INTRODUCTION
Diabetes mellitus (DM) is a group of metabolic disorders characterized by hyperglycemia due to insulin resistance or deficiency of insulin secretion or both (Punthakee et al., 2018). Globally, the prevalence of DM is estimated to be 9.3% among adults, and it is a leading cause of death (4.2 million deaths) (Saeedi et al., 2019). This disease may lead to complications like retinopathy (Ahmed et al., 2010), nephropathy, and neuropathy. A prolonged hyperglycemic condition is a complex, progressive disease leading to enhanced oxidative stress.
It has been suggested that the adverse effects of hyperglycemia can be attributed to several biochemical pathways, including activation of the protein kinase C pathway, an increase in glucose flux through the polyol pathway, the induction of high-advanced glycation end product production, the upregulated activity of the hexosamine pathway, and the generation of reactive oxygen species, leading to enhanced oxidative stress in a variety of tissues (Kang and Yang, 2020). The high oxidative stress from the lack of appropriate compensation for the endogenous antioxidant defense system leads to the activation of stress-sensitive intracellular signaling pathways. Consequently, the formation of gene products causes cellular damage contributing to the late complications of diabetes (Son, 2012).
Oxidative stress is involved in the development of morphological alterations in DM; thus, the use of plant-based remedies for the prevention and treatment of diabetic complications is believed to improve or prevent cellular damage from oxidative stress in many organs (Parul et al., 2016). It has already been documented that plant medicines, as a rich source of natural antioxidants, have a positive effect on metabolic disorders like DM (Kataya et al., 2011). In the present study, Piper sarmentosum (PS) was used as an herb which has a protective property in DM and its related complications.
PS, known in Thai as Cha-plu, is widely used in Asian countries as a food and traditional herbal plant (Chanwitheesuk et al., 2005). Methanolic extracts show a high level of antioxidant activity, and the water extract is rich in flavonoids with several pharmacological properties such as antioxidant, antiosteoporosis, anti-inflammatory, and antibacterial activity and has also been reported to reduce blood glucose levels and prevent degenerative and necrotic changes in the cells (β-cell) of the islets of Langerhans, progression of diabetic nephropathy, and degenerative changes in the cardiovascular tissue in streptozotocin-(STZ) induced diabetic male Wistar rats (Hussan et al., 2013; Thent et al., 2012).
However, the antihyperglycemic effect and complications of the ethanolic extracts of this plant in STZ-diabetic rats are still unclear. This study was therefore designed to investigate body weight (BW), fasting blood glucose (FBG) levels, and blood chemistry in STZ-induced diabetic rats treated with ethanolic extracts from PS leaves.
MATERIALS AND METHODS
Preparation of extract
Leaves of PS were obtained from Lampang, Thailand. The plant leaves were dried and ground to a fine powder. The powder was extracted using 70% ethanol (v/v) with regular shaking overnight, and it was then separated using Whatman No. 1 filter paper. The filtrates were evaporated using an evaporator and lyophilized into powder. The Piper sarmentosum extract (PSE) was then stored at −20°C until use.
Animals
Wistar albino rats (BW 120–150 g) were obtained from the National Laboratory Animal Centre, Mahidol University, Salaya, Thailand. The rats were provided with free access to water and normal standard diet (Perfect Companion Co. Ltd., Thailand) throughout the experimental period. They were housed under a controlled temperature (25°C ± 2°C) and humidity (60% ± 5%), with a 12 light/12 dark cycle. The experiments were conducted following the approval of the Animal Ethics Committee, University of Phayao (protocol number 5701040017).
Induction of diabetes
The rats were randomly divided into six groups (n = 12 per group). Each group was assigned one of the following treatments:
Group I, healthy control rats received sodium citrate buffer alone.
Group II, healthy rats treated with 50 mg PSE/kg BW.
Group III, healthy rats treated with 200 mg PSE/kg BW.
Group IV, diabetic control rats.
Group V, diabetic rats treated with 50 mg PSE/kg BW.
Group VI, diabetic rats treated with 200 mg PSE/kg BW.
Diabetes was induced in rats by a single dose of 75 mg/kg BW of STZ (Sigma Aldrich, USA) given intraperitoneally in 100 mM sodium citrate buffer (pH 4.5). The blood glucose level after 7 days of treatment in STZ-diabetic rats was monitored. Rats with an FBG level of more than 250 mg/dl were included in the study as having diabetes.
Blood glucose level and in vivo biochemical assays
The extracts were orally administered at doses of 50 and 200 mg/kg BW for a total period of 60 days, following 7 days of diabetes induction. BW was determined before treatment and every 15 days. Fasting blood samples were collected from the tail vein before treatment every 15 days into sodium fluoride (NaF) to determine blood glucose and into ethylenediaminetetraacetic acid to determine glutathione (GSH), malondialdehyde (MDA), cholesterol (CHO), triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), and creatinine levels using clinical test kits (Randox Laboratories Limited, Antrim, UK). At the end of the experimental period (60th day), the animals were sacrificed by decapitation between 7 and 10 AM.
Statistical analysis
The data were expressed as the mean ± standard error of the mean (SEM). The statistical analysis was carried out using a one-way analysis of variance followed by Tukey’s test as the posttest.
RESULTS
Effect of PSE on BW
All rats were closely monitored, and all remained alive throughout the study period. No significant differences in BW were observed in any of the groups prior to the STZ induction. During the 60-day experiment, STZ-diabetic rats exhibited significant (p < 0.05) weight loss compared with the normal rats. However, the BW of the normal control and normal PSE-treated groups given doses of 50 and 200 mg/kg was significantly higher (p < 0.05) than that of the diabetic control group and diabetic PSE-treated rats, whereas these levels were not significantly different between the diabetic control and diabetic PSE-treated groups (Fig. 1).
FBG level
The mean FBG level was significantly elevated in the STZ-induced diabetic groups compared with the control group (p < 0.05). At the end of the 60-day treatment, FBG in normal rats and normal PSE-treated rats was not different. The FBG level of untreated diabetic rats increased steadily from 257.50 ± 26.29 mg/dl before treatment to 380.92 ± 58.03 mg/dl at the end of the experiment. The FBG level reduced significantly (p < 0.05) in both PSE-treated groups, from 256.10 ± 28.07 to 248.92 ± 35.63 mg/dl at the dose of 50 mg/kg and from 240.83 ± 20.85 to 229.15 ± 28.36 mg/dl at the dose of 200 mg/kg as shown in Table 1. It can be concluded that long-term PSE intake reduces blood sugar in STZ-diabetic rats.
Effect of PSE on TGs and CHO
Administration of STZ 7 days prior to PSE treatment produced a significant increase in serum TG level in rats as compared to normal controls. However, serum TG levels were significantly reduced in the normal group treated with 50 mg/kg PSE (p < 0.05) compared to control rats after 60 days of PSE treatment. Administration of PSE at a dose of 200 mg/kg in diabetic rats significantly (p < 0.05) reduced serum TG level, which could be observed after 30 days of treatment in comparison with the diabetic control (Table 2). However, the serum TG level decreased significantly (p < 0.05) after 60 days in the group treated with 50 mg/kg PSE. The result demonstrates that long-term use of low-dose PSE can reduce the level of TGs.
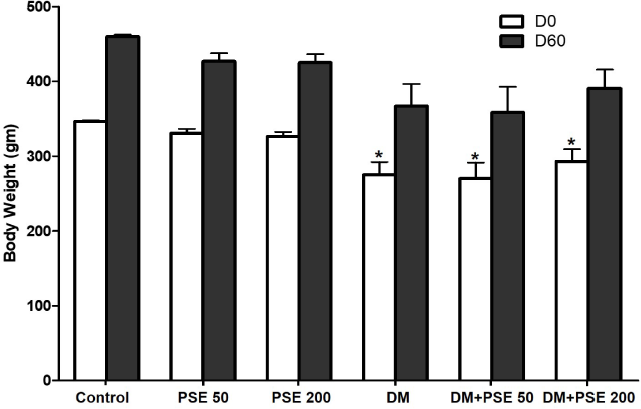 | Figure 1. Effect of PSE on BW (g) of STZ-diabetic rats. Values represent mean ± SEM (n = 12). *p < 0.05, as compared to the control group. [Click here to view] |
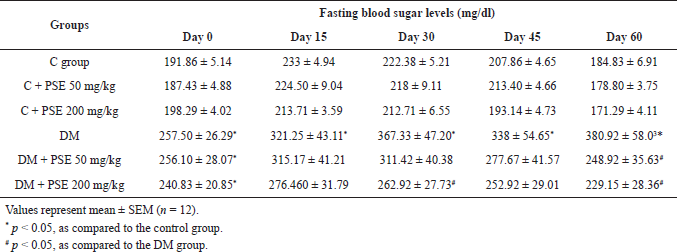 | Table 1. Effect of PSE on fasting blood sugar levels in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
The CHO level in the diabetic group was 84.67 ± 6.41 mg/dl, significantly higher than that of normal control rats (p < 0.05), as shown in Table 3. However, this level was not significantly different between the PSE-treated groups and the normal control group. When diabetic rats were treated with PSE at a dose of 200 mg/kg for 15 days, CHO levels were reduced (p < 0.05) compared to diabetic control rats. These changes were significantly attenuated (p < 0.01) after 60 days of PSE treatment, at which point the rats had CHO levels close to those of normal control rats. Furthermore, the diabetic group treated with 50 mg/kg PSE displayed a decreased level of CHO, but the difference was not significant.
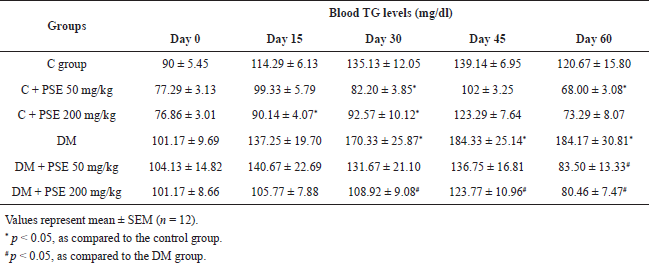 | Table 2. Effect of PSE on TGs in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
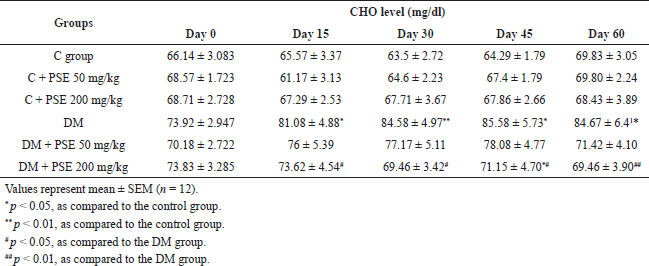 | Table 3. Effect of PSE on CHO in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
Effect of PSE on antioxidant level
The results showed that STZ-diabetic rats had a marked reduction (p < 0.01) in GSH levels as compared to normal control rats (p < 0.05), whereas the GSH levels in the group treated with PSE (50 and 200 mg/kg) were significantly elevated (p < 0.05) when compared with the untreated diabetic rats (Fig. 2). Moreover, GSH levels were significantly increased in the control group treated with 200 mg/kg PSE (p < 0.05) compared to control rats.
The levels of plasma MDA in the diabetic group were significantly elevated (p < 0.005), from 3.11 ± 0.27 to 9.51 ± 0.71 μM, in comparison to those in normal rats (Table 4). The MDA level in the PSE-treated groups was similar to that in the normal control group. In diabetic rats treated with PSE at doses of 50 and 200 mg/kg for 45 days, MDA levels were reduced compared to diabetic control rats (p < 0.05) and remained significantly lower (p < 0.001) after 60 days of PSE treatment.
Liver function test
The levels of AST, ALT, and ALP were significantly elevated (p < 0.05, 0.002) in the STZ-induced diabetic group compared to the normal control group, as shown in Table 5A–C. Comparison between the diabetic group and the treatment group showed a significant decrease of AST level observed after 45 days of PSE 50 mg/kg treatment and after 30 days of PSE 200 mg/kg treatment (Table 5A). Although the levels of AST at day 60 were slightly reduced compared with the untreated diabetic rats on the 60th day, there was no significant difference. Hence, the AST level was not improved after PSE treatment. Unfortunately, AST levels were significantly reduced in the control group treated with 200 mg/kg PSE (p < 0.05) compared to control rats after 45 days of treatment.
Administration of PSE at doses of 50 and 200 mg/kg in diabetic rats caused a significant (p < 0.05) lowering of ALT level after 15 days in comparison with diabetic control rats (Table 5B). These changes were significantly attenuated (p < 0.05, 0.001) after 60 days of PSE treatment. The result clearly demonstrates that the use of PSE can reduce the level of ALT. However, these levels were not significantly different between the PSE-treated groups and the normal control group.
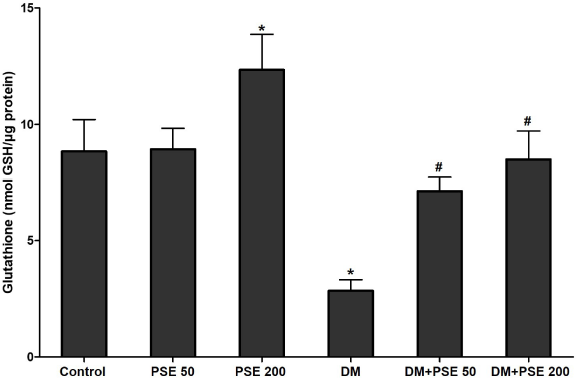 | Figure 2. Effect of PSE on GSH in normal rats, PSE-treated diabetic rats, STZ-diabetic rats, and PSE-treated diabetic rats. Values represent mean ± SEM (n = 12). *p < 0.05, as compared to the control group; #p < 0.05, as compared to the DM group. [Click here to view] |
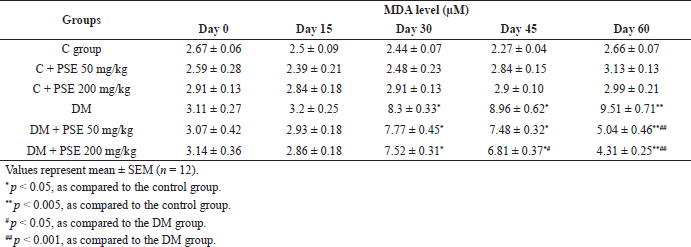 | Table 4. Effect of PSE on lipid peroxidation in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
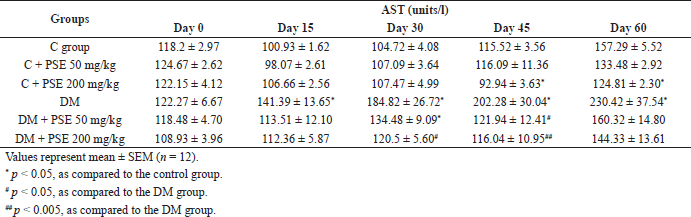 | Table 5A. Effect of PSE on AST in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
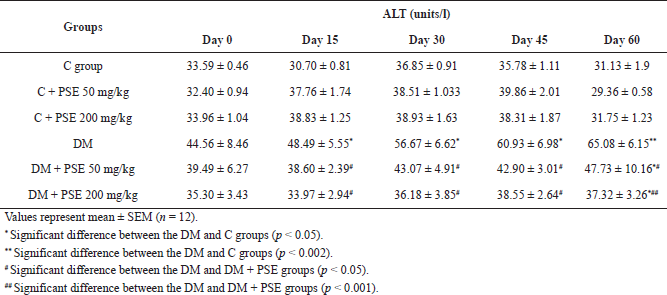 | Table 5B. Effect of PSE on ALT in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
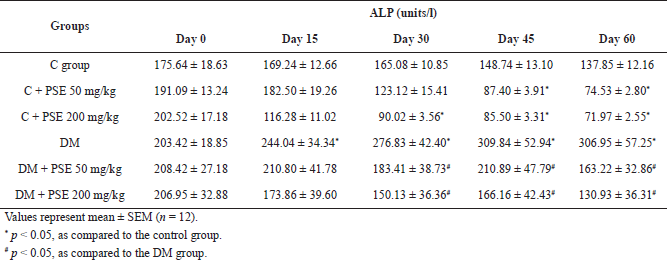 | Table 5C. Effect of PSE on ALP in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
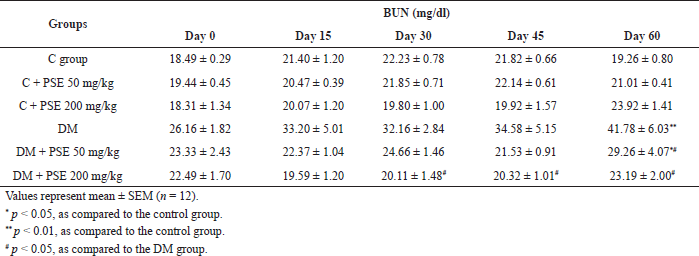 | Table 6A. Effect of PSE on BUN level in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
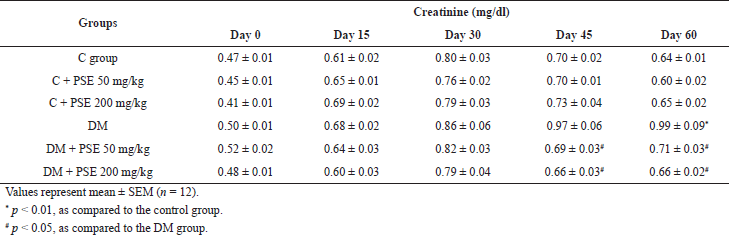 | Table 6B. Effect of PSE on creatinine level in normal rats, PSE-treated rats, STZ-diabetic rats, and PSE-treated diabetic rats. [Click here to view] |
Administration of PSE at 50 and 200 mg/kg caused a significant reduction (p < 0.05) in ALP levels (Table 5C). These results could be observed after 30 days of treatment, and the levels reduced consistently until the end of the experiment. It is clearly shown that PSE treatment can reduce the level of ALP. However, ALP levels were also significantly reduced in the control PSE-treated group (p < 0.05) compared to control rats after 45 days of treatment with 50 mg/kg PSE and after 30 days with 200 mg/kg PSE.
Renal function test
The levels of BUN and creatinine were markedly increased (p < 0.05, 0.01) in the untreated diabetic rats compared to the normal control group (Table 6A and B). The BUN level in the PSE-treated groups was similar to that in the normal control group. Administration of PSE at a dose of 200 mg/kg in diabetic rats caused a significant (p < 0.05) lowering of BUN level in comparison with the diabetic control (Table 6A). These results could be detected after 30 days of treatment. However, the serum BUN level decreased significantly (p < 0.05) after 60 days in the group treated with 50 mg/kg PSE. The result demonstrates that long-term use of low-dose PSE can reduce the level of BUN.
At the end of 60 days of treatment, the creatinine levels in normal rats and normal PSE-treated rats were not different. However, the PSE treatment at 50 and 200 mg/kg caused a significant decrease (p < 0.05) in creatinine levels (Table 6B). These findings could be observed after 45 days of treatment, and the creatinine levels reduced consistently until the end of the experiment. It is clearly shown that PSE treatment can decrease the creatinine level.
DISCUSSION
Diabetes is a diagnostic term for a metabolic disease characterized by a deficiency in the production of insulin and its action, resulting in high blood glucose levels or hyperglycemia, which can lead to many serious life-threatening complications. This study aimed to investigate the effect of PSE in STZ-induced diabetes in rats. STZ is a diabetogenic agent that induces irreversible β-cell damage, causing DNA alkylation and subsequent cell necrosis by entering the pancreatic islet via glucose transporter-2. Hence, STZ can contribute to the failure of β-cells to produce and adequately secrete sufficient insulin (Wu and Yan, 2015). The β-cell destruction and insulin secretory disorder in the diabetic state cause physiometabolic abnormalities such as BW loss while increasing blood glucose, blood TG, and CHO levels. The decrease in BW in diabetic rats might be attributed to excessive breakdown of tissue proteins (Qian et al., 2015). We demonstrated that hyperglycemia developed within a week after STZ induction of diabetes, and it remained the same for 60 days throughout the entire period of this study. A similar result was observed for hyperlipidemia, another characteristic of insulin-dependent diabetes: the TG and CHO levels also increased in diabetic rats after a single STZ injection (Islam and Loots, 2009).
In the present study, BW was significantly decreased in STZ-induced diabetic rats compared to the normal group. Although there were no significant differences in BW between the diabetic rats and the PSE-treated diabetic group, the PSE-treated group showed a significant decrease in blood glucose levels when compared with the untreated STZ-induced diabetic rats. Apparently, PSE had no effect in normal rats since BW and FBG in the normal rats treated with PSE did not differ from that in normal controls. A previous study on PSE revealed an effect on STZ-induced diabetic rats from the presence of phytochemical constituents. A high level of these phytochemical constituents like polyphenols, alkaloids (piperine), flavonoids (quercetin), and amides was present in the ethanol extracts (Ee et al., 2009; Hafizah et al., 2010; Thent et al., 2012). Piperine is found in the ethanol extracts of all parts of PS (Hussain et al., 2009). Quercetin in PSE has also been reported to regenerate the damaged pancreatic islets in STZ-induced diabetic rats and stimulate insulin secretion (Rahman et al., 2016; Shi et al., 2019). Therefore, the mechanism underlying the antihyperglycemic effect of this plant is partly due to the chemical compositions of PS. These trends were similar to the findings of Hussan et al. (2013) and Thent et al. (2012). Moreover, the crude extract of PS was also revealed to reduce the blood glucose level in high-fat diet-induced obese rats (Pornthep et al., 2019).
Importantly, benzoic acid was found in PS to be an inhibitor of insulinase and enhance the effect of insulin (Ignarro et al., 2006). These can promote glucose use and decrease lipolysis in adipose tissue and therefore reduce acetyl Coenzyme A transport to the liver and decrease TG and CHO synthesis. It has been mentioned earlier that the extract of PS was shown to attenuate hyperlipidemia in highly cholesterolemic experimental rabbits (Amran et al., 2012, 2010). In this study, we observed a significant increase in the levels of TGs and CHO in diabetic rats, and the 60-day administration of PSE in diabetic rats decreased the TG and CHO levels. It is promising that the PSE exhibited a hypolipidemic effect by inhibiting cellular CHO and TG synthesis, suggesting that PSE may prove beneficial to diabetes with hyperlipidemia. Interestingly, administration of PSE lowered the serum levels of TGs in control rats, suggesting that this extract has potent hypolipidemic effects due to the beneficial effects of piperine in lowering the level of TGs (Peela et al., 2012).
In addition, excess levels of serum BUN and creatinine are indicators of renal function, indicating the occurrence of diabetic nephropathy with renal hyperfiltration induced by STZ. Sixty-day administration of PSE reduced albuminuria and inhibited renal function loss, and improved glomerular hyperfiltration in STZ-diabetic rats. These findings are similar to those of a previous study in which PS ethanolic extract reduced the raised BUN level (Talubmook et al., 2013).
Raised AST, ALT, and ALP levels are used in the prognosis of liver damage and dysfunction. Increased enzyme activity was observed in diabetic rats. Treatment with PSE reduced the activity of these enzymes and thus attenuated the liver damage caused by STZ. These findings are consistent with those of Hussain et al. (2010) who found that PS ethanolic extract lowered the elevated markers of the liver against CCl4-induced toxicity. Unfortunately, the decreased AST and ALP levels in the PSE-treated group might be due to favorable effects in long-term use. However, a previous study demonstrated that oral administration of PS aqueous extract at a very high dose of 2 g/kg, 10 times higher than in this study, does not exhibit subacute toxicity in rats (Mohd Zainudin et al., 2013).
Oxidative stress arises from hyperactivity of the hyperglycemia-induced polyol pathway and acts as a major risk factor for diabetic complications (Yan, 2018). The oxidative stress evaluated in diabetes contributes considerably to excess production of free radicals in association with diabetic complications. It has been found that hyperglycemia in DM and high glucose levels can lead to decreased glucose-6-phosphate dehydrogenase (G6PD) activity and hence decreased levels of nicotinamide adenine dinucleotide phosphate (NADPH) and GSH (Sridevi et al., 2007; Xu et al., 2005). Our study showed that the level of MDA increased while the level of GSH decreased, as revealed by the control STZ-diabetic rats. These changes are commonly seen in DM-related complications. The PSE treatment decreased the MDA level; in contrast, the GSH level was increased by treatment. Administration of PSE caused a decrease in glucose level, leading to an increase in the activity of the cytoplasmic enzyme G6PD; thus, increased NADPH levels raised GSH levels. Similar findings were observed in previous studies, which showed a reduced MDA level in PSE-treated diabetic rats (Hafizah et al., 2010; Rahman et al., 2012). These findings suggest that PSE may be able to ameliorate the effects of chronic oxidative stress. Since naringenin is obtained from PSE, it has been shown to possess a potent antioxidant effect (Subramaniam et al., 2003).
CONCLUSION
Various studies have highlighted the herbal medicines used for preventing oxidative stress causing disorders like DM. The present study proves the efficacy of PSE in significantly reducing STZ-induced blood glucose, TGs, CHO, oxidative stress, and liver and renal function biomarkers. Administration of PSE also improved antioxidant capacity, affecting the induction of GSH in STZ-induced diabetic rats. Consumption of PSE may prove to have the potential for effective management of hyperglycemia that is associated with the development of diabetic complications.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
ACKNOWLEDGMENTS
This work was supported by the University of Phayao, Thailand.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
FUNDING
This research was funded by a University of Phayao research grant and by the Unit of Excellence of Research and Development for Cancer Therapy (Grants nos. UoE62015 and FF64-RIB006).
ETHICAL APPROVAL
The study protocol was approved by the Animal Ethics Committee, University of Phayao, Thailand (protocol number 5701040017).
REFERENCES
Ahmed KA, Muniandy S, Ismail IS. Type 2 diabetes and vascular complications: a pathophysiologic view. Biomed Res, 2010; 21:147–55.
Amran AA, Zakaria Z, Othman F, Das S, Al-Mekhlafi HM, Raj S, Nordin NAMM. Effect of methanolic extract of Piper sarmentosum leaves on neointimal foam cell infiltration in rabbits fed with high cholesterol diet. EXCLI J, 2012; 11:274–83; doi:10.17877/DE290R-5763
Amran AA, Zakaria Z, Othman F, Das S, Raj S, Nordin NA. Aqeous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids Health Dis, 2010; 9:1–6; doi:10.1186/1476-511X-9-44 CrossRef
Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem, 2005; 92:491–7; doi:10.1016/j.foodchem.2004.07.035 CrossRef
Ee GCL, Lim CM, Lim CK, Rahmani M, Shaari K, Bong CFJ. Alkaloids from Piper sarmentosum and Piper nigrum. Nat Prod Res, 2009; 23:1416–23; doi:10.1080/14786410902757998 CrossRef
Hafizah AH, Zaiton Z, Zulkhairi A, Mohd Ilham A, Nor Anita MMN, Zaleha AM. Piper sarmentosum as an antioxidant on oxidative stress in human umbilical vein endothelial cells induced by hydrogen peroxide. J Zhejiang Univ Sci B, 2010; 11:357–65; doi:10.1631/jzus.B0900397 CrossRef
Hussain K, Ismail Z, Sadikun A. Evaluation of ethanol extracts of leaves and fruit of piper Sarmentosum for in vivo hepatoprotective activity. Lat Am J Pharm, 2010; 29:1215–20.
Hussain K, Ismail Z, Sadikun A, Ibrahim P. Antioxidant, anti-TB activities, phenolic and amide contents of standardised extracts of Piper sarmentosum Roxb. Nat Prod Res, 2009; 23:238–49; doi:10.1080/14786410801987597 CrossRef
Hussan F, Bt Mat Zin NN, Zullkefli MR Bin, Choon YS, Bt Abdullah NA, Lin TS. Piper sarmentosum water extract attenuates diabetic complications in streptozotocin induced sprague-dawley rats. Sains Malays, 2013; 42:1605–12.
Ignarro LJ, Byrns RE, Sumi D, de Nigris F, Napoli C. Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric Oxide Biol Chem, 2006; 15:93–102; doi:10.1016/j.niox.2006.03.001 CrossRef
Islam MS, Loots DT. Experimental rodent models of type 2 diabetes: a review. Methods Find Exp Clin Pharmacol, 2009; 31:249–61; doi:10.1358/mf.2009.31.4.1362513 CrossRef
Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol, 2020; 37:101799; doi:10.1016/j.redox.2020.101799 CrossRef
Kataya HH, Hamza AA, Ramadan GA, Khasawneh MA. Effect of licorice extract on the complications of diabetes nephropathy in rats. Drug Chem Toxicol, 2011; 34:101–8; doi:10.3109/01480545.2010.510524 CrossRef
Mohd Zainudin M, Zakaria Z, Megat Mohd Nordin NA, Othman F. Does oral ingestion of Piper sarmentosum cause toxicity in experimental animals? Evid Based Complement Altern Med, 2013; 2013; doi:10.1155/2013/705950 CrossRef
Parul R, Alam MJ, Rana MS. Neuroprotective effect of Cinamomum zeylanicum in streptozotocin induced diabetes in mice. Indian J Pharm Biol Res, 2016; 4:63–73; doi:10.30750/ijpbr.4.1.8 CrossRef
Peela JR, Elshaari F, Elfrady M, Shakila S, Awamy HME, Singh R, Belkheir A, Zaidi SH, Barassi IF, Ramanujam R. Effect of piperine on lipid profile of non-transgenic mice. Int Rev Biophys Chem, 2012; 3:15–9.
Pornthep T, Arunporn I, Weerachai P, Chaisanucha S. Inhibitory effect on alpha-glucosidase activity of Benjakul, Soros Benjakul and their plant components. Thammasat Med J, 2019; 19:645–53.
Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes, 2018; 42:S10–5; doi:10.1016/j.jcjd.2017.10.003 CrossRef
Qian K, Zhong S, Xie K, Yu D, Yang R, Gong DW. Hepatic ALT isoenzymes are elevated in gluconeogenic conditions including diabetes and suppressed by insulin at the protein level. Diabetes Metab Res Rev, 2015; 31:562–71; doi:10.1002/dmrr.2655 CrossRef
Rahman NA, Mastura K, Mohd K. Piper sarmentosum influences the oxidative stress involved in experimental diabetic rats. Internet J Herb Plant Med, 2012; 1:1–8; doi:10.5580/1203 CrossRef
Rahman SFSA, Sijam K, Omar D, Wahab MZA. Identification of phenolic compounds and evaluation of antibacterial properties of Piper sarmentosum Roxb. against rice pathogenic bacteria. Malays J Microbiol, 2016; 12:475–84.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract, 2019; 157:107843. CrossRef
Shi GJ, Li Y, Cao QH, Wu HX, Tang XY, Gao XH, Yu JQ, Chen Z, Yang Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: a systematic review of the literature. Biomed Pharmacother, 2019; 109:1085–99; doi:10.1016/j.biopha.2018.10.130 CrossRef
Son SM. Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes Metab J, 2012; doi:10.4093/dmj.2012.36.3.190 CrossRef
Sridevi S, Chandramohan G, Pugalendi KV. Protective effect of Solanum surattense leaf-extract on blood glucose, oxidative stress and hepatic marker enzymes in STZ-diabetic rats. Asian J Biochem, 2007; 2:247–55; doi:10.3923/ajb.2007.247.255 CrossRef
Subramaniam V, Adenan MI, Ahmad AR, Sahdan R. Natural antioxidants: Piper sarmentosum (Kadok) and Morinda elliptica (Mengkudu). Malays J Nutr, 2003; 9:41–51.
Talubmook C, Nopparat B, Buddhakala N. Bioactivities of extract from Tinospora crispa stems, Annona squamosa leaves, Musa sapientum flowes, and Piper sarmentosum leaves in diabetic rats. Int J Adv Res Technol, 2013; 2:1–6.
Thent ZC, Seong Lin T, Das S, Zakaria Z. Effect of piper sarmentosum extract on the cardiovascular system of diabetic sprague-dawley rats: electron microscopic study. Evid Based Complement Altern Med, 2012; 2012; doi:10.1155/2012/628750 CrossRef
Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes Targets Ther, 2015; doi:10.2147/DMSO.S82272 CrossRef
Xu Y, Osborne BW, Stanton RC. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Ren Physiol, 2005; 289:F1040–7; doi:10.1152/ajprenal.00076.2005 CrossRef
Yan L. Redox imbalance stress in diabetes mellitus: role of the polyol pathway. Anim Model Exp Med, 2018; 1:7–13; doi:10.1002/ame2.12001 CrossRef