INTRODUCTION
About 3.3 million deaths globally were attributable to alcohol use and abuse in 2012. In the Philippines, 4.6% of males and females aged 15 and above are diagnosed with alcohol use disorder, and 2.9% are categorized under alcohol dependence. However, no national guidelines, action plans, or national monitoring systems yet exist in the Philippines for alcohol use and abuse. Hence, it is mandatory to invest in research on alcohol to influence and promote therapy and management in this field (World Health Organization, 2014).
Of the acute concerns, hangovers and hospital management prove to be the most immediate. Hangovers can lead to lower performance, less efficiency at work, and risks for catastrophes such as in driving or navigation (Penning et al., 2010). Meanwhile, intoxicated patients admitted to the hospital with comorbid concerns can be challenging to manage. In an article published in the Emerging Medicines Journal, alcohol-related emergency department attendances are at 12%–15% on average and peak on Friday and Saturday evenings, when up to 70% of all attendances can be alcohol-related (Irving et al., 2017). Therefore, performing triage while subsequently taking history and providing management can be cumbersome, due to decreased sensorium and motor function.
Fortunately, ginger (Zingiber officinale) has been traditionally used to promptly manage the symptoms of hangovers, often taken after drinking to reduce nausea and vomiting. In fact, ginger has been shown to be as effective as metoclopramide in preventing nausea and vomiting (Ernst and Pittler, 2000), which may point to its use for hangovers. In 2014, ginger was also shown to prevent morphine-induced addiction in mice (Torkzadeh-Mahani et al., 2014), suggesting that ginger might be capable of managing both the acute and chronic effects of substance use and abuse.
In this study, we aimed to explore these effects of ginger rhizome extract (GRE) on alcohol-induced behaviors and withdrawal symptoms in Caenorhabditis elegans (C. elegans) and to establish methods to allow its use as a high-throughput screening model for drugs with therapeutic value on alcohol use and abuse, which is supported by previous reports (Davies et al., 2003; Lee et al., 2009). Here, we show that GRE is a consistently acute depressant at 1,000 µg/ml but promotes tolerance over time compared with EtOH and vehicle alone. Furthermore, GRE restores deficits in locomotion induced by acute withdrawal of EtOH after prolonged exposure and disrupts learned preference of C. elegans to EtOH coupled with OP50 as a desirable stimulus.
MATERIALS AND METHODS
All works were carried out in the Biological Models Laboratory of the Department of Biochemistry and Molecular Biology, College of Medicine, University of the Philippines Manila. The study was conducted at 20°C and under the precautions of Biosafety Level 1, as approved by the biosafety committee of the university.
Collection of plant material
Two kilograms of Z. officinale rhizomes was obtained from Legazpi Sunday Market, Makati, which directly sources ginger rhizomes from La Trinidad, Benguet, Philippines. A sample of the rhizome was then sent to the Bureau of Plant Industry for taxonomic classification.
Preparation of ethanolic GRE
Ginger was minced and air-dried for 7 days, prior to soaking in 95% ethanol for another 7 days to minimize dilution of the solvent, in a ratio of approximately 2.05 kg:2 l ethanol (EtOH). The solvent was then decanted and subsequently filtered via double-layered Whatman twice and was then sent for rotary evaporation at the Natural Products Laboratory of the Department of Biochemistry and Molecular Biology, College of Medicine, University of the Philippines Manila. The moist extract was air-dried for 2 days and then heated for 2–3 hours at 55°C, with the dry weight obtained for dissolution in KPO4 buffer of pH 6.0. In general, seven (7) solutions were prepared: (-) vehicle (1M KPO4 pH 6.0 only), (+) vehicle (1M KPO4 + 500 mM EtOH), 1 µg/ml + 500 mM EtOH, 10 µg/ml + 500 mM EtOH, 100 µg/ml + 500 mM EtOH, 500 µg/ml + 500 mM EtOH, and 1,000 µg/ml + 500 mM EtOH. Phytochemical analysis of the extract was not carried out.
Preparation of nematode growth media (NGM)
Nematodes were maintained in NGM plates prepared at the Biological Models Laboratory of UP Manila. Briefly, 3 g NaCl, 2.5 g peptone, and 20 g agar were dissolved in 1 l of dH2O and autoclaved at 121°C for 1 hour and then cooled to 60°C prior to the addition of 1 ml each of 1M MgSO4, 1M CaCl2, and 5 mg/ml cholesterol in ethanol. After thorough mixing, the medium was then poured into Petri plates using sterile techniques and stored at 4°C until further use. NGM plates were then seeded with Escherichia coli strain OP50 on the day of assaying.
Age synchronization of C. elegans strain N2
Nematodes (N = 20) at stage L4 to young adulthood were transferred into prepared NGM plates seeded with E. coli strain OP50. This range in age is considered as a limitation in the morphological characteristics of the nematode at this time, with minimum variation in physiology. Worms were worm-picked until each treatment group had N = 20 per trial. In each assay, each worm acted as a biological replicate.
Lethality assay in C. elegans
Toxicity assay was based on the protocols of Williams and Dusenbery (1988) and Katiki et al. (2011), with slight modifications (Katiki et al., 2011; Williams and Dusenbery, 1988). Briefly, C. elegans (N = 20) were exposed separately to 10-fold dilutions in concentration (0.1, 1, 10, 100, 500, and 1,000 ug/ml) or vehicle (1% DMSO) and were followed up after 24 hours to check for acute exposure, to determine if the concentrations chosen did not cause significant attrition and affect statistical power. Percent attrition of 20% or more leads to lower statistical power because of attrition bias (Dumville et al., 2006); therefore, concentrations were used for subsequent assays only if the survival rate was >85% after 24 hours. All concentrations were nonlethal in this study.
Working concentrations of ethanol exposure
All C. elegans nematodes were exposed to 500 mM ethanol, which corresponds to a nematode internal concentration of ~22 mM (Davies et al., 2003). The minimum blood ethanol concentration in humans to induce intoxication is 0.1% or 21.7 mM, which corresponds to the worm concentration. This concentration was therefore used to interpret the results of exposure more reliably.
Acute depression and tolerance
Twenty C. elegans worms were exposed to 500 mM ethanol (EtOH) for 20 or 40 minutes, respectively, either with vehicle (KPO4 buffer pH 6.0) or with GRE. The GRE concentrations used were serially diluted at 10-fold dilutions (1,000, 500, 100, 10, and 1 µg/ml), which were all coadministered with EtOH. Then, five parameters each for locomotion (body bending, short reversals, long reversals, total reversals, and omega turns) and sensation [plate tap, light touch (head), light touch (tail), nose touch, and harsh touch] were recorded per worm per treatment group. All parameters of locomotion were obtained by first transferring each nematode singly to blank NGM and allowing them to roam for 60 seconds to minimize startle behavior (Zhao et al., 2003). Then, each worm was individually videotaped for 20 seconds and scored for bending, reversals, and omega turns. For general sensation, plate tap was carried out by tapping the blank NGM plate five consecutive times and recording a reversal, where observed. Should a reversal not be observed, a second set of five consecutive taps were done before recording a negative score. For light touches to the head and tail, a strand of hair fixed to a sturdy handle was used to brush the area closest to the pharynx (head) and anus (tail). Similarly, nose touch was carried out by laying the hair in front of the worm in advance of its direction and recording its motion after touching the hair with its nose. Lastly, harsh touch was carried out by carefully poking the midbody region of the worm with a fixed nichrome wire, with the response (forward or backward motion) similarly recorded (Fig. 1).
Alcohol withdrawal
To induce withdrawal, the method of Scott et al. (2017) was adopted, with slight modifications. Briefly, worms were pretested for locomotion and were then exposed to EtOH ± GRE for 24 hours. Then, each worm was withdrawn to a neutral NGM seeded with OP50 for 1 hour. After that, each worm was transferred individually to blank NGM plates and tested for the five locomotion parameters similarly aforementioned in the depression and tolerance assays.
Induction of ethanol preference
Ethanol dependence in C. elegans was induced using the methods of Lee et al. (2009), with slight modifications. Briefly, stage L4 to young adult worms were age-synchronized into NGM Petri plates and starved for 12 hours. After 12 hours, all the plates were half-seeded with OP50, and varying concentrations of GRE ethanolic concentrations (1, 10, 100, 500, and 1,000 µg/ml) with 500 mM of ethanol were placed per plate. The control plate contained KPO4 with 500 mM ethanol. All plates were sealed and taped for at least 6 hours.
Assaying for ethanol preference
Worms induced to prefer ethanol were transferred to plates with four quadrants each according to the protocol of Lee et al. (2009). Briefly, two quadrants diagonal to each other (labeled B and C) contained a chemoattractant—10 µl of 0.1 M sodium acetate (NaCH3COO)—while two other quadrants diagonal (A and D) contained 10 µl of 500 mM EtOH. Worms were then placed in the middle of the plate and were allowed to roam the quadrants for 1 hour, after which the proportion of nematodes in the ethanol quadrants was scored. In our preference assay, a preference index (PI) is calculated as [(number of animals in quadrants A and D)—(number of animals in quadrants B and C)] / total tested animals. In all quadrants, 1 µl of a paralytic—1M sodium azide (NaN3)—was placed near the attractants to immobilize the worms shortly after the quadrant had been entered. Furthermore, plates in the queue for scoring were placed at −40°C to prevent worms outside any quadrant from contributing to the overall PI after the allotted time (1 hour). The PI was then calculated as follows (Fig. 1).
Statistical analyses
For all treatment groups, data were statistically analyzed for normality via the Shapiro–Wilk test, followed by a one-way analysis of variances post hoc Bonferroni–Holm test for parametric data and a Kruskal–Wallis post-hoc Conover test for nonparametric data, N = 20 for each treatment. “*” denotes significance at p < 0.05. Data are presented as mean ± SE.
RESULTS
Acute exposure to GRE in C. elegans
When C. elegans was exposed acutely (20 minutes) to 500 mM ethanol, no significant changes were observed in body bending, short reversals, and omega turn rates—a finding that contrasted previous findings. However, an increase in reversal rate was observed, accounted for almost exclusively by an increase in long reversal frequency (Fig. 2). It has been previously shown that the probability of omega turns increases with reversal duration, so expectedly omega turn rate should increase (Zhao et al., 2003). The lack of increase in omega turn rates suggests (1) a loss of coordination between long reversals and omega turns and (2) the presence of long reversals without a change in direction, which indicates an increase in meaningless locomotion. The concentration of ethanol used in this study (500 mM) was shown to result in an internal concentration of approximately 0.1% in C. elegans; in alcohol intoxication, a blood concentration of 0.1% leads to symptoms indicative of either euphoria or excitement—characterized by a delayed reaction time, loss of coordination, and vision and balance problems, among many (McIntire, 2010; Olson et al., 2013). It is interesting to note that these were also observed in the C. elegans model upon ethanol exposure, which suggests conservation of behaviors during alcohol intoxication.
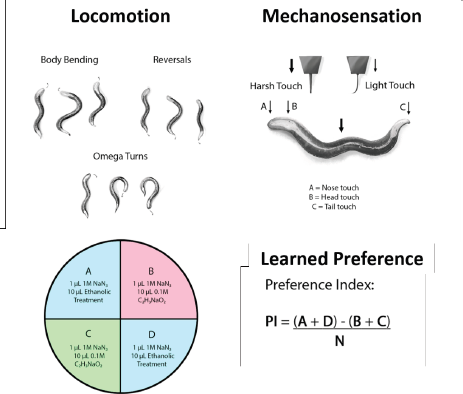 | Figure 1. Locomotion and mechanosensation is monitored in C. elegans by tracking voluntary movements and responses to mechanical stimuli while preference is determined via chemotaxis. [Click here to view] |
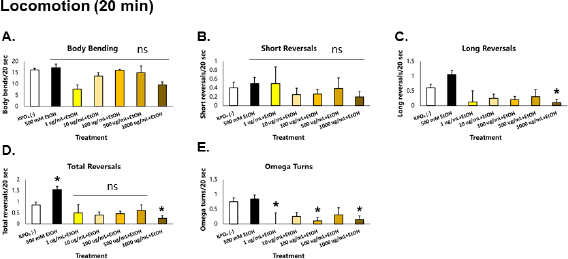 | Figure 2. GRE acts as an acute depressant of locomotion in C. elegans. Asterisks (*) denote significance at p < 0.05 versus vehicle. [Click here to view] |
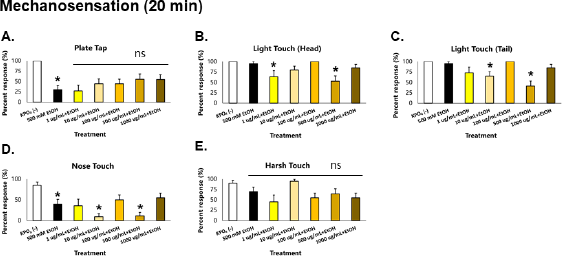 | Figure 3. GRE acts as an acute depressant of mechanosensation in C. elegans. Asterisks (*) denote significance at p < 0.05 versus vehicle. [Click here to view] |
When a response was elicited via physical stimulation, worms acutely exposed to ethanol showed a striking 75% reduction in plate tap response, which indicates loss of general sensation (Fig. 3). This suggests depression in the mechanoreceptor neurons ALML, ALMR, AVM, PLML, PLMR, and PVM which mediate general sensation and light touch (Goodman, 2006). More than a 50% reduction in nose touch response was also observed, which suggests depression of the ASH neuron—whose function is monitored via the nose touch response. Minor reduction in harsh touch response was also observed, which suggests depression of the PVD neuron in C. elegans, which is a nociceptor neuron in the chosen animal model. These mechanoreceptors deliver signals to AVA, AVD, AVB, and PVC interneurons: AVA and AVD deliver signals to DB and VB motor neurons which causes backward motion; AVB and PVC deliver signals to DA and VA motor neurons which causes forward motion (Goodman, 2006).
In worms exposed to GRE, significant decreases in the magnitude of all parameters of locomotion were observed, especially in the 1,000 ug/ml group, which were also greater than those exposed to ethanol alone. In addition, all parameters of sensation were depressed but were greater in the 500 ug/ml group. These results suggest that GRE is generally a depressant, which seems more potent than the concentration of ethanol used. Likewise, GRE cannot rescue C. elegans from acute ethanol-induced aberrations in sensation and locomotion because ethanol is also a depressant at moderate to high doses.
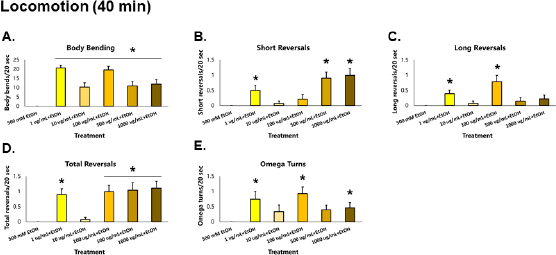 | Figure 4. GRE improves the recovery of locomotion in C. elegans possibly by improving tolerance. Asterisks (*) denote significance at p < 0.05 versus EtOH alone. [Click here to view] |
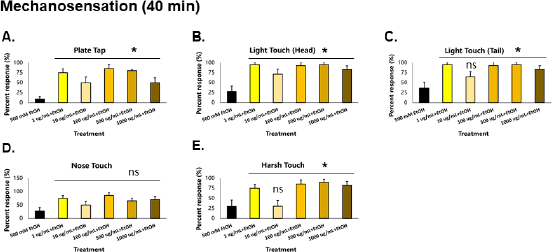 | Figure 5. GRE also improves the recovery of mechanosensation in C. elegans. Asterisks (*) denote significance at p < 0.05 versus EtOH control. [Click here to view] |
Among all parameters of locomotion, there were no observed movements in the 500 mM ethanol control group. Comparing results from the motor depression assay conducted after 20 minutes of alcohol exposure (Fig. 3A–E), a drastic decrease in locomotion from levels suggestive of reversal duration and omega turn rate coordination loss and meaningless locomotion to levels of complete absence of movement signifies that ethanol tolerance has yet to be induced in the worms. This finding follows that of a previous study which showed that the N2 strain of C. elegans develops alcohol tolerance slowly (Davies et al., 2004).
Extended exposure to GRE to assess tolerance in C. elegans
At 40 minutes, nematodes exposed to GRE were again assayed for both locomotion and sensation versus those exposed to ethanol alone, to determine the effects of GRE on worm tolerance. For body bends, all treatment groups were significantly higher than the ethanol group (p < 0.000001) after the Kruskal–Wallis post-hoc Conover test. For short reversals, the 1, 500, and 1,000 µg/ml treatment groups were significantly higher than the ethanol group (p = 0.000218), while treatments 1 and 100 µg/ml for long reversals were significantly higher (p = 0.001490). Total reversals showed significantly higher results across all treatments (p = 0.000138) except for 10 µg/ml. Lastly, omega turns were shown to be significantly higher in treatments 1, 100, and 1,000 µg/ml (p = 0.012692). Significantly higher locomotion parameters for the tolerance assay were seen most consistently in the 1 µg/ml followed by the 100 and 1,000 µg/ml treatment groups. Other treatment groups not able to generate significant difference with the ethanol control group, especially the 10 µg/ml group, could be attributed to a sampling error, wherein the worms might have been injured in the process of worm-picking, leading to decreased movement, if any. Nevertheless, GRE treatment significantly increased the recovery of locomotion to an otherwise depressed worm with only ethanol as its exposure (Fig. 4).
Similar to locomotion, mechanosensation parameters (plate tap, light touch head, light touch tail, nose touch, and harsh touch) of the control group worsened compared to those taken at 20 minutes (Fig. 5A–E). This signifies that ethanol tolerance has yet to be induced in the worms, which is also consistent with a previous study in 2004 (Davies et al., 2004). All treatment groups were significantly higher than the control for plate tap and light touch to the head (p < 0.01). All treatment groups except for those given 10 µg/ml GRE were significantly higher than control in light touch tail and harsh touch responses (p < 0.01). Only the nose touch parameter was not significantly different from the control, which may suggest that the neurons providing this sensation, which are also responsible for sensing noxious chemicals and repellants (ASH), are unaffected by GRE. Nonetheless, GRE treatments significantly increased recovery of mechanosensation to an otherwise depressed worm given only EtOH.
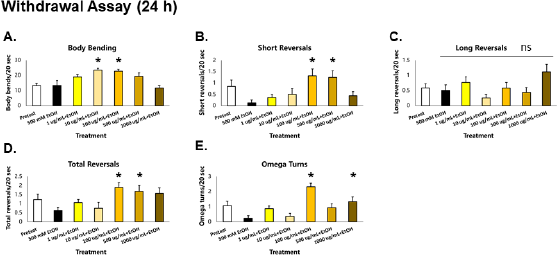 | Figure 6. GRE protects locomotion from deficits induced by acute EtOH withdrawal after a 24 h exposure in C. elegans. Asterisks (*) denote significance at p < 0.05 versus vehicle. [Click here to view] |
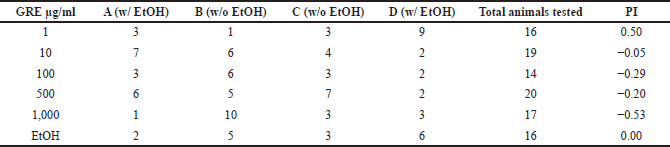 | Table 1. Coadministration of EtOH with GRE disrupts learned preference to EtOH in C. elegans. [Click here to view] |
Assessment of locomotion in ethanol-exposed C. elegans after stimulus withdrawal
When worms were withdrawn acutely (1 hour) from EtOH after prolonged exposure (24 hour), significant decreases in total reversals and omega turns were observed compared to preexposure conditions (white bar), indicating that withdrawal of EtOH results in deficits in foraging and roaming movements in C. elegans (Fig. 6D–E). Of the total reversals, short reversals were decreased, with no observable decreases in the long reversals (Fig. 6B–D). This implies that withdrawal also leads to incoordination between omega turns and long reversals—a pattern previously observed in the depression assay (Fig. 2D–E). Meanwhile, no decreases in body bending rate were seen (Fig. 6A), which was in contrast with a previous report showing reduction of crawl speed upon withdrawal (McIntire, 2010). Although this is the case, it is argued that the difference in effects with body bending is a function of exposure difference with previous studies. In this study, EtOH was applied directly to the worms after worm-picking; meanwhile, studies on alcohol withdrawal in C. elegans applied EtOH in the media and allowed for equilibration. The former method was carried out to ensure that exposure times among all worms were the same, which can be easily confounded by worm-picking.
Learned preference assay in C. elegans
In the learning preference assay, EtOH (500 mM) coupling with OP50 as a desirable stimulus after a minimum of 12 hours starvation led to an ethanol PI of zero. Compared with sodium acetate that is a natural chemoattractant to C. elegans, this indicates that the nematodes displayed equal preferences for sodium acetate and the previously neutral stimulus (EtOH), suggestive of a successful associative learning.
Meanwhile, exposure to GRE led to a dose-dependent decrease in preference to EtOH, with the greatest loss of preference observed with exposure to 1,000 µg/ml GRE (Table 1). At 1 µg/ml, the preference for EtOH increased twofold. Since GRE is a consistent acute depressant at high concentrations, the observed increase in EtOH preference may be due to suboptimal depression of excitatory neurons in the nematode, leading to rebound excitation that can in fact promote learning.
However, while GRE is effective at higher concentrations, acute lethality at 24 hours showed that GRE is most toxic at the highest concentration (1,000 µg/ml). While all other concentrations are relatively nontoxic at the threshold survival rate of 85%, a question of benefits versus risks arises with GRE at higher doses, which may possibly be a concern in future work with higher mammalian models and in future therapy and management.