INTRODUCTION
Several literatures have earlier reported the application of the most versatile C18 based chromatographic technique for the individual and concurrent quantification of saxagliptin (SXP) and metformin (MET) from the pharmaceutical product. However, as realized, the utilization of this C18 is very challenging for several reasons. Such as first, most negatively charged polar pharmaceutical amines do not retain in ion pairing mode (Gedawy, Al-salami & Dass, 2019; Gabhane et al., 2020) and, therefore, owing to their hydrophilic characteristics, it lowers their binding capacities to the ODS (Eva et al., 2016). Second, eventhough to encourage the drug–ODS interaction, the “ion suppression effects” with additional basic buffers further exhibit the peak tailing or fronting effects and, therefore, it disrupt the elution order and selectivities (Buszewska-Forajta et al., 2018). These challenges specifically observed in simultaneous investigation of two or more polar aliphatic amines (Porwal & Talele, 2017). Nonetheless, some other analytical techniques were recommended and demonstrated which predominantly included the hydrophilic interaction liquid chromatography (HILIC) (Peng et al., 2018), capillary zone electrophoresis, high performance thin layer chromatography (Maher et al., 2019; Patel et al., 2017), and supercritical fluid chromatography (Bui et al., 2008) but their significance in polar amines separation is still questionable.
Considering these aspects, strong cation exchange based liquid chromatography offers concurrent estimation of several amines to ensure and validate the comprehensive analytical studies. This strong cation exchange (SCX) based chromatography is complementary to the RP-HPLC which however did not use the ion exchange mechanism. Moreover, multiple pharmaceutical drugs and natural products analysis usually required highly sophisticated methods, owing to the involvement with their complexities and most often, low abundance of UV-Visible detection sensitivity (Prasad et al., 2015; Yunoos and Sankar, 2015).
Therefore, the importance of this study is to ensure the effectiveness of SCX chromatography as an alternative analytical tool towards simultaneous estimation of two selected antidiabetic drugs, SXP and MET from pharmaceutical formulation which were not reported earlier with this technique. In fact, SXP is not reported in latest pharmacopeia, whereas the MET was analyzed by the non-aqueous titration method where the formic acid along with and 0.1 M perchloric acid was used as an additive and the end point determination was reported by the potentiometric titration (The British Pharmacopoeia, 2010; United States Pharmacopeia and National Formulary, 2012).
MET, 1, 1-Dimethylbiguanide monohydrochloride (Fig. 1) is the first line drug recommended for the type-2 diabetic mellitus. Importantly, it not only reduces the sugar production in liver but also it decreases its absorption/reabsorption in gastro-intestinal tract to increase the insulin sensitivity in target cells (Bonfigli et al., 1999; Scarpello & Howlett, 2008). SXP, (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexan-3-carbonitril (Fig. 2) is a dipeptidyl peptidase-4 inhibitor, anti-diabetic drug. It is used as a single drug therapy or recommended with MET or thiazolidinediones when it is not enough to exhibit glycemic control (Dhillon, 2015).
MATERIALS AND METHODS
Materials
Generous gifts of standards; SXP and MET were received from UltraChrom Innovatives Pvt. Ltd, India. The Riax-M® XR tablets, containing 5 mg of SXP and 500 mg of MET, manufactured by Dr. Reddy’s Laboratories Ltd, were purchased from pharmacy. All HPLC grade chemicals and solvents were purchased from Merck (Mumbai, India). The HPLC columns included ProSwift® SCX-1S (50 × 4.6 mm ID; monolith); SiliaBond® Propylsulfonic Acid (SCX-2); Phenomenex-Luna® SCX-3 (100 × 2.1 mm i.d., 5μ) purchased from UltraChrom Innovatives Pvt. Ltd. (Nagpur, India). HPLC analysis was performed on Shimadzu Class A-10 VP instrument, equipped with UV-Vis detector (SPD-10A VP), binary pumps (LC-10AT VP), system controller (SCL-10A VP) with manual rheodyne injector (20 μl), controlled by LC-solution software. Analytical balance (ME-205, Mettler-Toledo), pH meter (FiveEasy-A211, Mettler-Toledo), and sonicator (Labman®, PCI) were used throughout the analysis.
Chromatographic conditions
HPLC analysis was performed on Shimadzu HPLC system. Mobile phases A and B were water and methanol, respectively. Both contained 15 mM ammonium formate (AF). SXP and MET were eluted with AF (15mM): MeOH in ratio 13:87 v/v for 10 minutes with considering isocratic elution at 1 ml/minute flow rate. All separations were performed at 28°C and recorded at 228 nm wavelength.
Preparation of analytical solutions
Standard preparation
Accurately weighed 25 mg of each standard, SXP and MET were diluted with 25 ml blank eluents separately in 50 ml volumetric flask and sonicated for 20 minutes. Furthermore, the stock solution was filtered through 0.20 μm nylon filters and volume was adjusted to 50 ml with relevant solvents to make 500 ppm. Furthermore, serial dilutions of different concentrations were made by mixing both standards to determine their validation parameters.
 | Figure 1. Moleculer structures of (A) SXP and (B) MET. [Click here to view] |
Sample preparation
Twenty Riax-M® XR tablets were weighed separately and accurately. They were crushed to fine powder and then weighed accurately equivalent to “5 mg SXP and 500 mg MET” were transferred to a 100-ml beaker. Each powder was then mixed with 25–50 ml methanol with continuous stirring for 10 minutes, followed by filtering through 0.20 μm nylon membrane filters into a 100-ml volumetric flask and then volume was adjusted with same eluent. Further serial dilutions were made and then developed SCX-HPLC methods were evaluated for SXP and MET in their pharmaceutical formulation. Furthermore, the analyte concentration was calculated from their corresponding regression equations.
Method validation procedures
Precision
Precision results were expressed in relative standard deviation (RSD). In repeatability, standard stock solution of SXP (500 μg/ml) and MET (100 μg/ml) was injected six times a day and their resultant peak areas and RSD were determined. Similarly, in intraday and intermediate precision (three different days) the triplicate of standard stock solution containing 500, 250, and 125 μg/ml of SXP; and 100, 50, and 25 μg/ml of MET were injected thrice and their respective RSD were calculated.
Linearity and range
Linearity was determined by using the calibration curve of MET for the concentration between 250 and 15.62 μg/ml (15.62, 31.25, 62.5, 125, 250 μg/ml) and SXP in the range of 31.25–500 μg/ml (31.25, 62.5, 125, 250, 500 μg/ml). Prior to that, both standards MET and SXP were independently dissolved in 15 Mm AF-MeOH eluent to make the concentration of 1 mg/ml and then they were mixed and diluted with the same eluent to obtain the serial dilutions. Linearity of peak area against the concentration was calculated to get regression values and correlation coefficient (r2).
Limit of detection (LOD) and quantification
LOD and limit of quantification (LOQ) were determined by injecting the homologous mixture of SXP and MET standard solutions in the range of 0.05–1 μg/ml. Furthermore, the LOD and LOQ were calculated using the following formula:
 | Figure 2. Schematic representation of comparative retention capacity of SCX-1, SCX-2 and SCX-3 phases. [Click here to view] |
LOD = 3.3 × (Std. Deviation of intercept/slope)
LOQ = 10 × (Std. Deviation of intercept/slope)
Robustness
The robustness studies involved the small variations in selected separation parameters such as changes in temperature (±2°C), flow rate (±0.2 ml/minutes), and wavelength (±2 nm) were tested and evaluated. The flow rate of the eluent was changed from 1 ml/minutes to 0.8 and 1.2 ml/minutes; the concentration of acetonitrile was changed from 87% to 85% and 89% and the wavelength was changed from 228 to 226 and 230 for SXP; and from 235 nm to 233 and 237 nm for MET. Furthermore, the results derived were evaluated for any changes in capacity factor (k’), resolution (R), theoretical plates (N), and tailing factor (T).
Accuracy
The accuracy was determined by mixing the fixed concentration of standards, SXP (2.5 μg/ml) and MET (250 μG/ml) with varying concentrations of Riax-M® XR tablets as 2 μg, 2.5 μg, and 3 μg to make the 80%, 100%, and 120%, respectively. The analysis was performed in a triplicate with data calculated to determine the percentage (%) drug recovery, mean ± SD, and percentage (%) RSD.
Degradation studies
Acid, Alkali, oxidation and thermal degradation studies
Forced degradation studies of SXP and MET were performed as per the International Conference on Harmonization (ICH) guideline (ICH, 2003). 8 ml of freshly prepared homologous mixture of stock solution, containing SXP (500 μg.ml−1) and MET (100 μg.ml−1), prepared in H2O–Methanol eluents was equally distributed into 4 different 25 ml volumetric flasks and further diluted with equal volume of H2O, 0.1 N HCl, 0.1N NaOH, and 3% H2O2 to get final concentration of 250 μg.ml−1 and 50 μg.ml−1 of SXP and MET, respectively. Sample prepared in 3% H2O2 was kept at room temperature for 6 hours whereas the acid-base and neutral hydrolyzed samples were kept at 60°C for 6 hours. Furthermore, all samples were sonicated, filtered through 0.20 μm nylon filters and then twenty μL of each sample was analyzed by HPLC using specified chromatographic method mentioned in chromatographic condition.
RESULTS AND DISCUSSION
Previously established chromatographic methods were evaluated in terms of their capabilities for concurrent estimation of both SXP and MET; included RP-HPLC (Gedawy et al., 2019), HILIC (AbuRuz et al., 2003; Peng et al., 2018), and capillary electrophoresis (CE) (Maher et al., 2019) techniques. Importantly, the selected amines resolving capacity; resolution (Rs) and capacity factor (K’) was the optimum in SCX method (60.52%), followed by RP-HPLC (50.79%) and CE while the lowest was observed for HILIC (50.83%); even it had the worst orthogonal selectivities relative to the RP-HPLC. In all attempted C18 based separation, as more hydrophilic characteristics of MET, it hardly retained with any eluents composition. Even its elution was erratically delayed with wide asymmetric peak of co-existing SXP was observed (Pednekar et al., 2014), moreover, in few articles MET was eluted with the dead volume (Vasudevan et al., 2001; Aburuz et al., 2005; Pednekar et al., 2014; Yunoos and Sankar, 2015; Eva et al., 2016; Merey et al., 2017; Kant et al., 2019). Similar incidences were also noticed in HILIC techniques (AbuRuz et al., 2003).
The SCX adsorbents included three main strong cation exchangers, such as sulfonic acid (SCX-1), propyl sulfonic acid (SCX-2), and benzene sulfonic acid (SCX-3) were tested and evaluated and as represented (Fig. 2A–C) their performances were varied in terms of selected amines separation. Among these, benzene sulfonic acid (SCX-3) proved the most efficient adsorbent among the tested cation exchangers (Fig. 2C). Perhaps, due to the existence of aromatic phenyl ring, SCX-3 has more hydrophobic and electrostatic characteristics, therefore enhancing nonpolar secondary interostatic affinity and increase binding strength with resultant analytes. Beside these, SCX-3 might also have some other characteristics features which make it enable for slightly better separation of aliphatic amines (Table 1). Considering these benefits, this proposed study was repeated to perform the system suitability studies for simultaneous estimation of SXP and MET, using SCX-3, achieved in 15 mM AF-methanol (13:87% v/v) and the results are demonstrated in Figure 3.
The SCX-3; modified with benzene sulfonic acids is negatively charged in aqueous buffers to exhibit stronger binding with basic analytes. The SCX-3 residues then interact with counter-ion from buffers to replace and elute the amines. Importantly, these amines elute at low pH buffer between 2.7 and 3. Therefore, at this pH, those analytes have the net charges of ≤+1 eluted with void volume (t0) whereas others, mostly amines/peptides characterized by ≥+2 net charges, retained in the column (Edelmann, 2011). That is why, SCX chromatography is the most preferred technique to isolate the peptides/phytoamines/pharmaceutical amines from acids and neutral hydrophobic compounds. Although, this depends on the type of eluent and buffer selected for the separation. AF (AF; pKa = 3.7) would be preferred over ammonium acetate (pKa = 4.7) since it is volatile and behaves as a salt rather than un-dissociated acid like acetate. Importantly, the TFA, formic acid as well as the exposure above pH 7 would drastically affect the SCX column performance. SCX based chromatography of amines is based on the fact that between these MET and SXP; MET is enriched with N-terminal free primary amines which contributes to its stronger retention and enables to elute slightly later than SXP (Fig. 2A–C). Even both drugs revealed good resolution and peak selectivities, most likely because of the differences in their amino functionalities where their separation was achieved within four minutes which is comparatively quite shorter than previously reported articles (Fig. 2C).
 | Table 1. Comparative retention pattern of SXP and MET. [Click here to view] |
System suitability studies
The proposed cation-exchange HPLC method for the simultaneous quantification of SXP and MET was validated as per the ICH guidelines and therefore including system suitability studies, other variables such as linearity, accuracy, precision (intra/intermediate), robustness and specificity studies were tested, evaluated, and displayed in Table 2.
As demonstrated, the proposed HPLC method signifies a high degree of reproducibility for the simultaneous quantification of SXP and MET (Nadella et al., 2018). For SXP, this proposed method expressed average retention time (tR) of 1.33 minutes with mean k’ of 1.52 whereas the tR and k’ for MET were 2.44 minutes and 3.34, respectively (Table 2). The tailing factor (T) values <2 represented that the peak width is under the acceptance criteria as per the ICH guideline since both symmetric and asymmetric factors were found of equal magnitude. The separation factor (α) and resolution (Rs) for both SXP and MET were found significantly higher than the minimum requirement as per the ICH guidelines.
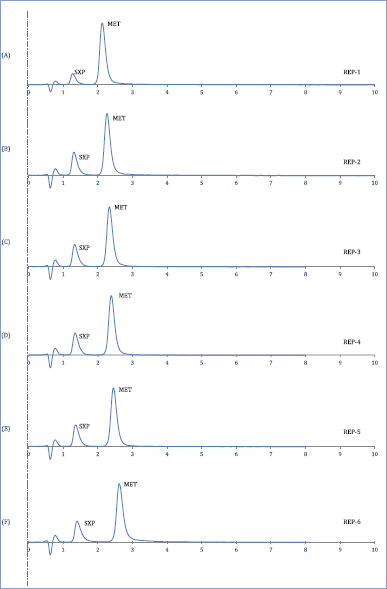 | Figure 3. Repeatability data (A–F) six replicates of SXP and MET at 228 nm wavelength. [Click here to view] |
 | Figure 4. (A) Linearity data of saxagliptin; (B) linearity data of and metformin. [Click here to view] |
Linearity and range
The linearity of any HPLC method represents its ability to explicit the results that should proportional to the concentration of studied analytes within a selected range (Nadella et al., 2018). Therefore, over the range of 32.5–500 μg.ml−1 for SXP and 16.25–250 μg.ml−1 for MET, significantly, higher proportionality was observed between the concentration against peak area with linear regression observed for SXP and MET were y = 5011.6x + 2,487.4 and y = 35,282x−36,065, respectively. Moreover, the regression coefficients (r2) were almost 0.999 for both samples; which itself represented a high degree of linearity (Fig. 4 and Table 2).
LOD and quantification
LOD and LOQ were calculated based on the standard deviation of the response and the slope of the regression equation. As observed, the LOD and LOQ of SXP were 4.67 and 14.17 μg.ml−1, whereas for MET they were 21.56 and 65.35 μg.ml−1, respectively (Table 2).
Accuracy
Percentage recoveries of three different concentrations (injected twice) to determine the SXP and MET were calculated to demonstrate the accuracy in RSD% for the selected pharmaceutical combination and reported in Table 3. Applying the calibration curve, the Y-intercept and the slope of the graph were used to determine the % recovery, attributed to the developed method for the simultaneous quantification. The achieved % RSD was 0.36, 0.63, and 0.36 for SXP and 0.63, 0.78, and 0.31 for MET, respectively, which are within the ICH and United States Pharmacopeia (USP) acceptance limit of ±2%. Above all, the proposed IEC method has proved the good accuracy from the obtained recovery data.
 | Table 2. System suitability parameters of SXP and MET. [Click here to view] |
 | Table 3. Drug Recovery data of SXP and MET. [Click here to view] |
Precision
The precision of HPLC method reflects its closeness to the agreement among the series of repetitive results, derived after multiple sampling of the same homogenous mixture of selected drugs under the given conditions (Nadella et al., 2018). As displayed in Table 2; both intra- and inter-day variabilities for precision studies, this method is significantly precise over the tested range of 10–30 μg/ml for SXP and 25–150 μg/ml for MET. Moreover, the peak area of the studied samples was also correlated with selected concentration; where the % RSDs were <2%. The RSDs were observed in the range of 0.44%–1.62% for SXP and 0.35%–0.87% for MET of the intra-day studies (Table 3); whereas the % RSDs were observed in the range of 0.26%–1.61% for SXP and 0.87%–1.52% for MET in the inter-day studies (Table 2) that reflects an acceptable precision with minimum variations of the proposed method.
Robustness
Robustness of HPLC method represents its ability to remain unaffected by small but deliberate variations in separation parameters to ascertain its reliability during routine analysis. In this method, robustness was established by making deliberate changes in flow rate (1.0 ± 0.2 ml/minutes), organic modifier (83% ± 2% ml), and temperature (28°C ± 2°C). Therefore, increased the flow rate by +0.2 ml/minutes, reduced the tR values to 1.59 and 3.34 mins of SXP and MET, respectively, whereas reduced the flow rate (−0.2 ml/minutes), extended the tR values to 2.50 and 6.78 minutes of similar drugs; although the variation was almost 27% (Fig. 5A). However, altering the concentration of methanol as mobile phase by 85% ± 2% has not made any significant changes in the retention pattern of both SXP and MET since the differences in their retention time were <10% (Fig. 5B). Similarly, deliberate but small variation in column temperature by 28°C ± 2°C has also not made any significant changes for both drugs since as observed their difference in tR values was <1% (Fig. 5C). Thus, increasing the flow rate, organic modifier, and temperature, both SXP and MET were appeared earlier whereas decreasing them, their elution order were elongated. Specifically, selecting the flow rate has made wide differences in retention values. It might presumably appear because of selecting the small column dimension (100 × 2 mm ID). Importantly, excluding the theoretical plates (N); other variables like capacity factor (k’), resolution (Rs), peak tailing (Tf) were almost unchanged which clearly signified that the proposed HPLC method obliged all minimum requirements led by the USP and ICH guidelines (Table 4).
 | Figure 5. Robustness data of SXP and MET. [Click here to view] |
 | Table 4. Robustness data of SXP and MET. [Click here to view] |
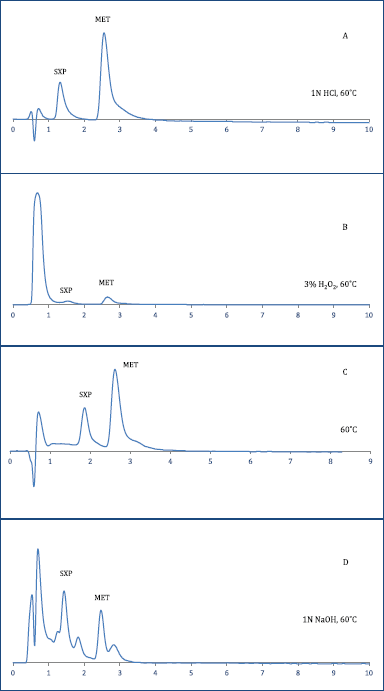 | Figure 6. Force degradation data of SXP and MET; A, acid induced stress effect; B, peroxide induced stress effect; C, thermal induced stress effect; D, alkali induced stress effect. [Click here to view] |
Forced degradation studies
The forced degradation studies using SCX chromatography revealed the possible disintegration of SXP and MET under the influence of stress factors like acid-base strength, peroxide, and thermal environment (Prasad et al., 2015) (Fig. 6). As observed, both SXP and MET were resistant to the 0.1N HCl treatment since no degradants were appeared in chromatograph (Fig. 6A). Similarly, the treatment under 3% H2O2 has not made any significant changes in stability as not any degradants were appeared in chromatograph (Fig 6B). Furthermore, when the homologous mixture was exposed to thermal stress at 60°C, as observed not any alteration in the retention and the development of new degraded products was observed for both SXP and MET (Fig. 6C). Nevertheless, comparatively, the treatment under 0.1N NaOH produced disintegration of the amines to the most severe form since as displayed, along with SXP, tR = 1.40 minutes and MET, tR = 2.44 minutes, few additional peaks were appeared at 0.68, 1.77, 2.78 minutes which collectively exhibit nearly 23% (Fig. 6D). However, the degradation mechanism is unpredictable but it might presumed to be the involvement of stabilization and destabilization (protonation and deprotonation) under the influence of 0.1N NaOH and 15 Mm AF. Therefore, future studies will involve the exact prediction of the chemical structures of quantified degraded products by using LC-MS/MS or LC-NMR for pharmaceutical formulations of SXP and MET.
CONCLUSION
The present HPLC method explicit the shortest run time for the simultaneous quantification of SXP and MET where the results of repeatability, linearity, accuracy, precision, and robustness and specificity were found acceptable and validated as per the ICH guidelines. The established method was found stable since there was no interference of degradants in force degradation was observed. Therefore, this established method is conducive for routine estimation and characterization of either SXP or MET or the combination of both with other co-existing drugs from bulk and finished pharmaceutical formulations. Collectively, this study further underlines the importance of strong cation exchange chromatography which was potentially useful in analysis of other relevant aliphatic/aromatic pharmaceutical amines based on their net positive charges and steric selectivities.
ACKNOWLEDGMENTS
This work is dedicated to Dr. Ravindranath B. Saudagar Sir. He was the Dean of Sapkal Knowledge Hub, Nashik, Maharashtra and he was the part of this study. Unfortunately, in the midway of this study, he passed away due to Colon cancer. This research is dedicated to his kindness, involvement, and most importantly his generous support throughout this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Not applicable.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
AbuRuz S, Millership J, McElnay J. Determination of metformin in plasma using a new ion pair solid phase extraction technique and ion pair liquid chromatography. J Chromatogr B, 2003; 798:203–9. CrossRef
AbuRuz S, Millership J, McElnay J. The development and validation of liquid chromatography method for the simultaneous determination of metformin and glipizide, gliclazide, glibenclamide or glimperide in plasma. J Chromatogr B, 2005; 817:277–86. CrossRef
Bonfigli AR, Manfrini S, Gregorio F, Testa R, Testa I, De-Sio G, Coppa G. Determination of plasma metformin by a new cation-exchange HPLC technique. Ther Drug Monit, 1999; 25:77–84. CrossRef
Bui H, Masquelin T, Perun T, Castle T, Kuo MS. Investigation of retention behavior of drug molecules in supercritical fluid chromatography using linear solvation energy relationships. J Chromatogr A, 2008; 1206:186–95. CrossRef
Buszewska-Forajta MF, Markuszewski MJ, Kaliszan R. Free silanols and ionic liquids as their suppressors in liquid chromatography. J Chromatogr A, 2018; 155920:17–43.
Dhillon S. Saxagliptin: a review in type 2 diabetes. Drugs, 2015; 75(15):1783–96. CrossRef
Edelmann MJ. Strong cation exchange chromatography in analysis of posttranslational modifications: innovations and perspectives. J Biomed Biotechnol, 2011; 2011:1–7. CrossRef
Eva T, Leonard D, Gezim B. Ion-pair HPLC method for the quantification of metformin in human urine. J Appl Bioanal, 2016; 2:16–24. CrossRef
Gabhane KB, Kamdi DB, Gulhane CA, Kharabe PM. Analytical validation and stability indicating studies for simultaneous estimation of benidepine and metoprolol by strong cation exchange (SCX) chromatography. Pharm Methods, 2020; 11:6–12. CrossRef
Gedawy A, Al-Salami H, Dass CR. Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and Gliclazide. J Food Drug Anal, 2019; 27:315–22. CrossRef
ICH. ICH Q1 A (R2) stability testing of new drug substances and products. International Conference on Harmonization, Geneva, Switzerland, 2003.
Kant R, Bodla RB, Kapoor G, Bhutani R. Optimization of a single HPLC-PDA method for quantifying metformin, gliclazide, pioglitazone, dapagliflozin, empagliflozin, saxagliptin, linagliptin and teneligliptin using central composite design. Bioorg Chem, 2019; 91:103–111. CrossRef
Maher HM, Abdel Rahman AE, Alzoman NZ, Aljohar HI. Stability-indicating capillary electrophoresis method for the simultaneous determination of metformin hydrochloride, saxagliptin hydrochloride, and dapagliflozin in pharmaceutical tablets. J Liq Chromatogr Relat Technol, 2019; 42:161–71. CrossRef
Merey HA, Ramadan NK, Diab SS, Moustafa AA. Chromatographic methods for the simultaneous determination of binary mixture of Saxagliptin HCl and Metformin HCl. Bull Fac Pharm Cairo Univ, 2017; 55:311–7. CrossRef
Nadella NP, Ratnakaram V, Srinivasu N. Development and validation of UPLC method for simultaneous quantification of carvedilol and ivabradine in the presence of degradation products using DoE concept. J Liq Chromatogr Relat Technol, 2018; 40(3):1–11. CrossRef
Patel D, Kumar P, Sharma S, Dwivedi J. Analytical methods for metformin estimation. Crit Rev Anal Chem, 2017; 47(5):405–17. CrossRef
Pednekar S, Lokhande R, Sutar R, Kolhal S, Surve S, Gudekar S. Simultaneous determination of metformin, sitagliptin, saxagliptin, linagliptin and vildagliptin in multicomponent pharmaceutical preparations by RP-HPLC. Int J Pharm Sci Rev Res, 2014; 24:128–33.
Peng Y, Chang Q, Yang N, Gu S, Sun J. Quantitative determination of metformin, saxagliptin and 5-hydroxy saxagliptin simultaneously by hydrophilic interaction liquid chromatography - electrospray ionization mass spectrometry and its application to a bioequivalence study with a single-pill combination in human. J Chromatogr B, 2018; 10821:109–17. CrossRef
Porwal PK, Talele GS. Development of validated HPLC-UV method for simultaneous determination of metformin, amlodipine, glibenclamide and atorvastatin in human plasma and application to protein binding studies. Bull Fac Pharm Cairo Univ, 2017; 55:129–39. CrossRef
Prasad PBN, Satyanaryana K, Krishnamohan G. Development and validation of a method for simultaneous determination of metformin and saxagliptin in a formulation by RP-HPLC. Am J Analyt Chem, 2015; 06:841–50. CrossRef
Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diabetes Vasc Dis Res, 2008; 5:157–67. CrossRef
The British Pharmacopoeia. Her majesty’s stationery office. The British Pharmacopoeia, London, 2010.
United States Pharmacopeia and National Formulary. United States Pharmacopeia and National Formulary (USP 35-NF 30). United States Pharmacopeia Convention, Rockville MD, 2012.
Vasudevan M, Ravi J, Ravisankar S, Suresh B. ION-pair liquid chromatography technique for the estimation of metformin in its multicomponent dosage forms. J Pharm Biomed Anal, 2001; 25:77–84. CrossRef
Yunoos M, Sankar DG. Stability indicating quantitative RP-HPLC method development and validation for simultaneous determination of metformin hydrochloride and saxagliptin in bulk and combined tablet dosage form. J Chem Pharm Res, 2015; 7:346–55.