INTRODUCTION
In medical chemistry, pyrimidine compound derivatives are well known for their application in various types of treatment (Mohamed et al., 2011; Tyli?ska et al., 2021). Pyrimidine core is an important pharmacophore in medical chemistry (Severina et al., 2019). The synthesis of pyrimidine derivatives attracts attention in modern drug discovery because it has broad benefits and has high potential (Cahyana et al., 2020; Sharma et al., 2014). One of the potential pyrimidine derivatives in the field of medicine is dihydropyrimidinones (DHPMs). DHPMs are heterocyclic compounds that have important bioactivity, such as anticancer, antimicrobial, antihypertensive, anti-inflammatory, antitubercular, antimalarial, and antioxidant activities (Beena et al., 2016). The compound was identified as a promising anticancer agent, of which monastrol is one of the most recognized elements (Mostafa and Selim, 2018).
DHPMs and their derivatives are heterocyclic compounds that have been synthesized through multicomponent reactions such as the Biginelli reaction (Matos et al., 2018). Multicomponent reactions are defined as reactions carried out into three or more reactants mixed in one reaction container to form products containing new properties or like the reactants used in the reaction (Poddar et al., 2017). The synthesis of previous DHMPs derivatives has traditionally been carried out with three-component cyclocondensation catalyzed using HCl but has major disadvantages such as low yield (20%–60%) and longer reaction times (24–36 hours). Research on the synthesis of DHPM derivatives continues to be developed with various types of catalysts to provide more optimal and environmentally friendly results (Bahekar et al., 2017b).
Multicomponent reactions (MCRs) are used in the synthesis of DHPMs, in which the synthesis of DHPMs is carried out with three reagent components reacted in one flask. The three components of the reagent are the urea compound, the β-ketoester compound, and the aromatic aldehyde compound (Bahekar et al., 2017a). Multicomponent reactions are widely used in the synthesis of organic compounds because they have high efficiency over the timing and stages of the reaction (Cahyana et al., 2021; Hapsari et al., 2020). The reaction on MCRs has a more complex reaction mechanism than reactions that have different reaction stages with two reagents to form the same product compound but can produce high-yield products (Qu et al., 2013). The Biginelli multicomponent reaction for the synthesis of pyrimidine derivatives in this study was a catalysis process using organic catalyst l-proline nitrate.
The l-proline is a natural amino acid that has a neutral pH and a very high solubility, is nontoxic at high concentrations, and exerts a high osmotic pressure (Zhang et al., 2016). Some previous studies have used l-proline and its derivatives as catalysts in direct reactions for asymmetric Diels–Alder reactions, asymmetric α-amination of ketones, asymmetric aldol reactions, and synthesis of coumarins (Azimi, 2015). The catalysis reaction will be easier when a catalyst has great mobility. The l-proline is reacted with nitric acid to form a liquid phase on l-proline nitrate, so it has higher mobility than the solid phase (Hirose et al., 2019). Lately, ionic liquids have been widely used in synthetic organic chemistry because they have been shown to function as new green reaction media. Excess ionic liquids include nonvolatile properties, irregularities in some solvents, and their ability to dissolve catalysts, making them easy to separate (Loh et al., 2002; Sheldon, 2001; Zhao et al., 2002).
In this research, the l-proline nitrate catalyst which is an amino acid ionic liquid is used to synthesize dihydropyrimidinones which are known to have many bioactivities. The l-proline nitrate is an environmentally friendly catalyst and has many benefits such as higher yields and shorter reaction times, is economical and recyclable, and can be reacted under simple conditions (Liu et al., 2008). The catalysis process using l-proline nitrate can be carried out at simple conditions, meaning it does not require a high-temperature reaction. The use of the l-proline nitrate catalyst was previously used by Tao et al. (2006) and Agrawal et al. (2015) to synthesize functional piperidine using the Diels–Alder reaction. Also, in the research of Bahekar et al. (2017a, 2017b), l-proline nitrate could facilitate electrophilic activation of enon sulfonamide with acetonitrile with an optimal dielectric constant of the solvent which can increase thiophenol nucleophilicity.
EXPERIMENT
Materials and instrumentations
All reagents and chemicals in this study were obtained from Merck or Sigma-Aldrich Chemical Company without the refining process. Some chemicals used were l-proline (Sigma-Aldrich, ≥99%), nitric acid (Merck, 65%), benzaldehyde (Sigma-Aldrich, 98%), 4-hydroxybenzaldehyde (Sigma-Aldrich, 98%), 3-methoxy benzaldehyde (Sigma-Aldrich, 98%), thiourea (Sigma-Aldrich, ≥99%), ethyl acetoacetate (Sigma-Aldrich, 99%), methanol p.a. (Sigma-Aldrich, 99.9%), distilled water, n-hexane (Merck, ≥99%), and ethyl acetate (Merck, 96%). For analysis, several instruments were used: Merck grade thin layer chromatography (TLC) plate, visualized by UV light; a melting point apparatus; the Fourier transform infrared (FTIR) IRPrestige-21 Shimadzu spectrophotometer that is registered in the 4,000–400 cm-1 region; the Shimadzu UV-Vis UV-2450 spectrophotometer; and the Agilent HP 6890 for gas chromatography mass spectroscopy (GCMS).
Synthesis of l-proline nitrate ionic liquids
The l-proline nitrate catalyst was synthesized by mixing an l-proline reagent with nitric acid with a stoichiometric mol ratio of 1:1. First, the l-proline was dissolved in deionized water for 1 hour until homogeneous. Then, nitric acid was added to the l-proline solution and stirred constantly for 4 hours. After being homogeneous, the solution was evaporated at room temperature. The evaporation process was carried out for 40 days to form a transparent yellow liquid. The synthesized product was ready to be characterized for its functional group using FTIR.
Synthesis of pyrimidine derivatives
In general, the synthesis of pyrimidine derivatives was carried out by mixing urea or thiourea (2 mmol), aromatic aldehydes (2 mmol), β-ketoester (2 mmol), and l-proline nitrate (0.2 mmol) in a 2 ml solvent at room temperature (Fig. 1). The reaction was carried out at room temperature using a magnetic stirrer for 60 minutes. The reaction process was monitored by TLC. After completion of the reaction, the mixture was evaporated and recrystallized with hot ethanol a few times. Next, the product was analyzed and characterized using a melting point apparatus, FTIR spectrophotometer, UV-Vis spectrophotometer, and GCMS.
RESULTS AND DISCUSSION
The synthesis of the l-proline nitrate catalyst was carried out by the method reported in Yogam et al. (2012), with some modifications. In this work, the precursors used to make ionic liquid l-proline nitrate are l-proline and nitric acid. The advantages of the used synthesis method are that the formation process does not utilize thermal energy. In other words, the synthesis process is carried out at room temperature, so the process is more environmentally friendly. An early indicator of the success of the synthesis of l-proline nitrate as an ionic liquid can be observed through color changes. Initially, colorless precursors will turn into pale yellow. According to the report of Yogam et al. (2012), the catalyst l-phenylalanine chloride (from l-phenylalanine and hydrochloric acid) was successfully synthesized for 25 days and formed a transparent yellow crystal. However, this study took 40 days to obtain l-proline nitrate in the form of a pale yellow liquid. The physical form and structure of the l-proline nitrate catalyst can be seen in Figure. 2.
The vibration characterization of the l-proline functional group was carried out by the FTIR spectrophotometer. In the FTIR spectrum of l-proline nitrate (Fig. 3), there are several specific peaks. Bands between 3,600 and 3,000 cm−1 indicate the existence of O-H stretching vibrations from the carboxylic acid group found in l-proline nitrate. The vibration stretch of the N-H functional group is in the range of 3,350–3,310 cm−1 but is covered by a widening O-H peak due to additional hydrogen bonds between O on C carbonyl and hydrogen. In addition, there may still be residual water used as a solvent at the beginning of the experiment. Then, the C-H sp3 stretch vibration is in the area of 2,850 cm−1; the vibration at 1,325 cm−1 is thought to be the N-O peak region of the nitrate group. In addition, there are also vibration groups C=O, C-N, and C-O in the 1,743, 1,422, and 1,053 cm−1 regions, respectively. Based on the IR spectrogram data, it can be concluded that the resulting peak indicates all the groups contained in the l-proline nitrate catalyst.
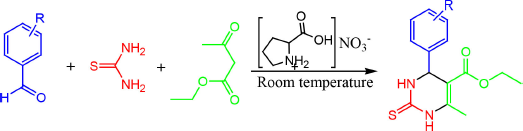 | Figure 1. General reaction of pyrimidine derivatives synthesis. [Click here to view] |
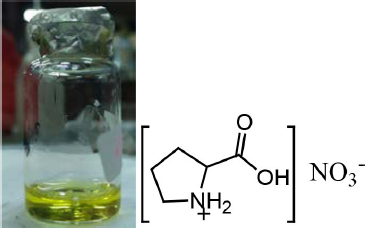 | Figure 2. Physical and molecular structure of l-proline nitrate. [Click here to view] |
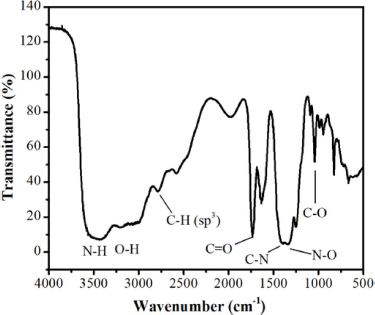 | Figure 3. FTIR spectrum of l-proline nitrate. [Click here to view] |
The evaluation of the catalytic ability of ionic liquids l-proline nitrate was carried out by synthesizing pyrimidine derivatives. The synthesis of pyrimidine compounds occurs through the reaction of the Biginelli multicomponent using the one-pot multicomponent reaction method. The proposed reaction mechanism that occurs is shown in Figure 4 (compound 1). The reaction begins with a nucleophilic addition reaction between benzaldehyde and thiourea using the l-proline nitrate catalyst and the methanol solvent in the reaction. At this stage, the catalyst l-proline nitrate attacks the nucleophilic benzaldehyde group and forms an iminium catalyst intermediate. Then, in the next stage, thiourea is assisted by iminium to form acyl imine intermediates. On the other hand, there is a reaction between ethyl acetoacetate and the l-proline nitrate catalyst to form ethyl-3-hydroxy-2-butenoate intermediates. Furthermore, the final step of this reaction is the reaction between the intermediate acyl iminium and ethyl-3-hydroxy-2-butenoate to form pyrimidine derivatives.
Monitoring of reaction conditions of pyrimidine synthesis, variations in solvent type, and catalyst concentration was studied by reacting benzaldehyde, thiourea, and ethyl acetoacetate (compound 1) in a 1:1:1 molar ratio as a reaction model. Treating optimization of the reaction can be seen in Table 1 under reaction conditions at room temperature for 60 minutes. The use of methanol as a solvent is the best choice for this reaction with a yield of 86.74%. The effect of the reaction with the catalyst and without the catalyst on the methanol solvent was significantly different. Without the use of catalysts, the yield obtained is 33.04%, while the addition of a 10% mol ionic liquids l-proline nitrate catalyst obtained a yield of 86.74%. The ability of catalytic ionic liquids l-proline nitrate as a catalyst is more than 2.5 times that of noncatalysts. To obtain the optimal solvent reaction, this study also uses water as a reaction medium. Under the same conditions as methanol (10% catalyst, room temperature for 60 minutes), the yield was much lower at only 12.56%.
With the encouraging result from the optimization process of the reaction, compound 2 and compound 3 were synthesized by replacing the benzaldehyde with 4-hydroxybenzaldehyde and 3-methoxybenzaldehyde. The three names of pyrimidine-derived compounds are ethyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (compound 1), ethyl 4-(4-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (compound 2), and ethyl 4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (compound 3). Product characterization and yield data are as illustrated in Table 2.
Experimental evidence in support of the structural evaluation and determination was conducted by melting point, functional group vibration, maximum wavelength, and molecular weight by a melting point apparatus, FTIR, a UV-Vis spectrophotometer, and GCMS, respectively. The FTIR spectrum of compound 1 (Fig. 5a) shows several peaks that indicated compound 1 vibration. The peak at 3,330 cm−1 is secondary N-H stretching vibrations. Peaks 3,172 cm−1 and 3,106 cm−1 are stretching vibrations of C-H sp2, while the peak at 2,985 cm−1 is stretching vibrations of C-H sp3. In addition, there are also stretching vibration peaks of C=O, C=C, C-N, and C-O at 1,680, 1,587, 1,465, and 1,202 cm−1, respectively. The maximum wavelength absorption test with a UV-Vis spectrophotometer found that compound 1 is absorbed at a wavelength of 309 nm (Fig. 5b). At the maximum absorption wavelength, a transition from n to π* occurs due to the presence of an aromatic chromophore.
Confirmation of the molecular weight of pyrimidine derivatives in this study was carried out by GCMS analysis. The single peak of compound 1 on the peak chromatogram was obtained at a retention time at 14,592 minutes. Then, the peak was analyzed by the mass spectrum and obtained a spectrum with parent peak m/z 276.1 (Fig. 6). The molecular weight of compound 1 with the molecular formula C14H16N2O2S is 276 g/mol. Thus, it can be said that the compound 1 pyrimidine derivative has been successfully synthesized. As additional information, fragments formed from the mass spectrum of compound 1 also support the formation of these compounds with base peak m/z 199 (Fig. 7). In this situation, the benzene group is released from the main compound. In addition, other fragments supporting the formation of this compound are at m/z 247, 171, 153, 128, 103, 77, and 42.
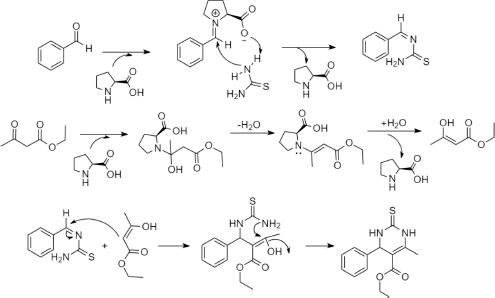 | Figure 4. The proposed reaction mechanism of compound 1 synthesis assisted by l-proline nitrate. [Click here to view] |
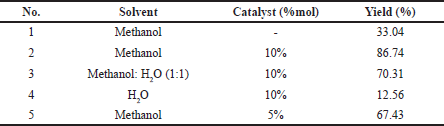 | Table 1. Optimization of reaction conditions. [Click here to view] |
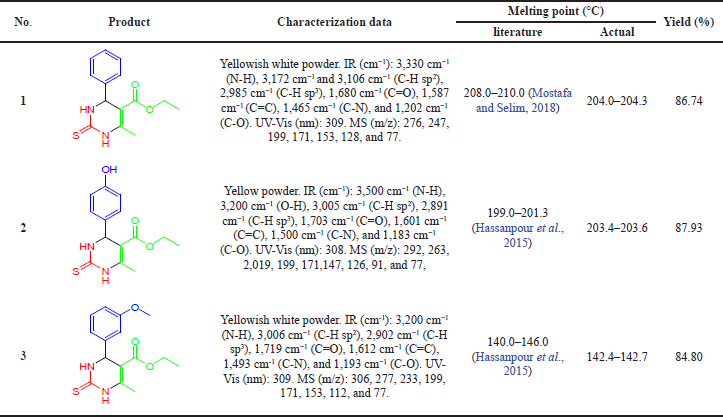 | Table 2. Characterization data and yield of pyrimidine derivatives. [Click here to view] |
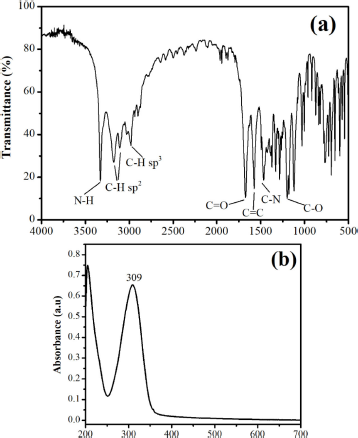 | Figure 5. (a) FTIR and (b) UV-Vis spectrum of compound 1. [Click here to view] |
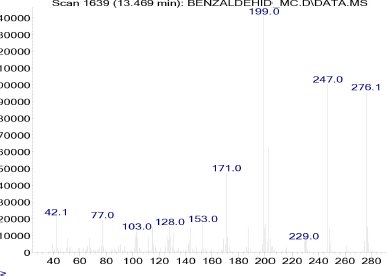 | Figure 6. Mass spectrum of compound 1. [Click here to view] |
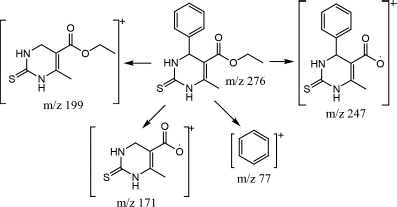 | Figure 7. Some fragments of compound 1 based on mass spectrum. [Click here to view] |
CONCLUSION
In summary, a series of pyrimidine derivative compounds were successfully synthesized. The analysis of products was carried out by a melting point apparatus, FTIR, UV-Vis, and GCMS, and identified that the compound formed was pyrimidine derivative compounds. The three pyrimidine derivatives are ethyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (compound 1), ethyl 4-(4-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (compound 2), and ethyl 4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (compound 3). The catalytic ability of ionic liquids l-proline nitrate as a catalyst is more than 2.5 times that of noncatalysts. The best condition of the reaction was carried out with a methanol solvent at a catalyst concentration of 3% mol with a yield of 86.74%.
ACKNOWLEDGMENT
This research was supported by Universitas Indonesia, Program Publikasi Terindeks Internasional (PUTI) Q2 Nomor: NKB-4263/UN2.RST/HKP.05.00/2020.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agrawal NR, Bahekar SP, Sarode PB, Zade SS, Chandak HS. L-proline nitrate: a recyclable and green catalyst for the synthesis of highly functionalized piperidines. RSC Adv, 2015; 5(58):47053–9; doi:10.1039/c5ra08022c CrossRef
Azimi, S. C. L-Proline catalyzed synthesis of naphthopyranopyrimidines via multicomponent reaction. Iranian J Cat, 2015; 5(1):41–8.
Bahekar SP, Agrawal NR, Sarode PB, Agrawal AR, Chandak HS. L-proline nitrate: an amino acid ionic liquid for green and efficient conjugate addition of thiols to sulfonamide chalcones. ChemistrySelect, 2017a; 2(29), 9326–9; doi:10.1002/slct.201701891 CrossRef
Bahekar SP, Sarode PB, Wadekar MP, Chandak HS. Simple and efficient synthesis of 3,4-dihydropyrimidin-2(1H)-thiones utilizing L-proline nitrate as a proficient, recyclable and eco-friendly catalyst. J Saudi Chem Soc, 2017b; 21(4):415–9; doi:10.1016/j.jscs.2015.09.004 CrossRef
Beena KP, Suresh R, Rajasekaran A, Manna PK. Dihydropyrimidinones-a versatile scaffold with diverse biological activity. J Pharm Sci Res, 2016; 8(8):741–6.
Cahyana AH, Liandi AR, Safitri Y, Yunarti RT. Synthesis of 1,4-dihydropiridine with aromatic of cinnamaldehyde compound using NiFe2O4 mnps catalyst and the activity test as antioxidant. Rasayan J Chem, 2020; 13(3):1491–7; doi:10.31788/RJC.2020.1335700 CrossRef
Cahyana AH, Liandi AR, Yulizar Y, Romdoni Y, Wendari TP. Green synthesis of CuFe2O4 nanoparticles mediated by Morus alba L. leaf extract: Crystal structure, grain morphology, particle size, magnetic and catalytic properties in Mannich reaction. Ceramics Int, 2021; 47(15):21373–80; doi:10.1016/j.ceramint.2021.04.146 CrossRef
Hapsari M, Cahyana AH, Oktavia SH, Liandi AR. Synthesis of spirooxindole-pyrrolizidine compounds using Fe3O4-GO catalyst and their bioactivity assays. Rasayan J Chem, 2020; 13(4):2317–24; doi:10.31788/RJC.2020.1345583 CrossRef
Hassanpour A, Khanmiri RH, Abolhasani J. ZnO nanoparticles as an efficient, heterogeneous, reusable, and ecofriendly catalyst for one-pot, three-component synthesis of 3,4-dihydropyrimidin-2(1 H)-(thio)one derivatives in water. Synt Comm, 2015; 45(6):727–33; doi:10.1080/00397911.2014.987350 CrossRef
Hirose M, Sugisaki S, Suga K, Umakoshi H. Detection of L-proline-catalyzed Michael addition reaction in model biomembrane. J Chem, 2019; 2019:1–8; doi:10.1155/2019/4926435 CrossRef
Liu XH, Fan JC, Liu Y, Shang ZC. L-Proline as an efficient and reusable promoter for the synthesis of coumarins in ionic liquid. J Zhejiang Univ: Sci B, 2008; 9(12):990–5; doi:10.1631/jzus.B0820079 CrossRef
Loh TP, Feng LC, Yang HY, Yang JY. L-proline in an ionic liquid as an efficient and reusable catalyst for direct asymmetric aldol reactions. Tetrahedron Lett, 2002; 43(48):8741–3; doi:10.1016/S0040-4039(02)02104-4 CrossRef
Matos LHS, Masson FT, Simeoni LA, Homem-de-Mello M. Biological activity of dihydropyrimidinone (DHPM) derivatives: a systematic review. Eur J Med Chem, 2018; 143:1779–89; doi:10.1016/j.ejmech.2017.10.073 CrossRef
Mohamed MS, Awad SM, Ahmed NM. Synthesis and antimicrobial activities of New Indolyl -pyrimidine derivatives. J Appl Pharm Sci, 2011; 1(5):76–80.
Mostafa AS, Selim KB. Synthesis and anticancer activity of new dihydropyrimidinone derivatives. Eur J Med Chem, 2018; 156:304–15; doi:10.1016/j.ejmech.2018.07.004 CrossRef
Poddar R, Jain A, Kidwai M. Bis[(L)prolinate-N,O]Zn: a water-soluble and recycle catalyst for various organic transformations. J Adv Res, 2017; 8(3):245–70; doi:10.1016/j.jare.2016.12.005 CrossRef
Qu H, Li X, Mo F, Lin X. Efficient synthesis of dihydropyrimidinones via a three-component Biginelli-type reaction of urea, alkylaldehyde and arylaldehyde. Beilstein J Org Chem, 2013; 9:2846–51; doi:10.3762/bjoc.9.320 CrossRef
Severina H, Skupa O, Khairulin A, Voloshchuk N, Georgiyants V. Synthesis and anticonvulsant activity of 6-methyl-2-thioxo-2, 3-dihydropyrimidin-4(1H)-one acetamides. J Appl Pharm Sci, 2019; 9(2):12–9; doi:10.7324/JAPS.2019.90202 CrossRef
Sharma V, Chitranshi N, Agarwal AK. Significance and biological importance of pyrimidine in the microbial world. Int J Med Chem, 2014; 2014:1–31; doi:10.1155/2014/202784 CrossRef
Sheldon R. Catalytic reactions in ionic liquids. Chem Comm, 2001; 1(23):2399–407; doi:10.1039/b107270f CrossRef
Tao G-H, He L, Liu W-S, Xu L, Xiong W, Wang T, Kou Y. Preparation, characterization and application of amino acid-based green ionic liquids. Green Chem, 2006; 8(7):639–46. doi:10.1039/b600813e CrossRef
Tyli?ska B, Wiatrak B, Czy?nikowska ?, Cie?la-Niechwiadowicz A, G?barowska E, Janicka-K?os, A. Novel pyrimidine derivatives as potential anticancer agents: Synthesis, biological evaluation and molecular docking study. Int J Mol Sci, 2021; 22(8); doi:10.3390/ijms22083825 CrossRef
Yogam F, Vetha Potheher I, Vimalan M, Jeyasekaran R, Rajesh Kumar T, Sagayaraj P. Growth and physicochemical properties of l-phenylalaninium maleate: a novel nonlinear optical crystal. Spectrochim Acta – Part A: Mol and Biomol Spect, 2012; 95:369–73; doi:10.1016/j.saa.2012.03.088 CrossRef
Zhang L, Xue X, Yan J, Yan LY, Jin XH, Zhu XH, He ZZ, Liu J, Li R, Qiao J. L-proline: A highly effective cryoprotectant for mouse oocyte vitrification. Sci Rep, 2016; 6:1–8; doi:10.1038/srep26326 CrossRef
Zhao D, Wu M, Kou Y, Min E. Ionic liquids: applications in catalysis. Catal Today, 2002; 74(1–2):157–89; doi:10.1016/S0920-5861(01)00541-7 CrossRef