INTRODUCTION
The silver metal was used as an antiseptic and a broad spectrum of biocidal activity (Ahmed et al., 2016). Furthermore, it is produced in nanosize to increase antibacterial activity and stability when used as an active ingredient in cosmetic preparation (Sakharwade, 2016). Nanosilver penetrates the bacterial cell wall and changes the structure and permeability of the cell membrane, thereby killing the bacteria (Prabhu and Poulose, 2012). The minimum effective concentration of nanosilver in cosmetic preparations is equivalent to 10 mg/kg w/w (Pulit-Prociak et al., 2019). Meanwhile, nanosilver with a size of 20–200 nm only penetrates the stratum corneum as far as 2–3 μm (stratum corneum is the outermost layer of the skin with a thickness of 15–20 μm), thereby preventing it from penetrating the systemic tract (Campbell et al., 2012). However, the Science Committee on Consumer Safety limits its concentration in the body not to exceed 10,000 ppm (Pulit-Prociak et al., 2019). Nanosilver in cosmetic preparations was designed to use topical antibiotics such as Clindamycin and Erythromycin to help cure skin problems, namely, acne (Fox et al., 2016). Therefore, long-term use of topical antibiotics is not recommended because it causes bacterial resistance (Coates et al., 2002).
The green synthesis method for the formation of nanosilver has advantages over physical and chemical because it is simple, cost-effective, environmentally friendly, and easier to upgrade on a larger scale (Dhupwer et al., 2012). The biosynthesis process of nanosilver requires a reducing and capping agent. Reductants reduce the particle size as well as stabilizing the size of nanosilver (Ahmed et al., 2016). Hydroxyl and carbonyl groups in plant extract are known as reducing and capping agents (Hembram et al., 2018). Sweet orange functions as a bioreducer (Citrus sinensis L. Osbeck), as shown by the previous research in reducing silver nitrate (AgNO3) by producing a particle size of 10–35 nm (Ahmed et al., 2016) and smaller size of nanosilver compared with the same genus as lemon (Citrus limon) and sweet lime (Citrus limetta). Furthermore, the biosynthesis process with C. limon produces nanosilver with a size of 17.3–61.2 nm, while C. limetta produces nanosilver with a size of 107 nm (Dutta et al., 2020; Nisha et al., 2014). Orange peel contains a flavonoid of 5.51 mg/g and citric acid of 53.67 mg/g (Canan et al., 2016; Liew et al., 2018), which have hydroxyl and carbonyl groups that are capable of forming nanosized particles of silver (Malassis et al., 2016). The results showed that the nanosilver using bioreducer of sweet orange peel infusion at 60°C has an inhibitory diameter against Escherichia coli of 16 mm, Pseudomonas aeruginosa of 13.4 mm, and Staphylococcus aureus 9.2 mm. Additionally, Logeswari et al. (2012) reported that 100 μl of nanosilver has a diameter of inhibition against S. aureus of 27 mm, P. aeruginosa of 18 mm, E. coli of 17 mm, and Klebsiella pneumoniae of 16 mm. Studies also showed that nanosilver has antibacterial activity against S. aureus with a diameter of inhibition of 28 mm, E. coli of 30 mm, Bacillus cereus 25 mm, and Salmonella typhimurium of 30 mm.
A peel-off mask is advantageous because it is easy to apply, leaves no residue when removed, and gives a clean sensation. Furthermore, its preparation optimizes the nanosilver as an antimicrobial agent when the polymer forms an occlusive layer on the stratum corneum skin surface (Velasco et al., 2014). Humectants in peel-off mask preparations have two functions, which include hydrating the skin by pulling water from the inner layer to the outermost layer and preventing water evaporation for a more stable viscosity (Baki and Alexander, 2015). They also complement the function of polyvinyl alcohol (PVA) to form a soft and sturdy film in humid conditions (Ogur, 2005). Polyethylene glycol 400 (PEG 400) and glycerin are humectants and function as moisturizers with a concentration of 0.01%–20% (Liu, 2018), while PEG 400 is a material that maintains the viscosity of cosmetics. Furthermore, glycerin prevents skin irritation and maintains skin moisture in the long term (Benson et al., 2019). This study aims to determine the effect of the combined humectants on the physicochemical character, stability, and antibacterial activity. The expected result is to obtain the formula of nanosilver peel-off mask that meets the requirements of quality, stability, and broad-spectrum antibacterial activity with a strong category.
MATERIALS AND METHODS
Materials and instruments
The materials used are sweet orange fruits from Pacitan, Central Java, Indonesia, silver nitrate powder (AgNO3) 99.8% (Merck, Darmstadt, Germany), PVA (Kurray Asia Pacific PTE LTD, Singapore), PEG 400 (PT. DOW Chemical, Indonesia), glycerin (P&G Chemical, Singapore), aquabidest as a solvent, phenoxyethanol (MakingCosmetics, USA), Mueller Hinton agar (MHA) media (Merck, German), Staphylococcus epidermidis ATCC 12228, P. aeruginosa ATCC 27853, S. aureus ATCC 25923, and E. coli ATCC 25922.
Meanwhile, the instruments used are pH meter (PH-009(I)A, China), analytical balance (Mettler Toledo AL204, d = 0.0001 g Colombus, Ohio), analytical balance (Precisa XB620C, d = 0.01 g Moosmattstrasse, Swiss), spectrophotometer UV-Vis (GenesysTM, Thermo Fisher Scientific, USA), centrifugation (Mini Spin Plus, Eppendorf AG, Jerman), viscometer (Viskotester VT-04, Kokubunji, Jepang), incubator (Memmert IN30, Mammert, Jerman), PSA is Particle Size Analyzer (HORIBA, USA), and SEM is Scanning Electron Microscope , ATCC is The American Type Culture Collection.
Biosynthesis process of silver ion using sweet orange infusion as bioreductor
Sweet orange plant harvested from Jetis Lor Village, Pacitan, Central Java, Indonesia, was determined in the Biology Laboratory, Faculty of Education Science, Universitas Muhammadiyah Surakarta, to ensure the correctness of the plant species. Fresh orange peel was washed using aquabidest and cut into smaller pieces, after which 4.0 g of it was mixed with 40 ml of aquabidest, boiled for 2 minutes, and filtered using Whatman paper No. 1 (Kaviya et al., 2011). Furthermore, a silver nitrate (AgNO3) solution of 1.0 mM was produced by dissolving 85 mg of silver nitrate powder into 500 ml aquabidest. The biosynthesis process was conducted by mixing 3.0 ml orange peel infusion with 40 ml of 1.0 mM AgNO3, and the mixed solution was heated at 60°C for 45 minutes which was also set at room temperature for 10 minutes. The success of colloidal nanosilver form is characterized by a color change from colorless to yellowish-brown (Kaviya et al., 2011).
Nanosilver characterization
The nanosilver is characterized using the instruments such as UV-Vis spectrophotometer, PSA, and SEM, which are used for monitoring the reduction from silver ions (Ag+) to nanosilver (Agº) with the maximum wavelength parameter that penetrates its SPR is Surface Plasmon Resonance range. After the biosynthesis process was completed and the water blanked, the samples were scanned at a wavelength range of 300–540 nm (Kaviya et al., 2011; Logeswari et al., 2012). Analysis was performed using PSA and SEM to determine the size and distribution of the nanosilver particles as well as show the morphology of the nanoparticles, respectively. Furthermore, the sample required for SEM analysis is a powder obtained by centrifuging the nanosilver colloidal solution at 10,000 rpm for 15 minutes. The precipitated pellet was heated using an oven at 60°C for 24 hours, and the dried nanosilver was then characterized using SEM (Kaushik and Joshi, 2015).
Nanosilver peel-off mask formulation
The amount of colloidal nanosilver as the active substance in the peel-off mask formula is 9.27 g, and it is obtained based on the minimum concentration of nanosilver as antibacterial activity. Silver nitrate used in the biosynthesis process is 170 ppm, and the silver (Ag) concentration in AgNO3 obtained by comparing the relative atomic mass of Ag with molecule mass of AgNO3 is 107.86 ppm. Also, the minimum effective concentration of nanosilver in cosmetic preparations is 10 mg/kg (Pulit-Prociak et al., 2019). A peel-off mask of 100 g was produced to obtain a 1.0 g minimum effective concentration value of nanosilver. Furthermore, a colloidal nanosilver of 9.27 g is equivalent to 1.0 mg of nanosilver. The effective concentration of nanosilver colloid used for the peel-off mask formula is three times the minimum effective concentration.
The concentration humectant used is a modification of the preparation of peel-off mask as stated by Badnore et al. (2019), where PEG 400 as a humectant was 0.5%. However, this study uses a combination of glycerin and PEG 400 with a total concentration of 0.5%. First, preheat distilled water at 80°C, then dissolve the PVA powder into hot water, stir until it is melted and homogeneous, and name it mixture 1. The comparison of water and PVA used is 4:1. Whereby the PVA solution is poured into the chamber, PEG 400 and glycerin are added. Afterward, aquabidest is added to mixture 1, together with the last nanosilver colloidal, after which the mixture was stirred until homogeneous (Table 1).
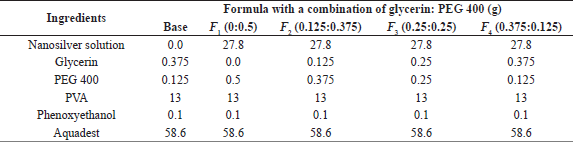 | Table 1. The peel-off mask formula of nanosilver using bioreductor sweet orange peel infusion with humectant combination. [Click here to view] |
Stability test of nanosilver peel-off mask using cycling test method
The cycling test was performed by storing the preparation at the temperature of 4°C for 24 hours and then storing it at 40°C for 24 hours. Before switching to a different temperature, the preparation stands at room temperature until the temperature decreases. This experiment was repeated for 6 cycles or 12 days, and the physicochemical properties of the preparation were compared before and after the experiment, although it was conducted with 3 replication.
Viscosity was measured using a Rion viscometer with spindle number 1, and the cup was filled with peel-off mask preparations while the rotor was placed in the center of the cup. After turning on the tool, wait for 1 minute and record the viscosity value (Puspitasari and Setyowati, 2019). However, the viscosity value that meets the requirement of gel preparations is 500–20,000 cPs.
The pH meter was calibrated using pH 4.01 and 6.86 buffers, and the electrode inserted into the mask preparation was then stirred until it showed a constant pH value. Afterward, the pH value was observed and recorded (Wulandari et al., 2019). Therefore, the pH value that meets the skin-friendly requirement is 4.5–6.5 (Budiman et al., 2017).
A dry time test was conducted by collecting 1.0 g of peel-off mask preparation and applying it to the skin over an area of 7 × 7 cm, and the time required for the mask to form a film was measured using a stopwatch (Armadany and Sirait, 2015). Meanwhile, the dry time for peel-off mask preparations is 15–30 minutes (Cahyani and Putri, 2018).
Antibacterial activity test of nanosilver peel-off mask
The equipment and materials were sterilized using an autoclave at 121°C with a pressure of 1.0 atm for 15 minutes. Also, an agar media was prepared by dissolving 8.5 g of MHA in 250 ml of distilled water (34 g/1,000 ml). Furthermore, the mixture was boiled until completely dissolved; then, it was poured into Erlenmeyer glass which was covered with cotton and then sterilized using autoclave at 121°C for 15 minutes at a pressure of 1.0 atm. MHA was poured into a Petri dish until it solidifies, and bacterial colonies were collected using a needle loop suspended in a sterile physiological NaCl solution and then homogenized. The turbidity of the measured suspension corresponds to the standard McFarland turbidity 0.5, and the bacteria suspensions were inoculated into MHA using the swabs method. Each 50 μl sample was pipetted and dropped into a well and then incubated at 37°C for 24 hours. Also, the diameter of the inhibition zone (clear area) was measured using a caliper. The clear area indicated the sensitivity of bacteria to antibiotics or other antibacterial substances, which is expressed by the width of the diameter of the inhibition zone. Tests were conducted on nanosilver solutions, peel-off mask preparations, AgNO3 solutions, antibiotics, and water, and the bacterias used were S. aureus, S. epidermidis, E. coli, and P. aeruginosa.
DATA ANALYSIS
The data were analyzed using Shapiro–Wilk to determine the distribution of the data, after which it shows a normal distribution, and then a paired sample t-test is used to determine the significant difference of antibacterial activity, pH, viscosity, dispersion, dry time, and antibacterial test of peel-off mask preparations before and after the stability test using the cycling test method. The one-way analysis of variance was used as data for the nanosilver, in which sweet orange peel extract was used as a bioreducer. Furthermore, the statistical analysis results showed a p-value of <0.05, which means that it is significantly different, while if the p-value is >0.05, it means that the results are not significantly different. Therefore, data which data do not meet the requirements of the normality are conducted by nonparametric testing using the Wilcoxon and Kruskal-Wallis method.
RESULTS AND DISCUSSION
Plant determination was used to discover the identity of a plant to prevent sample selection errors, and this was performed using the key of plant determination. Furthermore, this was conducted at the Biology Laboratory of the Faculty of Teaching and Education Science, Universitas Muhammadiyah Surakarta. The result shows that the species of sweet orange plant used is C. sinensis (L.) Osbeck.
Biosynthesis process of nanosilver using sweet orange peel infusion
The active compounds that are responsible for the biosynthesis process are citric acid and flavonoid, as these two compounds contain carbonyl and hydroxyl groups as well as high concentrations in sweet orange peels (Canan et al., 2016; Liew et al., 2018). Since flavonoids and citric acid are polar compounds, water is chosen as a solvent based on the “like dissolves like” principle of extraction (Corradini et al., 2011).
 | Figure 1. One possibility that can explain the mechanism of the reduction of silver ions and nanosilver chelation by the flavonoid Rutin (quercetin-3-O-rutinoside) found in sweet orange peel extract to form stable nanosilver. [Click here to view] |
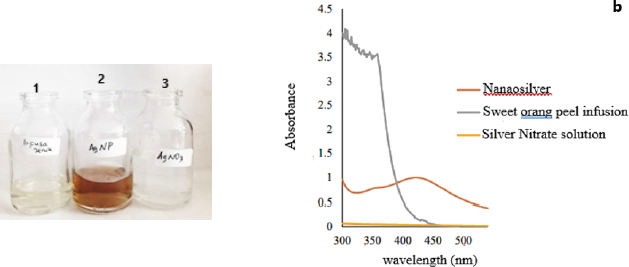 | Figure 2. The results of color change to yellow-orange at seven minutes and then the color of the mixture getting dark (a). The results of scanning the wavelength of silver nitrate solution before the biosynthesis process have no absorbance at a wavelength range of 300–540 nm. Sweet orange peel infusion has many peaks in the wavelength region of 300–380 nm. After the AgNO3 solution reacted with sweet orange peel infusion, a peak appears at a wavelength of 421–423 nm (b). [Click here to view] |
Biosynthesis of silver ion by a reduction reaction, in which 1.0 mM silver nitrate solution reacts with an infusion of sweet orange peel, leads to electron transfer. A reduction reaction is a reaction caused by a substance that accepts an electron, so that charge on the atom is reduced (decrease) (Kotz et al., 2010). The tautomeric transformation of flavonoid compounds from the enol to the keto, which may release reactive hydrogen, can be another mechanism in the formation of nanosilver from Ag+ ions; the mechanism can be described as follows: quercetin + 2 Ag+ ↔ quercetin +2Ag0 + 2H+ and one possibility that can explain the mechanism of the reduction of silver ions and nanosilver chelation by the flavonoid Rutin (quercetin-3-O-rutinoside) found in sweet orange peel extract to form stable nanosilver (Fig. 1). The biosynthesis process was performed at 60°C because the reaction completed faster and the resulting particle size was smaller compared to room temperature, as stated by Kaviya et al. (2011). In this research, the color changes to yellow-orange after seven minutes, and then the color of the mixture becomes darker. The silver nitrate solution and sweet orange peel infusion were colorless (bottle number 1,3), after the reaction process of 45 minutes, the color changes to yellowish-brown (bottle number 2) (Fig. 2). According to Ahmed et al. (2018), the successful formation of nanosilver from the biosynthesis process using sweet orange peel was characterized by a color change to yellowish-brown (2). Therefore, this study showed that, after 45 minutes of reaction, nanosilver colloidal had successfully formed.
Spectrophotometry UV-Vis is a method to see the optical properties of nanosilver (Shnoudeh et al., 2019), which is in the form of maximum absorbance in the SPR area. The mix solution after the biosynthesis process were scanned at a wavelength of Surface Plasmon Resonance range at 300–540 nm, where water as a blanko, and the SPR area appears in the range while water is the solvent used in the process. Meanwhile, the results of scanning the wavelength of AgNO3 solution before the biosynthesis process show no absorbance at a wavelength range of 300–540 nm. This is similar to the research conducted by Wei et al. (2012), where the AgNO3 solution shows no absorption at a wavelength of 300–700 nm. Sweet orange peel infusion has many peaks in the wavelength region of 300–380 nm; however, after its reaction with AgNO3 solution, a peak appears at a wavelength of 421–423 nm with an absorbance of 0.996. This SPR range shows that silver nanoparticles have been formed (Logeswari et al., 2012), and the nanosilver spectra produced are similar to that of Kaviya et al. (2011), namely, particle shape and maximum wavelength. The biosynthesis process of silver ions using sweet orange peel infusion at a temperature of 60°C has characteristic spectra with maximum absorption of more than 1.5 at a wavelength of 424 nm. Furthermore, the difference is in the maximum absorption, in which nanosilver spectra had an absorbance of 0.996, while Kaviya et al. (2011) had more than 1.5. The difference is probably caused by compounds such as citric acid and flavonoids that are responsible for the biosynthetic process and act as reducing and capping agents. However, the variation in the concentration of flavonoids and citric acid in sweet orange peel is influenced by internal factors such as plant varieties, as well as external factors, including environmental conditions, maintenance techniques, and harvest time. The phytochemical composition of sweet orange peel also is influenced by geographical conditions that affect soil type, light intensity, and humidity. The fluctuation of environmental temperature during the day and night has a significant effect on the decrease in flavonoid levels. Additionally, the duration of sun exposure to plants during the growth period correlated with flavonoid levels (Ghasemzadeh et al., 2018).
The other characterization used is the PSA, which is an instrument used to determine the particle size distribution of biosynthetic nanosilver. The result of the analysis is that nanosilver has a Z-average value of 83.17 ± 7.19 nm, which shows the average value of particle distribution at Figure 4 (HORIBA, 2017). Furthermore, the process of biosynthesis showed the infusion of sweet orange peel that produces nanosilver with particle size below 100 nm. Nanosilver biosynthetic in the study by Kaviya et al. (2011) has a particle size of 10 ± 1 nm, and the difference in size is caused by the levels of reducing agents. The study by Prathna et al. (2011) showed that the reduced content of citric acid in the process of biosynthesis affects the particle size of nanosilver. Therefore, this shows that the citric acid compound is responsible for the process biosynthetic through the use of sweet orange peel infusion. The study by Osonga et al. (2015) showed that flavonoids are present as reducing and capping agents in nanosilver biosynthetic. Citric acid and flavonoids have a maximum wavelength of 420 and 418 nm, respectively (Hamidu et al., 2018; Roukas and Kotzekidou, 2020). In this study, the absorption values at the wavelength of 418 and 420 nm are 0.667 and 0.689, respectively. However, the study by Kaviya et al. (2011) stated that the absorption value at the same wavelength is around 0.800, which shows that the lower the levels of citric acid and flavonoids in sweet orange peel, the bigger the particle size. The average polydisperse index obtained from this analysis is 0.522 ± 0.07; based on its interpretation, the value is less than 0.7, and hence the particles are categorized as monodisperse (homogeneous dispersed) (Sreeram et al., 2008). Therefore, the results of characterization using PSA showed that the biosynthesis process using sweet orange peel (C. sinensis) succeeded in forming a nanosilver.
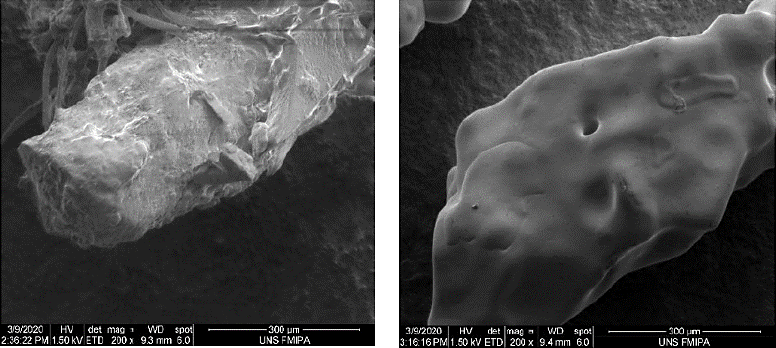 | Figure 3. The results of SEM analysis. SEM represents nanosilver morphology with a magnification of 200 times. The biosynthetic process in this study produces a rod-shaped nanosilver. [Click here to view] |
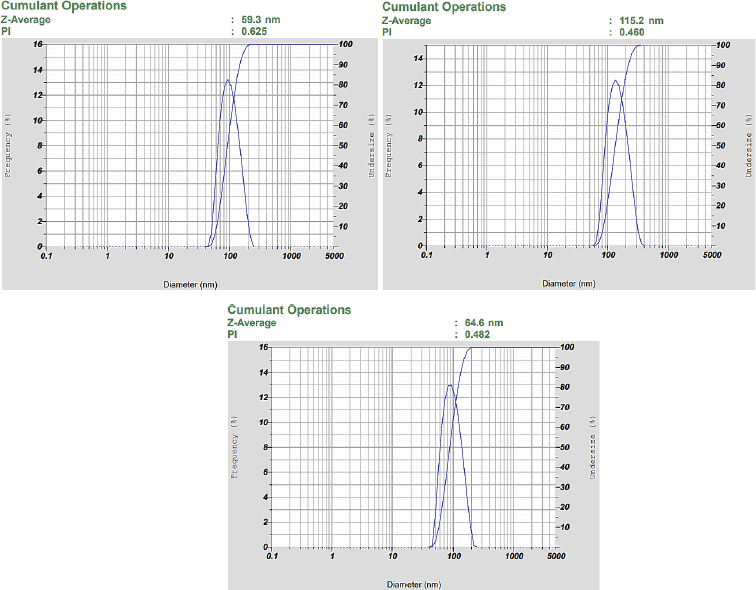 | Figure 4. The result of the analysis shows that nanosilver has aZ-average value of 79.7 ± 25.19 nm and the PDI is Polidispers Index value less than 0.7, so that particles are categorized as monodisperse. [Click here to view] |
Morphological characteristics of nanosilver biosynthetic using SEM instrument
In this study, the morphology of silver nitrate powder was used as a control, as SEM represents particle morphology with a magnification of 200 times. vw shows that the biosynthetic process in this study produces a rod-shaped nanosilver, and the significant difference between silver nitrate and nanosilver biosynthetic is on its surface. The particle size distribution on the silver surface is very smooth, and there are no large particles coating it. However, after the biosynthesis process, there is a biological material that coated the surface of the nanosilver formed. This material is derived from the sweet orange peel infusion that functions as a capping agent (Ibrahim, 2015).
Stability test of nanosilver peel-off mask
The total weight of glycerin and PEG 400 is 0.5 g, and both ingredients function as a humectant to maintain the viscosity of the preparation by preventing water evaporation and absorbing water from the environment. These two ingredients are combined because they have different characteristics, where glycerin is more viscous and absorbs more water than PEG 400. PVA is Polyvinyl Alcohol acts as a film-forming agent in this research, and polymers such as HPMC is Hydroxyl Propyle Methyle Cellulose and gelatin are used as film formers, but PVA has the advantage of forming good adhesive properties to provide a clean sensation. It also forms a film that is environmentally friendly and nontoxic (Kathe and Kathpalia, 2017) and interacts with humectants. Furthermore, it is influenced by moisture factors. At the low humidity, PVA forms a rigid and brittle film, but when humidity is improved, the physical properties of the film formed are soft and flexible (Ogur, 2005). The use of glycerin and PEG 400 are appropriate because they have hygroscopic properties, increasing the moisture in the preparation, and producing soft and flexible films. In addition, PVA contains adhesive after drying, which removes dirt and dead skin cells, and when mixed with distilled water with a ratio of 4:1 and then heated at a temperature of 80°C, it breaks the inter and intramolecular hydrogen bond. The bond will break because of heat energy (Briscoe and Luckham, 2000).
Preliminary studies on the physicochemical properties of the nanosilver peel-off mask preparation were conducted for 28 days of storage at room temperature. The results showed that the difference between the concentration of glycerin-PEG 400 in the nanosilver peel-off mask formula has a significant effect on the physical properties of the preparation, such as the viscosity, dispersibility, and dry time. Peel-off mask with a composition of glycerin-PEG 400 (0.125:0.375 g and 0.375:0.125 g) has viscosity, spreadability, and pH that were not significantly different during 28 days storage at room temperature. The formula with a ratio glycerin-PEG 400 of 0.125:0.375 was selected to test for stability and antibacterial activity against Gram-positive and Gram-negative bacterias.
Viscosity describes the resistance to the flow of the preparation. A higher viscosity leads to the high flow resistance of a preparation (Gunawan et al., 2012). Furthermore, the condition of viscosity value of a peel-off mask is 500–20,000 cPs, and the results show that all peel-off mask formulations meet the requirement of 500–20,000 cPs. The longer storage of the preparation decreases the viscosity value, but the value is not a significant difference. However, when the gel when is stored at high temperatures, the polymer chains formed will release the spherical roll (disentangle), hence decreasing the viscosity gel (watery). When the gel is stored at cold temperatures, the polymer chains will shorten and join each other. For a long time, the gel shrinks (entangle), thereby resulting in a change in viscosity.
Statistical test results show that there is no significant difference in the average pH value between the formulas before and after the cycling test. In topical preparations, the pH should not be too acidic because it causes skin irritation; besides that, the pH value should not be too alkaline because it causes the skin to become dry and scaly (Draelos and Lauren, 2006). The whole formula has a pH value in the range of 4.5–6.5, so the nanosilver peel-off mask formula in this study has met the requirements of gel mask preparation. In the peel-off mask preparation after the cycling test, there was a decrease in the pH, which is due to the influence of temperature. In addition, the difference in the test materials used in the peel-off mask gel also affects the stability of the pH of preparation. Despite the decrease in pH after storage using the cycling test method, all formulations and bases were still within the required pH range (Table 2).
Dry time of peel-off masks affects the comfort of use, where the longer it takes when it is too dry, the mask preparation is increasingly uncomfortable to use. The time required for peel-off mask preparations is 15–30 minutes (Cahyani and Putri, 2018). The test results show that the dry time of all formulas after the stability test with the cycling method at a temperature of 4°C and 40°C for six cycles meets the requirement of dry time. Also, the statistical analysis results for a dry time showed that during storage at 4°C and 40°C, the dry time of a peel-off mask increased, and it is caused by a temperature that absorbs water in the preparation. Other than the addition of humectants to the preparation, the dry time is also increased, and this is because PEG 400 and glycerin have hygroscopic properties that cause an absorption mechanism of water from the environment. The dry time of the peel-off mask is influenced by viscosity, where the low viscosity value affects the dry time by increasing it (Mahyun et al., 2018). Furthermore, the use of a high concentration of PVA increases the viscosity value but forms a film of better quality. Also, the peel-off mask preparation contains glycerin which is hygroscopic with a high affinity for attracting and holding water molecules and maintains stability by absorbing moisture from the environment by reducing evaporation of water from the preparation. The concentration of PVA is the important factor affecting the formation of film performance in a peel-off mask. Meanwhile, preparation at a high temperature has a long dry time because of the increased temperature, which increases the volatility of water. The function of water in gel preparations is to speed up the drying time, which then affects the preparations in the form of increased dry time (Beringhs et al., 2013).
ANTIBACTERIAL ACTIVITY TEST
Nanosilver peel-off mask is used for cosmetic and therapy in the treatment of acne that is caused by bacterial infection; therefore, this test aims to determine the ability of silver nanoparticle peel-off mask preparations to inhibit the growth of Gram-positive and Gram-negative bacteria under certain conditions before and after stability test. Antibacterial activity of nanosilver biosynthetic and peel-off mask preparations were conducted against Gram-positive and Gram-negative bacteria, and the good diffusion method was chosen because it is relatively easy and practical.
 | Table 2. The results of stability test using cycling test method of nanosilver biosynthetic. [Click here to view] |
 | Table 3. The results of diameter of inhibition zone of nanosilver biosynthetic. [Click here to view] |
The sample is in direct contact with the agar media; therefore, the inhibition zone is easily identified visually. Also, this is a clear area around the wells where bacteria are inhibited by antibacterial agents. The antibacterial test was performed for 1.0 mM silver nitrate solution, the antibiotics Vancomycin® 1% for Gram-positive bacteria, Chloramphenicol® 1% for Gram-negative, and water as a negative control. Therefore, the antibacterial activity test aimed to determine the diameter of the inhibition zone of nanosilver solutions and nanosilver using sweet orange peel as bioreduction peel-off mask preparations. Vancomycin® is chosen as a control for Gram-positive infections such as S. aureus and S. epidermidis. Furthermore, it offers a significant inhibition zone based on research by Vermeluen (2000). Imipenem® is chosen as a control for P. aeruginosa and E. coli bacteria, it is an antibiotic that has a broad spectrum that works against anaerobic and aerobic bacteria, and it is effective for Gram-positive and Gram-negative bacteria. The results showed that silver nitrate solution has a strong inhibition, while nanosilver solution and Vancomycin® 1% have a very strong category of inhibition. Meanwhile, the results of the antibacterial test against P. aeruginosa and E. coli showed that the AgNO3 solution, nanosilver, and control Chloramphenicol® 1% have a very strong category of inhibition. Observations showed that antibacterial activity decreased after being formulated into a peel-off mask preparation, and it is probably due to an active substance trapped in the gel polymer; therefore, it is difficult to passively diffuse to the agar medium (Table 3). The results show that all formulas are in the moderate category in inhibiting S. epidermidis, S. aureus, E. coli, and P. aeruginosa bacteria after the accelerated stability test. However, the decreasing value of the diameter of the inhibition zone after stability testing means that the storage temperature affects the inhibitory ability of the preparation.
A higher concentration of glycerin than PEG-400 increases the effectiveness of the active substance, and as a humectant, it also helps in the penetration of substances and helps the active substances inhibit bacteria. According to, glycerin also helps in maintaining excess water evaporation excess in the preparation, which is an advantage in the hot stability test for the active substance to function. The smaller size of nanosilver increases the antibacterial activity, and this corresponds to a study by Gajbhiye and Sakharwade (2016), which stated that the smaller particle size increases the surface area as well as the effectiveness. Furthermore, the mechanism of nanosilver in inhibiting microbial growth is by binding to proteins on the cell wall membrane so that the process of cellular respiration and production does not occur. The direct contact of the affected microbes with nanosilver damages the microbial cell wall and causes the difference in diameter resulting from the inhibition zone for each bacterium.
Flavonoids in plants belong to the phenol group that is known to have antibacterial activity by inhibiting the synthesis of nucleic acids, a function of the cytoplasmic membrane, and metabolism energy. It works by denaturing proteins that cause cell metabolic activity, which is catalyzed by an enzyme. Subsequently, it forms extracellular protein complexes that dissolve with the cell wall to prevent microorganisms from adhering and invading the cells. The diameter of the inhibition zone produced by nanosilver using sweet orange peel as a bioreducer on Gram-positive bacteria is not as large as that of the inhibition zone in Gram-negative bacteria. This is necessary because antibacterial compounds in the form of organic acids have greater inhibition against Gram-negative bacteria (Ermawati et al., 2020). Sweet orange peel contains a class of phenolic acid compounds, organic acids, and flavonoids (Liew et al., 2018), and the difference in the structure of the bacterial cell wall Gram-positive and Gram-negative affects the sensitivity to antibacterial. The cell wall of Gram-positive bacteria consists of about 40 layers of peptidoglycan, thereby reaching 70% of the dry mass of the cell wall, therefore making it thick and stiff. Conversely, Gram-negative bacteria have peptidoglycan, which is about 10% of the dry mass, resulting in thinner cell walls.
CONCLUSION
The sweet orange peel infusion produces nanosilver with a size of 79.7 ± 25.19 nm and SPR absorption of 421–423 nm. Different concentrations of glycerin and PEG 400 affect the physical stability of the preparation, namely viscosity and dry time. Also, the preparation of a peel-off mask with a composition of glycerin-PEG 400 (0.375:0.125 g) has stability when the pH value, viscosity, and dry time show no significant difference compared to the conditions before and after the cycling test. This has the most optimum antibacterial activity with the diameter of inhibition zone against S. epidermidis bacteria of 14.57 mm, P. aeruginosa of 18.16 mm, S. aureus of 20.22 ± 0.122 mm, and E. coli of 23.51 ± 0.36 mm. Therefore, the antibacterial activity of the nanosilver biosynthetic solution has a strong category, while the peel-off mask preparation inhibits Gram-negative and Gram-positive bacteria with a moderate category before and after the stability test using the cycling method.
ACKNOWLEDGMENTS
The authors would like to thank the Research Institute and Community Service (LPPM), Universitas Sebelas Maret, Surakarta, for providing research funding “Hibah Non APBN UNS 2021” with a contract no. 349.1/UN27/HK/2021.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res, 2016; 7:17–28. CrossRef
Ahmed S, Kaur G, Sharma P, Singh S, Ikram S. Fruit waste (peel) as bio-reductant to synthesize silver nanoparticles with antimicrobial, antioxidant and cytotoxic activities. J Appl Biomed, 2018; 16:221–31. CrossRef
Armadany FI, Sirait M. Formulasi sediaan masker gel peel-off antioksidan dari ekstrak sari tomat (Solanum lycopersicum L. var. cucurbita). Majalah Farmasi, Sains dan Kesehatan, 2015; 1:29–32.
Badnore AU, Sorde KI, Datir KA, Ananthanarayan L, Pratap AP, Pandit AB. Preparation of antibacterial peel-off facial mask formulation incorporating biosynthesized silver nanoparticles. Appl Nanosci (Switzerland), 2019; 9:279–87. CrossRef
Baki G, Alexander K. Introduction to cosmetic formulation and technology. Wiley, Hoboken, NJ, 2015.
Benson H, Roberts M, Leite-Silva V, Walters, K. Cosmetic formulation?: principles and practice. CRC Press, Boca Raton, FL, 2019. CrossRef
Beringhs AOR, Rosa JM, Stulzer HK, Budal RM, Sonaglio, D. Green clay and Aloe vera peel-off facial masks: response surface methodology applied to the formulation design. AAPS PharmSciTech, 2013; 14:445–55. CrossRef
Briscoe B, Luckham P. The effects of hydrogen bonding upon the viscosity of aqueous poly(vinyl alcohol) aolutions. Polymer, 2000; 41:3851–60. CrossRef
Budiman A, Aulifa DL, Kusuma ASW, Kurniawan IS, Sulastri, A. Peel-off gel formulation from black mulberries (Morus nigra) extract as anti-acne mask. Nat J Physiol Pharm Pharmacol, 2017; 7:987–94. CrossRef
Cahyani IM, Putri IDC. Formulation of peel-off gel from extract of curcuma heyneana val & zijp using carbopol 940. J Pharm Med Sci, 2018; 2:48–51.
Campbell CSJ, Contreras-Rojas LR, Delgado-Charro MB, Guy RH. Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. J Control Rel, 2012; 162:201–7. CrossRef
Canan I, Gundogdu M, Seday U, Oluk CA, Karasahin Z, Eroglu EC, Yazici E, Unlu, M. Determination of antioxidant, total phenolic, total carotenoid, lycopene, ascorbic acid, and sugar contents of Citrus species and mandarin hybrids. Turk J Agr Forest, 2016; 40:894–9. CrossRef
Coates P, Vyakrnam S, Eady EA, Jones CE, Cove JH, Cunliffe WJ. Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Brit J Dermatol, 2002; 146:840–8. CrossRef
Corradini E, Foglia P, Giansanti P, Gubbiotti R, Samperi R, Laganà A. Flavonoids: chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat Prod Res, 2011; 25:469–95. CrossRef
Dhuper S, Panda D, Nayak PL. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano Trend J Nanotechnol Appl, 2011; 13:16–22.
Draelos ZD, Lauren AT. Cosmetic formulation of skin care products. Taylor and Francis Group, New York, NY, 2016.
Dutta T, Nath N, Das M, Adhikary R, Mandal V, Chattopadhyay AP. Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract?: experimental and theoretical studies. J Environ Chem Eng, 2020; 8:1–9. CrossRef
Ermawati DE, Yuniastuti A, Fajrin HI. Effectiveness of nanosilver biosynthesis using inulin gembili tuber (Dioscorea esculenta L.) on variation of inulin solution towards particle sizes and antibacterial activities. In: Journal Physics: Conference Series, IOP Publishing, Bristol, UK, vol. 012042 p, 1912, 2021; doi:10.1088/1742-6596/1912/1/012042. CrossRef
Fox L, Csongradi C, Aucamp M, Du Plessis J, Gerber M. Molecules treatment modalities for acne. Molecules, 2016; 27:1–20. CrossRef
Gajbhiye S, Sakharwade S. Silver nanoparticles in cosmetics. J Cosmet Dermatol Sci Appl, 2016; 06:48–53. CrossRef
Ghasemzadeh A, Jaafar HZE, Bukhori, MFM, Rahmat MH, Rahmat A. Assessment and comparison of phytochemical constituents and biological activities of bitter bean (Parkia speciosa Hassk.) collected from different locations in Malaysia. Chem Cent J, 2018; 12:1–9. CrossRef
Gunawan A, Sihotang DE, Thoha MY. Volume larutan pemasak terhadap viskositas pulp dari ampas tebu. J Teknik Kimia, 2018; 18:1–8.
Hamidu L, Ahmad AR, Najib A. Qualitative and quantitative test of total flavonoid buni fruit (Antidesma bunius (L.) Spreng) with UV-Vis spectrophotometry method. Pharmacogn J, 2018; 10:60–3. CrossRef
Hembram KC, Kumar R, Kandha L, Parhi PK, Kundu, CN, Bindhani BK. Therapeutic prospective of plant-induced silver nanoparticles: application as antimicrobial and anticancer agent. Artif Cells Nanomed Biotechnol, 2018; 46:S38–51. CrossRef
HORIBA. A guidebook to particle size analysis. HORIBA, Kyoto, Japan, 2017.
Ibrahim HMM. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci, 2015; 8:265–75. CrossRef
Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharm Sci, 2017; 12:487–97. CrossRef
Kaushik U, Joshi SC. Silver nanoparticles: green synthesis, optical properties, antimicrobial activity and its mechanism using Citrus sinensis. Asian J Pharm Clin Res, 2015; 8:179–84.
Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc, 2011; 79:594–8. CrossRef
Kotz J, Treichel P, Townsend J. Chemistry and chemical reactivity. 7th edition, Thomson Higher Education, Belmont, CA, 2011.
Liew SS, Ho WY, Yeap SK, Bin Sharifudin SA. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. Peer J, 2018; 6:1–16. CrossRef
Liu W. Facial mask composition and its preparation method, 2018.
Logeswari P, Silambarasan S, Abraham J. Synthesis of silver nanoparticles using Plants extract and analysis of their antimicrobial property. J Saudi Chem Soc, 2012; 19:311–7. CrossRef
Mahyun F, Kusuma A, Tamhid HA. Formulation peel-off gel mask of impatiens balsamina l. as an antibactery against Staphylococcus aureus. Indo J Med Health, 2018; 9:1552–65. CrossRef
Malassis L, Dreyfus R, Murphy RJ, Hough LA, Donnio B, Murray CB. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv, 2016; 6:1–9. CrossRef
Nisha NS, Aysha OS, Syed Nasar Rahaman J, Vinoth Kumar P, Valli S, Nirmala P, Reena A. Lemon peels mediated synthesis of silver nanoparticles and its antidermatophytic activity. Spectrochim Acta A Mol Biomol Spectrosc, 2014; 124:194–8. CrossRef
Ogur E. Polyvinyl alcohol: materials, processing and applications. Rapra Tecnology, Shrewsbury, UK, 2005.
Osonga FJ, Kariuki VM, Yazgan I, Jimenez A, Luther D, Schulte J, Sadik OA. Synthesis and antibacterial characterization of sustainable nanosilver using naturally-derived macromolecules. Sci Total Environ, 2016; 563–564:977–86. CrossRef
Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Letters, 2012; 2:1–10. CrossRef
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle zize. Colloid Surf B Biointerfaces, 2011; 82:152–9. CrossRef
Pulit-Prociak J, Grabowska A, Chwastowski J, Majka TM, Banach M. Safety of the application of nanosilver and nanogold in topical cosmetic preparations. Colloid Surf B Biointer, 2019; 183. CrossRef
Puspitasari AD, Setyowati DA. Evaluasi karakteristik fisika kimia dan nilai SPF sediaan gel tabir surya ekstrak etanol daun kersen (Muntingia calabura L). J Phar Sci, 2019; 5:153–62. CrossRef
Roukas T, Kotzekidou P. Pomegranate peel waste: a new substrate for citric acid production by Aspergillus niger in solid-state fermentation under non-aseptic conditions. Environ Sci Pollut Res, 2020; 27:1–9. CrossRef
Sakharwade S. Silver Nanoparticles in cosmetics. J Cosm Dermatol Sci Appl, 2016; 6:48–53. CrossRef
Shnoudeh AJ, Hamad I, Abdo RW, Qadumii L, Jaber AY, Surchi H, Alkelany SZ. Synthesis, characterization and applications of metal nanoparticle. In: Tekade RK (ed.). Biomaterial and bionanotechnology, Academic Press, Cambridge, MA, pp 527–612, 2019. CrossRef
Sreeram KJ, Nidhin M, Indumathy R, Nair BU. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull Mater Sci, 2008; 31:93–6. CrossRef
Velasco MVR, Vieira RP, Fernandes AR, Dario MF, Pinto CASO, Pedriali CA, Kaneko TM, Baby AR. Short-term clinical of peel-off facial mask moisturizers. Int J of Cosm Sci, 2012; 36:355–60. CrossRef
Wei X, Luo M, Li W, Yang L, Liang X, Xu L, Kong P, Liu H. Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresource Technol, 2012; 103:273–8. CrossRef
Wulandar W, Ermawati DE, Yugatama A. Optimization SNEDDS (self-nano emulsifying drug delivery system) of ZnO that dispersed into Hydrogel Matrix as UV-Protective. IOP Publisher, Bristol, UK; doi:10.1088/1757-899X/578/1/012058. CrossRef