibition of signal transducers and transcription activator 3 and activation of Janus kinase 2 (Jo et al., 2012). Alonezi et al. (2016) demonstrated that chemoresistant cells (A2780CR) were highly sensitive to melittin and had a slightly lower IC50 value of 4.5 μg/ml compared to 6.8 μg/ml in the chemosensitive cells (A2780).
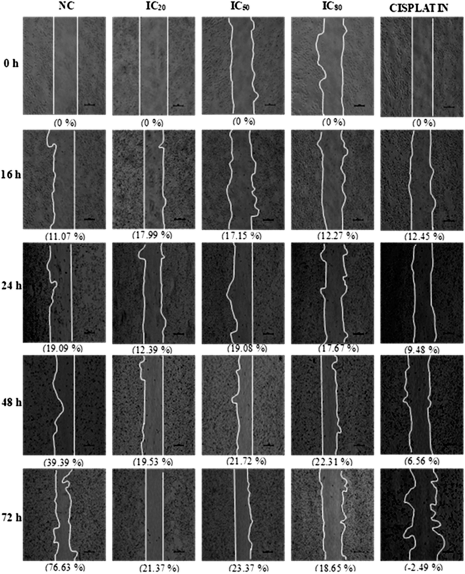 | Figure 4. Effect of GL methanol root extract on PEO1 ovarian cancer cell migration by scratch assay. The experiments were repeated thrice to determine the average percentages of cell migration. [Click here to view] |
The GL leaves and roots were extracted using different solvents (DCM, hexane, and methanol) of different polarities to obtain different groups of active compounds. DCM is a moderate polar organochloride solvent that is widely used to dissolve most organic compounds. Hexane is a nonpolar solvent commonly used in the extraction of nonpolar compounds and methanol is a polar and universal solvent. Due to these differences, the presence and quantity of the compounds will also affect the activity of the cells. The phytochemical analysis indicates the presents of goniodiol, 8-epi-9-deoxygoniopypyrone 9-deoxygoniopypyrone, digoniodiol, and GTN in the GL extract (Zohdi et al., 2017). Two new alkaloids, goniolanceolactam and 2-acetyl-3-amino-1,4-naphthoquinone, were isolated from the DCM root extract of the GL (Rasol et al., 2018a). Goniolanceolactam showed cytotoxic activity on human colon and lung cancer cell lines with IC50 values ranging from 5.32 to 9.91 μM. A new styryl lactone, 5R,6R-5-hydroxy-6-styryltetrahydropyrane-2-one, was isolated from the GL roots with cytotoxic activity of IC50: 2.38–7.59 µM against human colon and lung cancer cell lines (Rasol et al., 2018b).
PEO1 and PEO4 cells are primarily derived from patients with poorly differentiated serous adenocarcinoma at different stages of ovarian cancer. The cells were found as single adhesive cells or small clusters in vitro with approximately 37 hours of doubling times (Langdon and Lawrie, 2001). Cancer cell migration is generally associated with the alteration of the cell-matrix interface on the cell surface (Kim et al., 2012). Inhibition of cell migration prevents cancer metastasis and enhances patient survival in vivo (Helbig et al., 2003). Therefore, it was hypothesized that GL extracts could modulate cell migration while controlling disease progression. The scratch assay revealed that the GL extracts inhibited cell migration in a concentration-dependent manner compared to the negative control. Cells treated with a higher concentration of GL extracts inhibited cell migration more than with a lower concentration. Interestingly, suppression of cell migration by the GL extract was found to be dependent on the type of cell resistance, whereby it inhibited more chemoresistant cells than chemosensitive cells compared to the negative control cells.
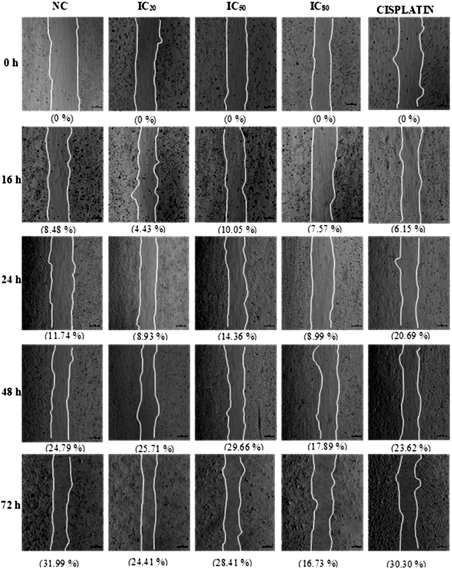 | Figure 5. Effect of GL DCM leaves extract on PEO4 ovarian cancer cell migration assessed by scratch assay. The experiments were repeated thrice to determine the average percentages of cell migration. [Click here to view] |
A previous study showed that GTN inhibits human lung cancer cell line migration (H1299) at a concentration of less than 10 μg/ml. The inhibition of migration is associated with decreased levels of metalloproteinase matrix (MMP-2 and MMP-9) activity (Chiu et al., 2011). Another study found that Goniolactone-C from G. cheliensis strongly inhibited PDGF-BB-induced vascular smooth muscle cell (VSMC) migration by the suppression of adhesion molecule expression (Sun et al., 2014). Similarly, auraptene and Kaempferia parviflora extract suppressed cell migration and invasion of ovarian cancer in vitro by inhibiting the MMP-2 and MMP-9 activities (Jamialahmadi et al., 2018; Paramee et al., 2018).
Apoptosis is essential in a multicellular organism and is a dominant tumor-suppressive pathway, which can potentially deplete cancer cells (Ghante and Jamkhande, 2019). Cancer cells have the ability to a range of modifications and responses. Mutation is one of the modifications that can cause dysfunction to the apoptotic machinery pathway, thus rendering the cancer cells to be resistant to the drug (Das et al., 2016). Apoptosis has been shown by the externalization of phosphatidylserine indicating early apoptosis in cell death with intact membrane integrity. Necrotic cells are the late apoptotic cells with damaged membranes (Foo et al., 2014). This study showed that GL extracts induced apoptosis in both cell lines; however, GL has a higher ability to induce apoptosis in chemoresistant cells compared to the chemosensitive cells.
This finding was similar to the previous studies that showed GTN from G. griffithii increased early apoptosis in MDA-MB-231 breast cancer cells and an increased level of caspases 3, 8, and 9 activities (Khaw-On et al., 2018). In addition, GTN from G.macrophyllus also induced early apoptosis and triggered S-phase arrest of HeLa cells after 24, 48, and 72 hours of treatment (Alabsi et al., 2012). It also induced apoptosis in MCF-7, HeLa, HepG2, and NIH3T3 cell lines, with IC50 values of 7.33, 14.8, 37.1, and 65.4 µM, respectively (Banjerdpongchai et al., 2016). Tangchirakhaphan et al. (2018) in another study revealed that GTN mediates chromatin condensation and apoptotic bodies in A375 skin cancer cells.
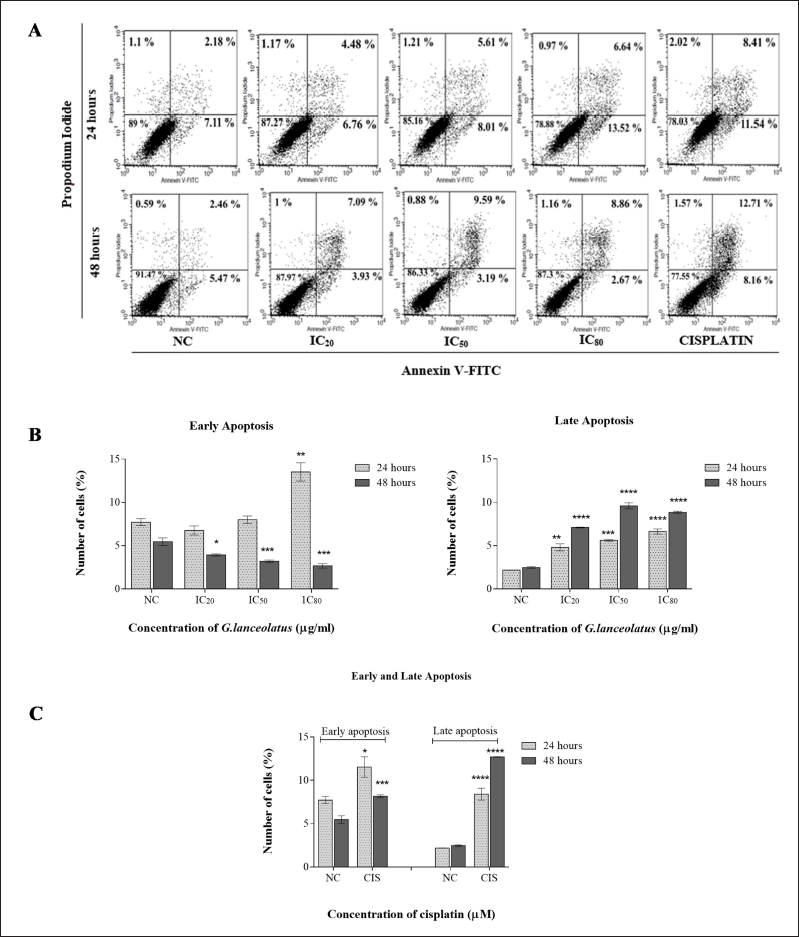 | Figure 6. Effect of GL methanol root extract on PEO1 cell apoptosis. (A) Cell populations are shown in quadrants: the proportion of viable cells, necrotic cells, early apoptotic cells, and late apoptotic cells is shown in the lower left, upper left, lower right, and upper right, respectively. (B) and (C) Percentages of early and late apoptosis shown in the bar graph. All data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ****p < 0.0001 versus negative control (NC). [Click here to view] |
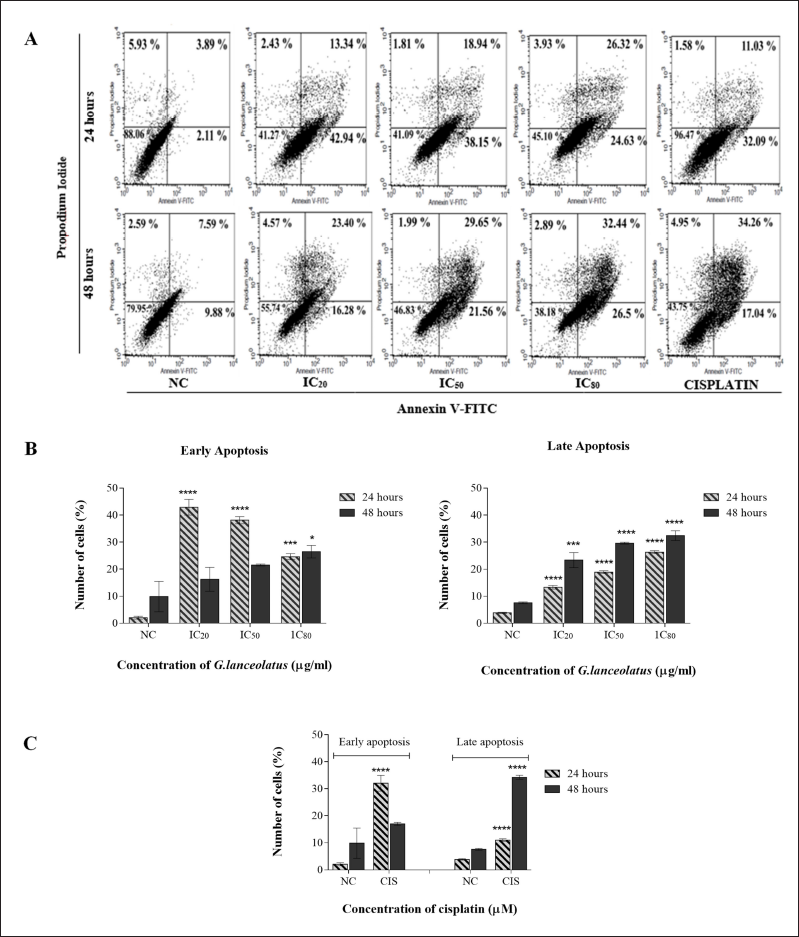 | Figure 7. Effect of GL DCM leaves extract on PEO4 cell apoptosis. (A) Cell populations are shown in quadrants; the proportion of viable cells, necrotic cells, early apoptotic cells, and late apoptotic cells is shown in the lower left, upper left, lower right, and upper right, respectively. (B) and (C) Percentages of early and late apoptosis shown in the bar graph. All data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ****p < 0.0001 versus negative control (NC). [Click here to view] |
CONCLUSION
Goniothalamus lanceolatus extracts exhibited a significant inhibitory effect on cell viability, cell migration, and apoptosis initiation in ovarian cancer cells (PEO1 and PEO4). Nonetheless, careful attention is needed to better understand these promising findings and their interaction as natural extract products containing a complex mixture of natural compounds and may involve multiple molecular targets and pathways.
ACKNOWLEDGMENTS
This research was supported by the Fundamental Research Grant Scheme [600-RMI/FRGS 5/3 (93/2015)] awarded by the Ministry of Education, Malaysia. Special thanks are due to Dr. Fazlin Mohd Fauzi from the Faculty of Pharmacy, UiTM, Selangor, for reviewing this manuscript.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This type of study does not require ethical approval.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alabsi AM, Ali R, Ali AM, Al-Dubai SA, Harun H, Abu Kasim NH, Alsalahi A. Apoptosis induction, cell cycle arrest and in vitro anticancer activity of gonothalamin in a cancer cell lines. Asian Pac J Cancer Prev, 2012; 13(10):5131–36. CrossRef
Alonezi S, Tusiimire J, Wallace J, Dufton MJ, Parkinson JA, Young LC, Clements CJ, Park JK, Jeon JW, Ferro VA, Watson DG. Metabolomic profiling of the effects of melittin on cisplatin resistant and cisplatin sensitive ovarian cancer cells using mass spectrometry and biolog microarray technology. Metabolites, 2016; 6(4):35. CrossRef
Banjerdpongchai R, Khawon P, Pompimon W. Phytochemicals from Goniothalamus griffithii induce human cancer cell apoptosis. Asian Pac J Cancer Prev, 2016; 17(7):3281–87.
Bihud NV, Rasol NE, Imran S, Awang K, Ahmad FB, Mai CW, Leong CO, Cordell GA, Ismail NH. Goniolanceolatins A-H, cytotoxic bis-styryllactones from Goniothalamus lanceolatus. J Nat Prod, 2019; 82(9):2430–42. CrossRef
Chiu CC, Liu PL, Huang KJ, Wang HM, Chang KF, Chou CK, Chang FR, Chong IW, Fang K, Chen JS, Chang HW, Wu YC. Goniothalamin inhibits growth of human lung cancer cells through DNA damage, apoptosis, and reduced migration ability. J Agric Food Chem, 2011; 59(8):4288–93. CrossRef
Cornelison R, Llaneza DC, Landen CN. Emerging therapeutics to overcome chemoresistance in epithelial ovarian cancer: a mini-review. Int J Mol Sci, 2017; 18(10):2171. CrossRef
Dar S, Chhina J, Mert I, Chitale D, Buekers T, Kaur H, Giri S, Munkarah A, Rattan R. Bioenergetic adaptations in chemoresistant ovarian cancer cells. Sci Rep, 2017; 7(1):8760. CrossRef
Das J, Samadder A, Das S, Paul A, Khuda-Bukhsh AR. Nanopharmaceutical approach for enhanced anti-cancer activity of betulinic acid in lung-cancer treatment via activation of PARP: interaction with DNA as a target: -anti-cancer potential of nano-betulinic acid in lung cancer. J Pharmacopuncture, 2016; 19(1):37–44. CrossRef
Foo JB, Yazan LS, Tor YS, Armania N, Ismail N, Imam MU, Yeap SK, Cheah YK, Abdullah R, Ismail M. Induction of cell cycle arrest and apoptosis in caspase-3 deficient MCF-7 cells by Dillenia suffruticosa root extract via multiple signalling pathways. BMC Complement Altern Med, 2014; 14:197. CrossRef
Ghante MH, Jamkhande PG. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: an overview on targets and underling mechanisms. J Pharmacopuncture, 2019; 22(2):55–67.
Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem, 2003; 278(24):21631–38. CrossRef
Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the us preventive services task force. J Am Med Assoc, 2018; 319(6):595–06. CrossRef
Jamialahmadi K, Salari S, Alamolhodaei NS, Avan A, Gholami L, Karimi G. Auraptene inhibits migration and invasion of cervical and ovarian cancer cells by repression of matrix metalloproteinasas 2 and 9 activity. J Pharmacopuncture, 2018; 21(3):177–84.
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet, 2014; 384(9951):1376–88. CrossRef
Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol, 2012; 258(1):72–81. CrossRef
Kaharudin FA, Zohdi RM, Mukhtar SM, Sidek HM, Bihud NV, Rasol NE, Ahmad FB, Ismail NH. In vitro antiplasmodial and cytotoxicity activities of crude extracts and major compounds from Goniothalamus lanceolatus. J Ethnopharmacol, 2020; 254:112657. CrossRef
Khaw-On P, Pompimon W, Banjerdpongchai R. Apoptosis induction via atm phosphorylation, cell cycle arrest, and er stress by goniothalamin and chemodrugs combined effects on breast cancer-derived mda-mb-231 cells. Biomed Res Int, 2018; 2018:7049053. CrossRef
Kim YH, Kwon HJ, Kim DS. Matrix metalloproteinase 9 (MMP-9)-dependent processing of betaig-h3 protein regulates cell migration, invasion, and adhesion. J Biol Chem, 2012; 287(46):38957–69. CrossRef
Langdon SP, Lawrie SS. Establishment of ovarian cancer cell lines. Methods Mol Med. 2001; 39:155–9. CrossRef
Li LK, Rola AS, Kaid FA, Ali AM, Alabsi AM. Goniothalamin induces cell cycle arrest and apoptosis in H400 human oral squamous cell carcinoma: a caspase-dependent mitochondrial-mediated pathway with downregulation of NF-kappabeta. Arch Oral Biol, 2016; 64:28–38. CrossRef
Noviyani R, Indrayathi PA, Budiana ING, Niruri R, Tunas K, Adnyani NMDD. Effect of paclitaxel-cisplatin chemotherapy towards hemoglobin, platelet, and leukocyte levels in epithelial ovarian cancer patients. J Appl Pharm Sci, 2019; 9(1):104–7. CrossRef
Paramee S, Sookkhee S, Sakonwasun C, Na Takuathung M, Mungkornasawakul P, Nimlamool W, Potikanond S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement Altern Med, 2018; 18(1):178. CrossRef
Petsophonsakul P, Pompimon W, Banjerdpongchai R. Apoptosis induction in human leukemic promyelocytic HL-60 and monocytic U937 cell lines by goniothalamin. Asian Pac J Cancer Prev, 2013; 14(5):2885–89. CrossRef
Rasol NE, Ahmad FB, Lim XY, Chung FFL, Leong CO, Mai CW, Bihud NV, Zaki HM, Ismail NH. Cytotoxic lactam and naphthoquinone alkaloids from roots of Goniothalamus lanceolatus Miq. Phytochem Lett, 2018a; 24:51–5. CrossRef
Rasol NE, Ahmad FB, Mai CW, Bihud NV, Abdullah F, Awang K, Ismail NH. Styryl lactones from roots and barks Goniothalamus lanceolatus. Nat Prod Commun, 2018b; 13(12):1575–8. CrossRef
Saunders RMK. A synopsis of Goniothalamus species (annonaceae) in peninsular malaysia, with a description of a new species. Bot J Linn Soc, 2003; 142(3):321–39. CrossRef
Seyed MA, Jantan I, Bukhari SN. Emerging anticancer potentials of goniothalamin and its molecular mechanisms. Biomed Res Int, 2014; 2014:536508. CrossRef
Sophonnithiprasert T, Mahabusarakam W, Nakamura Y, Watanapokasin R. Goniothalamin induces mitochondria-mediated apoptosis associated with endoplasmic reticulum stress-induced activation of JNK in HeLa cells. Oncol Lett, 2017; 13(1):119–28. CrossRef
Sun L, Zhao R, Lan X, Chen R, Wang S, Du G. Goniolactone C, a styryl lactone derivative, inhibits PDGF-BB-induced vascular smooth muscle cell migration and proliferation via PDGFR/ERK signaling. Molecules, 2014; 19(12):19501–15. CrossRef
Tangchirakhaphan S, Innajak S, Nilwarangkoon S, Tanjapatkul N, Mahabusrakum W, Watanapokasin R. Mechanism of apoptosis induction associated with ERK1/2 upregulation via goniothalamin in melanoma cells. Exp Ther Med, 2018; 15(3):3052–58. CrossRef
Umar-Tsafe N, Mohamed-Said MS, Rosli R, Din LB, Lai LC. Genotoxicity of goniothalamin in CHO cell line. Mutat Res, 2004; 562(1–2):91–02. CrossRef
Warden C, Barnett JM, Brantley MA, Jr. Taurocholic acid inhibits features of age-related macular degeneration in vitro. Exp Eye Res, 2020; 193:107974. CrossRef
Wiart C. Goniothalamus species: a source of drugs for the treatment of cancers and bacterial infections? Evid Based Complement Alternat Med, 2007; 4(3):299–311. CrossRef
Yang B, Zhu RB, Ding HB, Bouamanivong S, Tan YH. A new species and two new records of Goniothalamus (annonaceae) from lao PDR. PhytoKeys, 2020; 138:17–25. CrossRef
Zohdi RM, Mukhtar SM, Bihud NV, Rasol NE, Ahmad FB, Awang K, Ismail NH. In vivo antiplasmodial and toxicological effects of Goniothalamus lanceolatus crude extracts. Nat Prod Commun, 2017; 12(8):1251–4. CrossRef