INTRODUCTION
Fluoroquinolones are among the utmost commonly prescribed antimicrobial medicines because of their towering bioavailability and wide-ranging antimicrobial efficacy (Klein et al., 2018). Fluoro-quinolones possess excellent oral absorption, extensive distribution in vitro, near to the ground plas-ma protein binding potential, a comparatively long plasma t½-life, and minimum adverse drug reac-tions. Consequently, these antimicrobials have emerged as the medicine of choice to treat most Gram-negative bacterial infections (Blandeau, 1999; Mandell and Tillotson, 2002; Zhanel et al., 1999). However, because of their widespread use, including potentially inappropriate use, fluoro-quinolones resistance has developed in quite a lot of clinically relevant microbes that comprise En-terobacteriaceae (Dalhoff, 2012; de Lastours et al., 2014; Mitra et al., 2019). Plasmid-mediated quinolone resistance (PMQR) and the mutations within the chromosomal quinolone resistance-determining regions (QRDRs) contribute to the development of the quinolone-resistant mechanism of pathogenic microorganisms (Shetty et al., 2019; Tamang et al., 2012). PMQR includes various qnr genes, aac (6’)-Ib-cr, and qepA. The qnr genes, including qnrA, qnrB, qnrC, qnrD, qnrE, qnrS, and qnrVC, encode DNA protection gyrase and topoisomerase IV from quinolone inhibition (Jacoby et al., 2014; Poirel et al., 2012). The first qnrA (a PMQR determinant) inactivating quinolone was detected in Birmingham, Alabama, in 1998, among clinical specimens of Klebsiella pneumoniae (Martínez-Martínez et al., 1998). Multiple studies have subsequently reported that the presence of PMQR genes among Citrobacter freundii, Escherichia coli, Enterobacter cloacae, Enterobacter sa-kazakii, K. pneumoniae, Providencia stuartii, Salmonella spp., Enterobacter spp., and Klebsiella ox-ytoca across all continents, including Asia, Europe, Australia, and South America, in recent years (Cheung et al., 2005; Jonas et al., 2005; Minarini et al., 2008; Nazic et al., 2005; Nordmann and Poirel, 2005; Ode et al., 2009; Poirel et al., 2005; Rodriguez-Martinez et al., 2006; Wang et al., 2003).
Both PMQR genes and mutations within the QRDRs contribute to the advent of quinolone-resistant pathogens (Ferrari et al., 2013, Kotb et al., 2019). Gram-negative microbial DNA gyrase is further liable to impeding by quinolones than in topoisomerase IV (Jacoby, 2005). Bacteria evolve re-sistance by mutations in the QRDR of gyrA and parC genes, altering the structure of topoisomerase that subsequently reduces the enzyme’s affinity to quinolone antibiotics (Moon et al., 2010, Ruiz, 2003, Varughese et al., 2018). The QRDR lies on the DNA-binding surface of the DNA gyrase en-zyme where amino acid positions 83 and 87 remain the “hotspots” for mutations for fluoroquinolone resistance (Piddock, 1999). Substitutions of serine-83 (Ser-83) and asparagine-87 (Asp-87) in the gyrA gene are among the most repeatedly detected mutations in Enterobacteriaceae-resistant strains (Varughese et al., 2018). Conversely, the preliminary bull’s-eye mutations happen more frequently in parC in cases of moderately resistant Staphylococcus aureus or Streptococcus pneumoniae; however, their resistance phenomena increase with additional mutations found in gyrA and parE genes (Eliopoulos, 2004; Ng et al., 1996; Redgrave et al., 2014; Woodford and Ellington, 2007).
Quinolone and the fluoroquinolones include nalidixic acid, ciprofloxacin, ofloxacin, and lomefloxacin. They have been prescribed and consumed to treat urinary tract infections (UTIs) since their availability in the 1970s (Andersson and MacGowan, 2003; Oliphant and Green, 2002). The issue of fluoro-quinolones resistance among urinary pathogens not solitarily exists in several low- and middle-income countries (LMICs) but equally imposes health threats in high-income countries (Banerjee and Anupurba, 2016; Critchley et al., 2019; de Souza da-Silva et al., 2020; Odoki et al., 2020; Stapleton et al., 2020; Tchesnokova et al., 2019). Resistance is exacerbated significantly in LMICs by the ease of purchasing antibiotics over the counter without a prescription (Belachew et al., 2021; Bryce et al., 2016; Godman et al., 2021; Gravningen et al., 2020; Haque et al., 2019a, 2019b; Jacobs et al., 2019).
The rate of antimicrobial resistance (AMR) has progressively increased internationally. Multiple pieces of research have stated that imprudent prescribing and consumption of antimicrobials are the primary cause of microbial resistance in hospital and community settings (Haque and Godman, 2021a; Momanyi et al., 2019; Saleem et al., 2019a, 2019b). Multiple earlier research demonstrated that fluoroquinolone resistance remains an independent factor for high rates of mortality and poor clinical outcomes among patients with healthcare-associated infection (HCAIs) (Chong et al., 2014; Dalhoff, 2012; Haque et al., 2018; Lautenbach et al., 2010). Furthermore, the high prevalence of fluoroquinolone resistance raises concerns about whether this group of antimicrobials should be used for prophylaxis (Chong et al., 2014; Terahara and Nishiura, 2019). This is because fluoroquinolone resistance causes difficulties with treating many types of infections, including community-acquired UTIs and HCAIs UTIs, both community and HCAI respiratory infections, cystic fibrosis, chronic obstructive pulmonary disease, dermatological, intra-abdominal, and sexually transmitted infections, as well as traveler’s diarrhea (Dalhoff, 2012; Davidson et al., 2002; Fuller and Low, 2005; Pletz et al., 2005). This may be because the fluoroquinolones have become resistant to several bacterial pathogens (Xiao et al., 2008; Zou et al., 2003). These are also concerns with the development of fluoroquinolone resistance among TB bacilli (Takiff and Guerrero, 2011; Xu et al., 2009) driven by their imprudent use, i.e., monotherapy or without directly observed therapy (DOTS) (Xu et al., 2009).
In Bangladesh, self-purchasing of antibiotics is common, enhanced by affordability issues with see-ing a physician combined with a culture of self-medication (Darj et al., 2019; Do et al., 2021; Haque et al., 2020); it also exists in many neighboring countries of Bangladesh (Alghadeer et al., 2018; Aslam et al., 2020a, 2020b; Chautrakarn et al., 2021; Faqihi and Sayed, 2021; Gillani et al., 2021; Mandal et al., 2020; Nepal and Bhatta, 2018; Shamsudeen et al., 2018; Shrestha et al., 2021). This is apprehension as self-medication with antimicrobials increases their imprudent use and promotes re-sistance (Ayukekbong et al., 2017; Behzadifar et al., 2020; Godman et al., 2021; Haque et al., 2019b). Bangladesh also provides an appreciable migrant labor force in many realms around the world (Karim et al., 2020), consequently increasing the possibility of transmission fluoroquinolones resistant genes among these countries and vice versa.
We are aware of the many ongoing strategies, as well as the blossoming of national action plans (NAP) in Bangladesh, to try and reduce the rising AMR rates (Haque and Godman, 2021b). As part of these developments, this research was designed to assess the pervasiveness of three PMQR genes, qnrA, qnrB, and qnrS, and determine mutations in the gyrA gene quinolone resistance mechanism amid UTI enterobacteriaceae isolated in Bangladesh. This study further analyzed the alternation of fluoroquinolone drug affinity and phenotypic susceptibility related to the mutations in either gyrA or PMQR genes. We believe our findings will help direct future strategies as part of the Bangladesh NAP and other approaches to address high AMR rates in Bangladesh.
MATERIALS AND METHODS
Study design and specimen collection
A cross-sectional study was conducted between April 2017 and March 2018 among symptomatic UTI patients attending the outpatient departments at Gonoshasthaya Samaj Vittik Medical College Hospital, Savar, Dhaka, and Uttara Adhunik Medical College Hospital, Dhaka, Bangladesh. These are privately owned tertiary care teaching hospitals. All the patients who had no history of antibiotic treatment in the preceding 15 days were requested to take part in the study. Those patients who were diagnosed with immunocompromised diseases, different cancers, organ transplants, sexually trans-mitted infections, and renal disorders were excluded. Midstream clean catch urine samples were col-lected from 122 patients who met the study criteria for microbiological investigation. The urine spec-imens were instantly transported to the laboratory for further examination after collection. Patients were subsequently grouped by gender and age, in groups of 10 years, for comparative data analysis.
Bacterial isolation and identification
Urine samples were collected in sterile glass tubes and inoculated on a differential culture medium, MacConkey agar (Supplementary Fig. 1A), within 2 hours after collection. One loopful of urine was inoculated and incubated at 37°C for 24 hours. After performing quantitative urine cultures, 102 or 103 CFU/ml colony counts were considered to define a probable UTI infection. Colony counts of less than 102 CFU/ml were assumed as potentially contaminated. Etiologic proof of identity was con-firmed by a rapid biochemical test kit (API 20E, Biomerieux, Durham, NC) entailing a set of chro-mogenic panels, carbohydrate batteries, and enzymatic substrates (Supplementary Fig. 1B) after se-lecting the Gram-negative bacterial colony from the elective agar plat. Part of the bacterial identities was validated further by amplifying and sequencing the 16S rDNA gene (Supplementary Fig. 1C) (Van Der Zee et al., 2016). Sequencing services were obtained from a commercial service provider (Macrogen Inc., South Korea). The isolates were conserved in 30% glycerol at −20°C in trypticase soy broth for further research.
Antimicrobial susceptibility testing (AST)
This study analyzed the susceptibility pattern of quinolone and fluoroquinolones separately to each identified UTI species. Phenotypic antimicrobial susceptibilities of the isolates were tested by the disk diffusion method (Kirby–Bauer) on Mueller–Hinton agar (Oxoid, Basingstoke, UK) plates ac-cording to the Clinical and Laboratory Standards Institute (CLSI) guidelines (Weinstein and Lewis, 2020). Momentarily, a 4-hour bacterial suspension in the Mueller–Hinton broth was attuned to a den-sity of 0.5 McFarland equivalent and then squarely streaked on MHA plates to confirm steady growth. The quinolone disk, nalidixic acid (30 µg), and the fluoroquinolone disks, ciprofloxacin (5 µg), lomefloxacin (10 µg), and ofloxacin (5 µg), were positioned on the bacterial lawn and incubated at 37°C overnight to evaluate the sensitivity spectrum. Sensitive microbes formed a clear precinct around each disk, and the clear sector diameter was quantified and appraised per CLSI guidelines (Supplementary Fig. 1D). Escherichia coli ATCC 25922 was used as the susceptible control strain for the AST. In vitro antimicrobial potency of the commonly prescribed quinolone/fluoroquinolone antibiotics, including nalidixic acid, ciprofloxacin, ofloxacin, and lomefloxacin, were tested by the disk diffusion method against the 100 UTI Enterobacteriaceae. Antimicrobial disks were procured from Thermo Fisher Scientific Oxoid Ltd (Basingstoke, England) due to the easy availability of fluproquazones disks.
Detection of PMQR (qnrA, qnrB, and qnrS) genes
All the isolates were examined through a polymerase chain reaction (PCR) for genotypic endorsement of PMQR genes, including qnrA, qnrB, and qnrS. Specific primer sets for the respective genes were designated grounded on previous studies (Cattoir et al., 2007) and synthesized from Integrated DNA Technology (IDT, Singapore). For qnrA, the primer pair used was qnrA-F 5′-AGAGGATTTCTCACGCCAGG-3′ and qnrA-R 5′-TGCCAGGCACAGATCTTGAC-3′ with an expected amplicon size of 580 bp. For qnrB, the primer pair used was qnrB-F 5′-CCTGAGCGGCACTGAATTTAT-3′ and qnrB-R 5′-GTTTGCTGCTCGCCAGTCGA-3′ that produced a 390 bp amplicon. Primer pair sequences for qnrS were qnrS-F 5′-GCAAGTTCATTGAACAGGGT-3′ and qnrS-R 5′-TCTAAACCGTCGAGTTCGGCG-3′ with its product size of 428 bp. For each PCR reaction, the bacterial template DNA 2.0 µl was added to 12 µl of a 2X PCR pre-mixture (GeneON, Germany) and 5 pmol of each primer (1 µl), with deionized water subsequently added to make a final volume of 24 µl. Reactions underwent an initial denaturation at 95°C for 10 minutes, followed by 32 cycles of amplification (Applied Biosystems 2720 Thermal Cycler, Singapore), consisting of denaturation for 30 seconds at 94°C; annealing for 30 seconds at 52°C–56°C, depending on the primer sets; extension 1 minutes at 72°C; and a final 7-minute extension at 72°C. Amplicons were visualized under UV light after electrophoresis through 1.2% agarose gel at 100 volts for 30 minutes. The typical molecular weight marker was run corresponding to quantifying specific amplicon sizes (GeneRuler, ThermoFisher Scientific, MA) (Supplementary Fig. 2).
Amplification and sequence analysis of QRDR gyrA gene
Substitutions of nucleic acids and corresponding amino acids in gyrA proteins were studied by PCR amplification, followed by sequencing the gene in eight isolates carrying both quinolone-susceptible and quinolone-resistant phenotypes (Oram and Fisher, 1991). The obtained gyrA gene sequences of the isolates were compared to other published sequences available in the GenBank database. (http://www.ncbi.nlm.nih.gov). ClustalW multiple sequence alignment was carried out with the highest gyrA gene sequence similarity using BioEid software 7.0.
Docking analyses of quinolone
Mutations at two specific residues were considered for analysis: serine-83 and aspartate-87. Search-ing the Protein Data Bank (PDB) (Berman et al., 2000) we could find only the structure of E. coli gyrase complex bound to inhibitor YacG (PDB ID: 4TMA) (Supplementary Fig. 3), with no 3D structure of any Klebsiella spp. gyrase complex in PDB format. To predict 3D protein structures of Klebsiella pneumonia, we retrieved two protein sequences from UniProtKB (UniProt Consortium, 2019), namely K. pneumoniae gyrase subunit A (UniProt ID: R4Y7H5) and K. pneumoniae gyrase subunit B (UniProt ID: R4Y6T5). We submitted two protein sequences to the I-TASSER online server (Yang et al., 2015) for protein 3D structure prediction (Fig. 1A). Mutations at positions 83 (Ser to Leu) and 87 (Asp to Asn) of the amino acid sequences were unified into the normal protein (Fig. 1B). The structures of two quinolones, ciprofloxacin and ofloxacin, were retrieved in the struc-ture data file format from PubChem (Kim et al., 2019) database. These two antibiotics were docked as ligands against the wild-type and mutated gyrase complex receptors. The docking was undertaken in the YASARA platform (Krieger and Vriend, 2014) using the AutoDock Vina (Morris et al., 2009) docking module.
Statistical analyses
Data were substantiated; the key-in spreadsheet was scanned and explored using IBM SPSS statistics data editor (version 21). Missing data were omitted from the bivariate analysis. Descriptive and in-ferential statistical analyses were conducted to assess the carriage of the three PMQR genes and the gyrA mutation in UTI pathogens and their phenotypic attributes. Pearson’s chi-square test was con-ducted to examine any association between categorical data, and Yate’s correction for continuity was applied where necessary. When the chi-square test’s expected frequency cannot be assumed, Fisher’s Exact test results of the 2 × 2 contingency table were reported instead. Two-tailed p-values were cal-culated to determine statistical significance at the 0.05 level.
Ethical statement
The Ethics and Research Review Committee of the Jahangirnagar University (JU), Faculty of Bio-logical Sciences, approved this study [No. BBEC, JU/M 2017 3(4) dated 15.03.2017]. All the study protocols complied with the Declaration of Helsinki for enrolling human subjects for medical re-search. Written informed consent was obtained from each adult study patient for collecting their urine samples. Separate written informed consent was taken from parents or legal guardians for pa-tients under 18 years. Samples were coded to anonymize the study participants’ identities and other information.
RESULTS
UTI study patients and bacterial etiology
This research analyzed 122 urine samples from symptomatic patients. Of the total samples, 100 UTI bacteria were detected in 100 urine samples, and the remaining 22 samples had no growth; therefore, confirmed UTI recovery was in 82.0% of the patients (100/122). The recovered 100 UTI isolates with the respective study participants were subsequently analyzed in this study, and 22 subjects were excluded from the next level of analysis. All participants were self-reported symptomatic with either abdominal pain, painful urination, repeated urge to urinate, and an incomplete void feeling in their bladders. Frequency of the identified UTIs was elevated amid females (77.0%, n = 77) than their male counterpart (23.0%, n = 23).
The age range of the patients was from 8 to 76 years, with those aged between 21 and 30 years the most vulnerable to UTIs in both genders and accounting for 35.0% (35/100) of the total number of infections. In each 10-year tier, females had a greater prevalence of UTIs than males. There was no UTI detected among males below 20 years of age, although 18% of females of a similar age range had urinary infections (Table 1). However, the higher revealed UTI episodes in females in all the age groups were not statistically significant through the Chi-squared test (p = 0.084). Each urine sample from confirmed cases produced a single UTI pathogen. The most frequently identified UTI bacteria were E. coli (n = 28) and K. pneumoniae (n = 44). The other identified bacteria were 11 Proteus spp., 10 Enterobacter spp., 4 Pseudomonas spp., and 3 Staphylococcus spp.
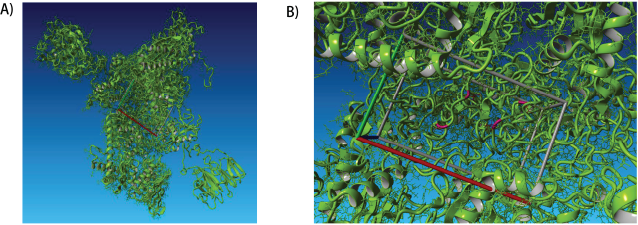 | Figure 1. The predicted 3D structure of K. pneumoniae gyrase complex. A). The K. pneumoniae gyrase complex’s predicted structure is shown. B) Mutated residues in the target site of S83L_D87H mutant K. pneumoniae gyrase are marked in red. [Click here to view] |
Quinolone/fluoroquinolone susceptibility profiling
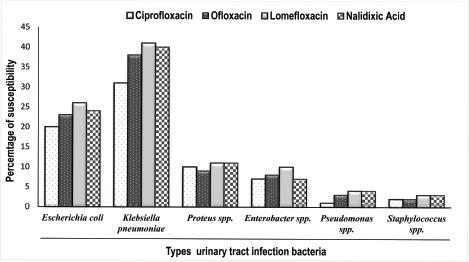
95.0% of the isolates exhibited resistance against lomefloxacin, which indicated the uppermost percentage of resistance among the quinolones tested. 89.0%, 83.0%, and 71.0% of the isolates, respectively, showed resistance to nalidixic acid, ofloxacin, and ciprofloxacin. Ciprofloxacin was the most effective fluoroquinolone among those tested, followed by ofloxacin against the UTI pathogens in this study. All four quinolones were found most effective against K. pneumoniae, followed by E. coli. They were intermediate effectiveness against Proteus spp. and Enterobacter spp. UTI pathogens Pseudomonas spp. and Staphylococcus spp. expressed most resistance in comparison to other bacteria tested (Fig. 2).
Phenotypic and genotypic assessment of PMQR genes
We used PCR to detect three PMQR, namely qnrA, qnrB, and qnrS. qnrS was the most prevalent plasmid-mediated gene detected in 54.0% of the UTI pathogens. However, the other two PMQR genes, qnrA and qnrB, were detected in 1.0% and 4.0% of the pathogens, respectively. All the identified qnrA and qnrB genes overlay with qnrS; consequently, complete co-carriage of two PMQR genes together with qnrA+qnrS or qnrB+qnrS was found. One E. coli carried all the qnr genes, while three K. pneumoniae possessed qnrS and qnrB. Intraspecies analyses revealed the highest carriage of PMQR in K. pneumoniae (63.6%), followed by Enterobacter spp. (60%), Proteus spp. (54.5%), and E. coli (46.4%). Pseudomonas spp. was found to carry 25% of the qnr genes, but none of Staphylococcus spp. carried any of the PMQR genes (Table 2).
As mentioned, we evaluated these UTI pathogens’ phenotypic quinolone/fluoroquinolone susceptibilities against nalidixic acid, ciprofloxacin, lomefloxacin, and ofloxacin. Subsequently, we assessed the associations of phenotypic susceptibilities with the carriage of PMQR genes. We did not find any statistically significant correlation with PMQR genes carried by the UTI pathogens with the phenotypic resistance phenomena of any of the four quinolone/fluoroquinolone antibiotics tested (Table 3). We further analyzed the association of phenotypic susceptibilities of quinolone/fluoroquinolone with PMQR genes for each UTI pathogen separately, and no statistically significant relation was detected (Supplementary Table 1).
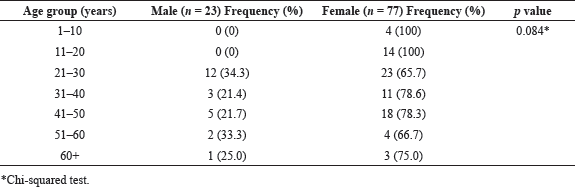 | Table 1. Gender and age-group distributions of urine culture-positive patients (n = 100). [Click here to view] |
 | Figure 2. The binding energy of quinolones as ligands for docking into gyrase protein within the QRDRs. [Click here to view] |
Mutations analysis of gyrA gene
Of the total number analyzed, 46% of UTI pathogens did not carry the qnrA, qnrB, or qnrS PMQR genes. However, 71.7% of these appeared resistant to at least two or more tested quinolones. We subsequently investigated mutations in the hotspot region of the chromosomal gyrA gene associated with the exhibited phenotypic quinolone/fluoroquinolone resistance in UTI isolates with and without the three qnr genes. gyrA gene from three UTI bacteria was amplified and sequenced. Afterward, their translated amino acid sequences were compared regarding the E. coli ATCC 25922 strain and R4Y7H5 K. pneumoniae strain. We examined gyrA gene sequences of four isolates carrying the PMQR (qnrS) gene. Two isolates were Proteus spp., E. coli, and K. pneumoniae. No mutation was observed in the gyrA region in any of the four isolates (Figure 3A). Of the other three isolates, one E. coli and one K. pneumoniae manifested double mutations at S83L (substitution of serine to leucine at position 83) and D87N (substitution of aspartic acid to asparagine at position 87). Another K. pneumoniae showed a single mutation at S83L (Fig. 3B).
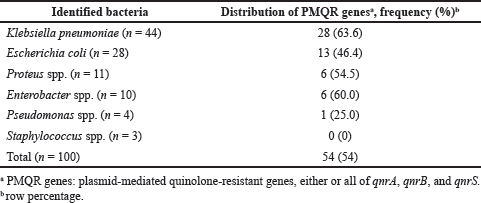 | Table 2. Identified PMQR genes, qnrA, qnrB, and qnrS in different UTI pathogens. [Click here to view] |
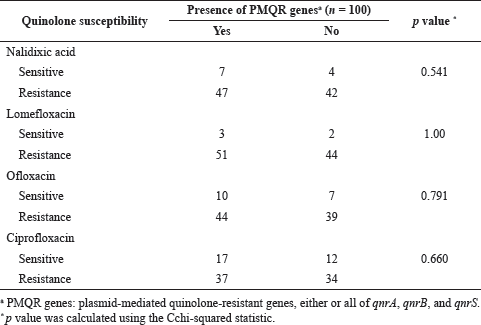 | Table 3. Association of phenotypic quinolone susceptibilities with PMQR genes. [Click here to view] |
The amino acid alignment of the gyrA gene covering the QRDR of UTI pathogens, K. pneumoniae and E. coli, was related to those of reference E. coli ATCC 25922 strain and R4Y7H5 K. pneumoniae strain. Genetic divergence of the QRDR of the uropathogens was determined by the pair-wise comparison to reference strains. DOTS indicate identity and letters represent substitutions in the UTI pathogens relative to the reference isolates. The findings suggest that gyrA sequences of the four bacteria carrying PMQR genes have been aligned, and no mutation was observed (Fig. 3A). The amino acid sequences of the gyrA gene from the three bacterial isolates without PMQR genes were aligned (Fig. 3B). One UTI E. coli (QN4) and one K. pneumoniae (QN5) manifested double mutations at S83L (substitution of serine to leucine at position 83) and D87N (substitution of aspartic acid to asparagine at position 87). Another K. pneumoniae (QN7) showed a single mutation at S83L.
The docking results of quinolones with both the reference and mutated E. coli gyrA are represented in binding energies. Higher binding energy signifies a more vital interaction amid the ligand and protein. Ciprofloxacin and ofloxacin docking results are shown in Fig. 4. The required binding energy was 8.055 and 8.666 kcal/mol for ciprofloxacin and ofloxacin for the wild-type protein complex, respectively. However, the binding energy became abridged to 6.973 kcal/mol and 7.417 kcal/mol in the case of a mutated protein complex at S83L and D87N, respectively (Fig. 4A).
Similarly, docking results were calculated in the case of K. pneumoniae as well. The binding affinity of ciprofloxacin for wild-type gyrA of K. pneumoniae was 7.969 kcal/mol, whereas an affinity for the mutant strain with S83L was 6.528 kcal/mol. The protein binding affinity went down to 6.203 kcal/mol for the strain with two mutations at S83L and D87N (Fig. 4B). For ofloxacin, the binding affinity was 7.092 kcal/mol in the case of wild-type protein; however, the affinity reduced to 6.934 and 6.957 kcal/mol for mutated strains with a single mutation at S83L and double mutations at S83L and D87N, respectively (Fig. 4B). Docking research revealed that the displacement of the quinolone binding sites in a mutated protein complex brings about lower binding energy than the wild one. The reduced affinity could cause the high resistance patterns displayed in this study.
The comparative binding energy for ciprofloxacin and ofloxacin to wild-type gyrase and mutant gyrase are shown in Figure 4. Figure 4A shows the binding energy of ciprofloxacin and ofloxacin for the wild-type gyrase protein complex and double-mutation (S83L and D87N) gyrase proteins of E. coli. Figure 4B shows the adhesive strengths of ciprofloxacin and ofloxacin for both single- (S83L) and double-mutation (S83L and D87N) gyrase proteins K. pneumoniae when compared with that wild type. For all cases, binding affinity was detected lower in the case of mutated gyrase proteins.
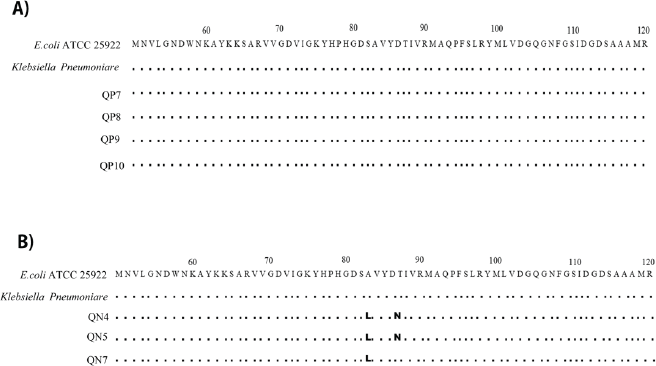 | Figure 3. TAnalyses of mutations in the gyrA gene within the QRDRs. [Click here to view] |
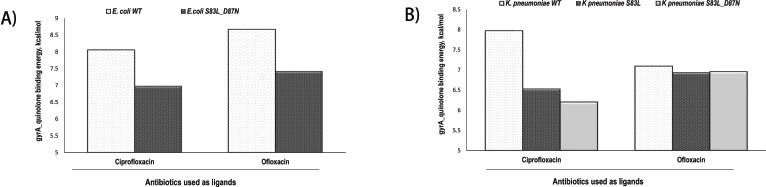 | Figure 4. The binding energy of quinolones as ligands for docking into gyrase protein within the QRDRs. [Click here to view] |
DISCUSSION
This study investigated phenotypic quinolone/fluoroquinolone susceptibility and carriage of three PMQR genes, namely qnrA, qnrB, and qnrS, in UTI bacteria in Bangladesh. Furthermore, we analyzed the association of mutations in the QRDR of the gyrA gene with the acquisition of quinolone resistance in some selected UTI pathogens.
Our results identified UTI bacteria in Bangladesh exhibiting a high prevalence of phenotypic resistance to four commonly used quinolone antimicrobials. The uropathogens showed higher resistance to lomefloxacin and nalidixic acid than ciprofloxacin and ofloxacin, which is similar to many other studies conducted in different countries (Cao et al., 2011; Colodner et al., 2008; Kim et al., 2020; Lee et al., 2018; Santiso et al., 2009). Ciprofloxacin was found to be the drug of choice to manage patients with UTIs among the four quinolones tested, with similar findings reported earlier in India, Nepal, Bangladesh, and Sri Lanka, as well as many other LMICs (Britto et al., 2018; Hooda et al., 2019; Saksena et al., 2018; Sedighi et al., 2015; Singh et al., 2019).
This study established a high abundance (54.0%) of PMQR genes dominated by qnrS in quinolone-resistant urinary Enterobacteriaceae, comparable to the earlier studies (Kim et al., 2009a; Poirel et al., 2006). The lower detection of qnrB (4.0%) and qnrA (1.0%) in clinical isolates was also consistent with other studies (Abd El Salam et al., 2020; Poirel et al., 2006). Despite the presence of different PMQR genes, our study did not find a statistically significant relationship between detected qnr genes and corresponding phenotyping quinolone/fluoroquinolone resistance.
A significant portion of UTI isolates without bearing qnr genes exhibited phenotypic resistance to the same sets of quinolone/fluoroquinolone antimicrobials. The inconsistency of the genotype-phenotype association could be explained by other PMQR genes, such as aac(6’)-Ib-cr, qepA, qnrC, qnrD, qnrE, and qnrVC, that were not investigated in this study (Jacoby et al., 2015; Strahilevitz et al., 2009).
Furthermore, this study characterized the gyrA gene mutation mediated quinolone-resistant mechanisms in circulating UTI pathogens in Bangladesh. We found one E. coli and K. pneumoniae with two substitutions (S83L and D87N) and one K. pneumoniae with one mutation (S83L) in the gyrA gene. Similar mutations were reported in some diarrheal enterotoxigenic E. coli in Bangladesh (Begum et al., 2016) as well as UTI pathogens from other countries (Betitra et al., 2014; Komp Lindgren et al., 2003; Varughese et al., 2018). In our study, these three strains were resistant to all tested quinolones without harboring PMQR genes. These results make available further evidence that chromosomal QRDR mutations in sequences encoding gyrA perform an indispensable role in quinolone resistance (Moon et al., 2010). The findings also suggest that S83L and D87N mutations in gyrA can hinder the broad-spectrum antibacterial activities of quinolones by restricting the DNA gyrase and topoisomerase IV activities (Piddock, 1999). Moreover, docking results of quinolones with wild-type and mutated gyrA protein from both E. coli and K. pneumoniae provided the principle of mutation-based QRDR (Ruiz, 2003). Mutated gyrA protein showed reduced ligand binding energy for both uropathogens, as observed in previous research reports (Chu et al., 2020; Varughese et al., 2018).
This high frequency of quinolone-resistant urinary pathogens is a concern, as quinolones are still the antimicrobials of choice for managing UTIs in Bangladesh and abroad. However, the excessive use of either oral or parenteral quinolones for UTIs and other infections in recent years may enhance high rates of AMR (Holmes et al., 2016). The increased resistance in any currently widely used antibiotic makes treatment decisions difficult. It imposed higher medical expenditure when primary recommended antibiotics do not produce the desired results and/or alternative antibiotics are prescribed (Strahilevitz et al., 2009). This study showed that K. pneumoniae and E. coli were the most typical pathogens causing complicated and uncomplicated UTIs, which is similar to other studies (Founou et al., 2017; Hofer, 2019; Haque and Godman, 2021b; Urmi et al., 2020). Several different bacteria identified known to cause UTIs, including Pseudomonas aeruginosa, Staphylococcus spp., Proteus spp., and Enterobacter spp., have been stated in earlier research reports (Linhares et al., 2013; Urmi et al., 2019). These findings can help to provide empirical guidance on the management of UTIs in Bangladesh and the wider prospect around the globe.
This study also identified urinary infections more commonly in females than males, with a 10-year stratified age grouping revealing a higher prevalence of UTIs among females in all the age classes. Reproductively active women aged 20–39 years accounted for most UTI presentations, similar to others (Ara et al., 2021; Moran et al., 2020; Smith et al., 2018; Urmi et al., 2020). We believe these combined research findings can be used to develop preventive strategies for managing recurrent UTIs among the general population in Bangladesh, especially among women, and we will be following this up.
We are aware of several limitations with this study. Firstly, this study was conducted under a cross-sectional design, and we are aware of the importance of perspectives regarding cross-sectional research. The purposive sampling was also only undertaken in a single community and urban hospital in Bangladesh. Thirdly, risk behavior data among the participants, including the prescribing physicians, were not studied in detail. Fourthly, we could not recruit cases from the initial stages of UTIs of the research subjects as we were typically dealing with recurrent UTIs. In Bangladesh, patients usually seek medical care when the disease process is more advanced. Fifthly, PMQR genes including aac (6’)-Ib-cr, qepA, qnrC, qnrD, qnrE, and qnrVC, were not investigated. However, we maintained the internal validity of our results by repeating independent experiments where necessary, enhancing the robustness of our findings.
CONCLUSION
Uropathogens circulating in Bangladesh are highly resistant to quinolone antibiotics. Ciprofloxacin was the most effective fluoroquinolone against tested UTI pathogens, while lomefloxacin appeared the least effective. Acquisition of the qnrS, qnrA, and qnrB genes carry the spurious association of quinolone resistance in UTI pathogens. However, our findings have disclosed shortcomings of molecular methods of identifying AMR in Bangladesh. The discordance of genotype and phenotype resistance necessitates further studies to ensure precision diagnosis, careful selection of antimicrobials, and rational therapeutic decisions to reduce future AMR rates. Possession of mutation in the QRDR confers quinolone resistance in uropathogens independently. The findings suggest urgent surveillance and national and global antimicrobial stewardship interventions as part of the NAP in Bangladesh to guide future management.
This study’s initiatives and protocols can help design further point prevalence surveys (PPS) from more sentinel sites to collect data on resistance and usage of quinolones and other antibiotics. Similar PPS studies can provide a relatively quick assessment of AMR or antimicrobial uses in low-resource settings where continuous surveillance is challenging to enhance future care. Notably, the protocols developed in this study can be applied to establish and maintain surveillance systems to collect and use data on AMR and antimicrobial use in hospitals and communities where most antimicrobials are used, and unbiased AMR rates are unknown chiefly.
CONFLICT OF INTEREST
None.
FUNDING
Professor Shamsun Nahar received research funding from the Grants for Advanced Research in Education (GARE), Bangladesh’s Ministry of Education (LS2017576). This grant had provided support in study design, data collection, and laboratory investigation. It did not secure any part to publish any manuscript. This study was further supported by a research grant from the Bangladesh Academy of Sciences, United States Department of Agriculture (BAS-USDA Endowment Fund, Fourth Phase) awarded to Dr. Salequl Islam (Award ID: JU HN28). This grant provided supports in laboratory investigation partly. The authors would like to thank the study participants for their active support.
CONSENT TO PARTICIPATE
All authors reviewed and approved the final version and have agreed to be accountable for all aspects of the work, including any issues related to accuracy or integrity
AUTHOR CONTRIBUTIONS
Conceptualization: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Data curation: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Formal analysis: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Funding acquisition: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Methodology: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Project administration: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Supervision: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Visualization: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Writing–original draft: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG; Writing–review and editing: TAN, ULU, ABMMKI, BA, SN, ASMM, HL, SK, DJ, NAAR, MH, SI, BG.
DISCLOSURE
The authors declare that they do not have any monetary connection or relationships with any organization, association, or entity directly or indirectly with the subject matter or materials presented in this article. This includes honoraria, expert testimony, employment, ownership of stocks or options, patents or grants received or pending, or royalties.
DATA SHARING
The data supporting the findings of this study will be made available by the corresponding author, SI, upon reasonable request
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abd El Salam M, Gamal D, El Said M, Aitta AA, El Gamal MS. Plasmid-mediated resistance genes among ciprofloxacin-resistant enterobacteriaceae isolates in neonatal and pediatric intensive care units. Int J Pharm Res Technol, 2020; 10:1–9.
Adam HJ, Hoban DJ, Gin AS, Zhanel GG. Association between fluoroquinolone usage and a dramatic rise in ciprofloxacin-resistant Streptococcus pneumoniae in Canada, 1997–2006. Int J Antimicrob Agents, 2009; 34:82–5. CrossRef
Alghadeer S, Aljuaydi K, Babelghaith S, Alhammad A, Alarifi MN. Self-medication with antibiotics in Saudi Arabia. Saudi Pharm J, 2018; 26:719–24. CrossRef
Almalki ZS, Alahmari AK, Guo JJ, Cavanaugh TM. Off-label use of oral fluoroquinolone antibiotics in outpatient settings in the United States, 2006 to 2012. Pharmacoepidemiol Drug Saf, 2016; 25:1042–51. CrossRef
Andersson MI, MacGowan AP. Development of the quinolones. J Antimicrob Chemother, 2003; 51 Suppl 1:1–11. CrossRef
Ara B, Urmi UL, Haque TA, Nahar S, Rumnaz A, Ali T, Alam MS, Mosaddek ASM, Rahman NAA, Haque M, Islam S. Detection of mobile colistin-resistance gene variants (mcr-1 and mcr-2) in urinary tract pathogens in Bangladesh: the last resort of infectious disease management colistin efficacy is under threat. Expert Rev Clin Pharmacol, 2021; 14(4):513–22. CrossRef
Aslam A, Gajdács M, Zin CS, Ab Rahman NS, Ahmed SI, Zafar MZ, Jamshed S. Evidence of the practice of self-medication with antibiotics among the lay public in low-and middle-income countries: a scoping review. Antibiotics, 2020a; 9:597. CrossRef
Aslam A, Gajdács M, Zin CS, Binti Abd Rahman NS, Ahmed SI, Jamshed SQ. Public awareness and practices towards self-medication with antibiotics among the Malaysian population. A development of questionnaire and pilot-testing. Antibiotics, 2020b; 9:97. CrossRef
Ayobola ED, Oscar WO, Ejovwokoghene EF. Occurrence of plasmid-mediated fluoroquinolone resistance genes amongst enteric bacteria isolated from human and animal sources in Delta State, Nigeria. AIMS Microbiol, 2021; 7:75. CrossRef
Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control, 2017; 6:1–8. CrossRef
Banerjee T, Anupurba S. Risk factors associated with fluoroquinolone-resistant enterococcal urinary tract infections in a tertiary care university hospital in North India. Indian J Med Res, 2016; 144:604.
Begum YA, Talukder KA, Azmi IJ, Shahnaij M, Sheikh A, Sharmin S, Svennerholm AM, Qadri F. Resistance pattern and molecular characterization of enterotoxigenic Escherichia coli (ETEC) strains isolated in Bangladesh. PLoS One, 2016; 11:e0157415. CrossRef
Behzadifar M, Behzadifar M, Aryankhesal A, Ravaghi H, Baradaran HR, Sajadi HS, Khaksarian M, Bragazzi NL. Prevalence of self-medication in university students: systematic review and meta-analysis. East Mediterr Health J, 2020; 26:846–57. CrossRef
Belachew SA, Hall L, Selvey LA. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control, 2021; 10:1–15. CrossRef
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res, 2000; 28:235–42. CrossRef
Betitra Y, Teresa V, Miguel V, Abdelaziz T. Determinants of quinolone resistance in Escherichia coli causing community-acquired urinary tract infection in Bejaia, Algeria. Asian Pac J Trop Med, 2014; 7:462–7. CrossRef
Blondeau JM. Expanded activity and utility of the new fluoroquinolones: a review. Clin Ther, 1999; 21:3–40. CrossRef
Britto CD, Dyson ZA, Duchene S, Carter MJ, Gurung M, Kelly DF, Murdoch DR, Ansari I, Thorson S, Shrestha S, Adhikari N. Laboratory and molecular surveillance of pediatric typhoidal Salmonella in Nepal: antimicrobial resistance and implications for vaccine policy. PLoS Neglect Trop Dis, 2018; 12:e0006408. CrossRef
Brown PD. Ciprofloxacin for the management of urinary tract infection. Women’s Health, 2006; 2:509–16. CrossRef
Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in pediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ, 2016; 352:i939. CrossRef
Bush NG, Evans-Roberts K, Maxwell A. DNA Topoisomerases. EcoSal Plus. 2015;6(2). doi: 10.1128/ecosalplus.ESP-0010-2014. CrossRef
Cao X, Cavaco LM, Lv Y, Li Y, Zheng B, Wang P, Hasman H, Liu Y, Aarestrup FM. Molecular characterization and antimicrobial susceptibility testing of Escherichia coli isolates from patients with urinary tract infections in 20 Chinese hospitals. J Clin Microbiol, 2011; 49:2496–501. CrossRef
Cattoir V, Poirel L, Rotimi V, Soussy CJ, Nordmann P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother, 2007; 60:394–7. CrossRef
Chautrakarn S, Khumros W, Phutrakool P. Self-medication with over-the-counter medicines among the working age population in metropolitan areas of Thailand. Front Pharmacol, 2021; 12:726643. CrossRef
Cheung TK, Chu YW, Chu MY, Ma CH, Yung RW, Kam KM. Plasmid-mediated resistance to ciprofloxacin and cefotaxime in clinical isolates of Salmonella enterica serotype Enteritidis in Hong Kong. J Antimicrob Chemother, 2005; 56:586–9. CrossRef
Chong Y, Shimoda S, Yakushiji H, Ito Y, Aoki T, Miyamoto T, Kamimura T, Shimono N, Akashi K.. Clinical impact of fluoroquinolone-resistant Escherichia coli in the fecal flora of hematological patients with neutropenia and levofloxacin prophylaxis. PLoS One, 2014; 9:e85210. CrossRef
Chu A, Wang D, Guo Q, Lv Z, Yuan Y, Gong Y. Molecular detection of H. pylori antibiotic-resistant genes and molecular docking analysis. FASEB J, 2020; 34:610–8. CrossRef
Colodner R, Kometiani I, Chazan B, Raz R. Risk factors for community-acquired urinary tract infection due to quinolone-resistant E. coli. Infection, 2008; 36:41–5. CrossRef
Correia S, Poeta P, Hébraud M, Capelo JL, Igrejas G. Mechanisms of quinolone action and resistance: where do we stand? J Med Microbiol, 2017; 66:551–9. CrossRef
Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One, 2019; 14:e0220265. CrossRef
Dalhoff A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis, 2012; 2012:976273. CrossRef
Darj E, Newaz MS, Zaman MH. Pharmacists’ perception of their challenges at work, focusing on antimicrobial resistance: a qualitative study from Bangladesh. Glob Health Action, 2019; 12:1735126. CrossRef
Davidson R, Cavalcanti R, Brunton JL, Bast DJ, de Azavedo JC, Kibsey P, Fleming C, Low DE. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med, 2002; 346:747–50. CrossRef
de Lastours V, Chau F, Roy C, Larroque B, Fantin B. Emergence of quinolone resistance in the microbiota of hospitalized patients treated or not with a fluoroquinolone. J Antimicrob Chemother, 2014; 69:3393–400. CrossRef
de Souza da-Silva AP, de Sousa VS, de Araújo Longo LG, Caldera S, Baltazar ICL, Bonelli RR, Santoro-Lopes G, Riley LW, Moreira BM. Prevalence of fluoroquinolone-resistant and broad-spectrum cephalosporin-resistant community-acquired urinary tract infections in Rio de Janeiro: impact of Escherichia coli genotypes ST69 and ST131. Infect Genet Evol, 2020; 85:104452. CrossRef
Dehbanipour R, Khanahmad H, Sedighi M, Bialvaei AZ, Faghri J. High prevalence of fluoroquinolone-resistant Escherichia coli strains isolated from urine clinical samples. J Prev Med Hyg, 2019; 60:E25.
Do NTT, Vu HTL, Nguyen CTK, Punpuing S, Khan WA, Gyapong M, Asante KP, Munguambe K, Gómez-Olivé FX, John-Langba J, Tran TK, Sunpuwan M, Sevene E, Nguyen HH, Ho PD, Matin MA, Ahmed S, Karim MM, Cambaco O, Afari-Asiedu S, Boamah-Kaali E, Abdulai MA, Williams J, Asiamah S, Amankwah G, Agyekum MP, Wagner F, Ariana P, Sigauque B, Tollman S, van Doorn HR, Sankoh O, Kinsman J, Wertheim HFL. Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob Health, 2021; 9:e610–9. CrossRef
Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev, 1997; 61:377–92. CrossRef
Eliopoulos GM. Quinolone resistance mechanisms in pneumococci. Clin Infect Dis, 2004; 38:S350–6. CrossRef
Faqihi AHMA, Sayed SF. Self-medication practice with analgesics (NSAIDs and acetaminophen), and antibiotics among nursing undergraduates in University College Farasan Campus, Jazan University, KSA. Ann Pharm Fr, 2021; 79(3):275–85. CrossRef
Ferrara AM. New fluoroquinolones in lower respiratory tract infections and emerging patterns of pneumococcal resistance. Infection, 2005; 33:106–14. CrossRef
Ferrari R, Galiana A, Cremades R, Rodríguez JC, Magnani M, Tognim MC, Oliveira TC, Royo G. Plasmid-mediated quinolone resistance (PMQR) and mutations in Brazil’s topoisomerase genes of Salmonella enterica strains. Braz J Microbiol, 2013; 44:657–62. CrossRef
Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PloS One, 2017; 12:e0189621. CrossRef
Fuller JD, Low DE. A review of Streptococcus pneumoniae infection treatment failures associated with fluoroquinolone resistance. Clin Infect Dis, 2005; 41:118–21. CrossRef
Gillani AH, Chang J, Aslam F, Saeed A, Shukar S, Khanum F, Jairoun A, Nicholson A, Mohamed Ibrahim MI, Fang Y. Public knowledge, attitude, and practice regarding antibiotics use in Punjab, Pakistan: a cross-sectional study. Expert Rev Anti Infect Ther, 2021; 19:399–411. CrossRef
Godman B, Egwuenu A, Haque M, Malande OO, Schellack N, Kumar S, Saleem Z, Sneddon J, Hoxha I, Islam S, Mwita J, do Nascimento RCRM, Dias Godói IP, Niba LL, Amu AA, Acolatse J, Incoom R, Sefah IA, Opanga S, Kurdi A, Chikowe I, Khuluza F, Kibuule D, Ogunleye OO, Olalekan A, Markovic-Pekovic V, Meyer JC, Alfadl A, Phuong TNT, Kalungia AC, Campbell S, Pisana A, Wale J, Seaton RA. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life, 2021; 11:528. CrossRef
Gravningen K, Field N, Blix HS, Asfeldt AM, Småbrekke L. Non-prescription purchase of antibiotics during travel abroad among a general adult population in Norway: findings from the seventh Tromsø Study. PloS One, 2020; 15:e0228792. CrossRef
Gutierrez A, Stokes JM, Matic I. Our evolving understanding of the mechanism of quinolones. Antibiotics, 2018; 7:32. CrossRef
Haque M, Godman B. Potential strategies to improve antimicrobial utilization in hospitals in Bangladesh building on experiences across developing countries. Bangla J Med Sci, 2021a; 20:469–77. CrossRef
Haque M, Godman B. Potential strategies to reduce inappropriate prescribing and dispensing of antimicrobials in Bangladesh building on the experiences in other developing countries. Bangla J Med Sci, 2021b; 20(4):700–6. CrossRef
Haque M, Islam S, Iqbal S, Urmi UL, Kamal ZM, Rahman A, Kamal M, Haque M, Jahan I, Islam Z, Hossain MM. Availability and price changes of potential medicines and equipment for the prevention and treatment of COVID-19 among pharmacy and drug stores in Bangladesh; findings and implications. Bangla J Med Sci, Special Issue on Covid19. 2020: S36-S50 CrossRef
Haque M, Rahman NAA, McKimm J, Binti Abdullah SL, Islam MZ, Zulkifli Z, Saidin NB, Azhar NIK, Binti Lutfi SNN, Binti Othman NSA. A cross-sectional study evaluating the knowledge and beliefs about and the use of antibiotics amongst Malaysian university students. Expert Rev Anti Infect Ther, 2019a; 17:275–84. CrossRef
Haque M, Rahman NAA, McKimm J, Kibria GM, Azim Majumder MA, Haque SZ, Islam MZ, Binti Abdullah SL, Daher AM, Zulkifli Z, Rahman S, Kabir R, Lutfi SNNB, Aishah Binti Othman NS. Self-medication of antibiotics: investigating practice among university students at the Malaysian National Defence University. Infect Drug Resist, 2019b; 12:1333. CrossRef
Haque M, Sartelli M, McKimm J, Abu Bakar M. Healthcare-associated infections–an overview. Infect Drug Resist, 2018; 11:2321. CrossRef
Hersh AL, Gerber JS, Hicks LA, Pavia AT. Lessons learned in antibiotic stewardship: fluoroquinolone use in pediatrics. Journal of the Pediatric Infectious Diseases Society, 2015; 4:57–9.
Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol, 2019; 17:3. CrossRef
Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, Guerin PJ, Piddock LJ. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet, 2016; 387:176–87. CrossRef
Hooda Y, Sajib MSI, Rahman H, Luby SP, Bondy-Denomy J, Santosham M, Andrews JR, Saha SK, Saha S. Molecular mechanism of azithromycin resistance among typhoidal Salmonella strains in Bangladesh identified through passive pediatric surveillance. PLoS Negl Trop Dis, 2019; 13:e0007868. CrossRef
Hooper DC, Jacoby GA. Mechanisms of drug resistance: quinolone resistance. Annals of the New York Academy of Sciences, 2015; 1354:12. CrossRef
Hooper DC, Jacoby GA. Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med, 2016; 6:a025320. CrossRef
Jacobs TG, Robertson J, van den Ham HA, Iwamoto K, Pedersen HB, Mantel-Teeuwisse AK. Assessing the impact of law enforcement to reduce over-the-counter (OTC) sales of antibiotics in low-and middle-income countries; a systematic literature review. BMC Health Serv Res, 2019; 19:1–15. CrossRef
Jacoby GA, Strahilevitz J, Hooper DC. Plasmid-mediated quinolone resistance. Microbiol Spectr, 2014; 2:10. CrossRef
Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis, 2005; 41:S120–6. CrossRef
Jacoby GA, Strahilevitz J, Hooper DC. Plasmid-mediated quinolone resistance. Microbiol Spectr. 2014;2(5):10.1128/microbiolspec.PLAS-0006-2013. CrossRef
Jonas D, Biehler K, Hartung D, Spitzmüller B, Daschner FD. Plasmid-mediated quinolone resistance in isolates obtained in German intensive care units. Antimicrob Agents Chemother, 2005; 49:773–5. CrossRef
Karim MR, Islam MT, Talukder B. COVID-19′ s impacts on migrant workers from Bangladesh: In search of policy intervention. World Dev, 2020; 136:105123. CrossRef
Kern WV, Klose K, Jellen-Ritter AS, Oethinger M, Bohnert J, Kern P, Reuter S, von Baum H, Marre R. Fluoroquinolone resistance of Escherichia coli at a cancer center: epidemiologic evolution and effects of discontinuing prophylactic fluoroquinolone use in neutropenic patients with leukemia. Eur J Clin Microbiol Infect Dis, 2005; 24:111–8. CrossRef
Kern WV, Andriof E, Oethinger M, Kern P, Hacker J, Marre R. Emergence of fluoroquinolone-resistant Escherichia coli at a cancer center. Antimicrob Agents Chemother, 1994; 38:681. CrossRef
Kim B, Seo MR, Kim J, Kim Y, Wie SH, Ki M, Cho YK, Lim S, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. Molecular epidemiology of ciprofloxacin-resistant Escherichia coli isolated from community-acquired urinary tract infections in Korea. Infect Chemother, 2020; 52:194. CrossRef
Kim HB, Park CH, Kim CJ, Kim EC, Jacoby GA, Hooper DC. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob Agents Chemother, 2009a; 53:639–45. CrossRef
Kim HB, Park CH, Kim CJ, Kim EC, Jacoby GA, Hooper DC. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob Agents Chemother, 2009b; 53:639. CrossRef
Kim JH, Lee HJ, Jeong OM, Kim DW, Jeong JY, Kwon YK, Kang MS. High prevalence and variable fitness of fluoroquinolone-resistant avian pathogenic Escherichia coli isolated from chickens in Korea. Avian Pathol, 2021; 50:151–60. CrossRef
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res, 2019; 47:D1102–9. CrossRef
Kirkegaard K, Wang JC. Mapping the topography of DNA wrapped around gyrase by nucleolytic and chemical probing of complexes of unique DNA sequences. Cell, 1981; 23:721–9. CrossRef
Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Nat Acad Sci, 2018; 115:E3463–70. CrossRef
Komp Lindgren P, Karlsson A, Hughes D. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrobial agents and chemotherapy, 2003; 47:3222–32. CrossRef
Kotb DN, Mahdy WK, Mahmoud MS, Khairy RMM. Impact of co-existence of PMQR genes and QRDR mutations on fluoroquinolones resistance in Enterobacteriaceae strains isolated from community and hospital acquired UTIs. BMC Infect Dis, 2019; 19:1–8. CrossRef
Krieger E, Vriend G. YASARA view—molecular graphics for all devices—from smartphones to workstations. Bioinformatics, 2014; 30:2981–2. CrossRef
Lautenbach E, Metlay JP, Mao X, Han X, Fishman NO, Bilker WB, Tolomeo P, Wheeler M, Nachamkin I. The prevalence of fluoroquinolone resistance mechanisms in colonizing Escherichia coli isolates recovered from hospitalized patients. Clin Infect Dis, 2010; 51:280–5. CrossRef
Lee DS, Lee SJ, Choe HS. Community-acquired urinary tract infection by Escherichia coli in the era of antibiotic resistance. Biomed Res Int. 2018;2018:7656752. CrossRef
Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med, 2005; 118:259–68. CrossRef
Linhares I, Raposo T, Rodrigues A, Almeida A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: a ten-year surveillance study (2000–2009). BMC Infect Dis, 2013; 13:1–14. CrossRef
Mammeri H, Van De Loo M, Poirel L, Martinez-Martinez L, Nordmann P. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob Agents Chemother, 2005; 49:71–6. CrossRef
Mandal J, Acharya NS, Buddhapriya D, Parija SC. Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin-resistant Escherichia coli. Indian J Med Res, 2012; 136:842.
Mandal NK, Rauniyar GP, Rai DS, Panday DR, Kushwaha R, Agrawal SK, Regmee P. Self-medication practice of antibiotics among medical and dental undergraduate students in a medical college in Eastern Nepal: a descriptive cross-sectional atudy. JNMA J Nepal Med Assoc, 2020; 58:328. CrossRef
Mandell L, Tillotson G. Safety of fluoroquinolones: an update. Can J Infect Dis, 2002; 13:54–61. CrossRef
Martínez-Martínez L, Pascual A, Jacoby GA. Quinolone resistance from a transferable plasmid. Lancet, 1998; 351:797–9. CrossRef
Mdluli K, Ma Z. Mycobacterium tuberculosis DNA gyrase as a target for drug discovery. Infect Disord Drug Targets, 2007; 7:159–68. CrossRef
Mérens A, Matrat S, Aubry A, Lascols C, Jarlier V, Soussy CJ, Cavallo JD, Cambau E. The pentapeptide repeat proteins MfpAMt and QnrB4 exhibit opposite effects on DNA gyrase catalytic reactions and on the ternary gyrase-DNA-quinolone complex. J Bacteriol, 2009; 191:1587–94. CrossRef
Minarini LA, Poirel L, Cattoir V, Darini AL, Nordmann P. Plasmid-mediated quinolone resistance determinants among enterobacterial isolates from outpatients in Brazil. J Antimicrob Chemother, 2008; 62:474–8. CrossRef
Mitra S, Mukherjee S, Naha S, Chattopadhyay P, Dutta S, Basu S. Evaluation of co-transfer of plasmid-mediated fluoroquinolone resistance genes and bla NDM gene in Enterobacteriaceae causing neonatal septicemia. Antimicrob Resist Infect Control, 2019; 8:1–15. CrossRef
Momanyi L, Opanga S, Nyamu D, Oluka M, Kurdi A, Godman B. Antibiotic prescribing patterns at a leading referral hospital in Kenya: a point prevalence survey. J Res Pharm Pract, 2019;8:149. CrossRef
Moon DC, Seol SY, Gurung M, Jin JS, Choi CH, Kim J, Lee YC, Cho DT, Lee JC. Emergence of a new mutation and its accumulation in the topoisomerase IV gene confers high levels of resistance to fluoroquinolones in Escherichia coli isolates. Int J Antimicrob Agents, 2010; 35:76–9. CrossRef
Moran J, Fitch TJ, Villanueva G, Quadir MM, Chien LC, Alamgir H. Urinary symptoms and infections among female garment factory workers in Bangladesh. Work, 2020; 65(4):1–12. CrossRef
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem, 2009;30:2785–91. CrossRef
Nambasa V, Ndagije HB, Serwanga A, Manirakiza L, Atuhaire J, Nakitto D, Kiguba R, Figueras A. Prescription of levofloxacin and moxifloxacin in select hospitals in Uganda: a pilot study to assess guideline concordance. Antibiotics, 2020;9:439. CrossRef
Nazic H, Poirel L, Nordmann P. Further identification of plasmid-mediated quinolone resistance determinant in Enterobacteriaceae in Turkey. Antimicrob Agents Chemother, 2005;49:2146–7. CrossRef
Nepal G, Bhatta S. Self-medication with antibiotics in WHO Southeast Asian Region: a systematic review. Cureus, 2018;10:e2428. CrossRef
Ng EY, Trucksis M, Hooper DC. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother, 1996;40:1881–8. CrossRef
Nordmann P, Poirel L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J Antimicrob Chemother, 2005; 56:463–9. CrossRef
Ode T, Saito R, Kumita W, Sato K, Okugawa S, Moriya K, Koike K, Okamura N. Analysis of plasmid-mediated multidrug resistance in Escherichia coli and Klebsiella oxytoca isolates from clinical specimens in Japan. Int J Antimicrob Agents, 2009; 34:347–50. CrossRef
Odoki M, Aliero AA, Tibyangye J, Maniga JN, Eilu E, Ntulume I, Wampande E, Kato CD, Agwu E, Bazira J. Fluoroquinolone resistant bacterial isolates from the urinary tract among patients attending hospitals in Bushenyi District, Uganda. Pan Afr Med J. 2020;36:60. CrossRef
Oliphant CM, Green GM. Quinolones: a comprehensive review. Am Fam Physician, 2002; 65:455.
Oram M, Fisher LM. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother, 1991; 35:387–9. CrossRef
Patel SN, McGeer A, Melano R, Tyrrell GJ, Green K, Pillai DR, Low DE; Canadian Bacterial Surveillance Network. Susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Antimicrob Agents Chemother, 2011; 55:3703. CrossRef
Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Med, 2006; 34:S20–8. CrossRef
Piddock LJ. Mechanisms of fluoroquinolone resistance: an update 1994–1998. Drugs, 1999; 58:11–8. CrossRef
Pletz MW, McGee L, Burkhardt O, Lode H, Klugman KP. Ciprofloxacin treatment failure in a patient with resistant Streptococcus pneumoniae infection following prior ciprofloxacin therapy. Eur J Clin Microbiol Infect Dis, 2005; 24:58–60. CrossRef
Poirel L, Cattoir V, Nordmann P. Plasmid-mediated quinolone resistance; interactions between human, animal, and environmental ecologies. Front Microbiol, 2012;3:24. CrossRef
Poirel L, Leviandier C, Nordmann P. Prevalence and genetic analysis of plasmid-mediated quinolone resistance determinants QnrA and QnrS in Enterobacteriaceae isolates from a French university hospital. Antimicrob Agents Chemother, 2006; 50:3992–7. CrossRef
Poirel L, Van De Loo M, Mammeri H, Nordmann P. Association of plasmid-mediated quinolone resistance with extended-spectrum β-lactamase VEB-1. Antimicrob Agents Chemother, 2005; 49:3091–4. CrossRef
Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol, 2014; 22:438–45. CrossRef
Reece RJ, Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol, 1991; 26:335–75. CrossRef
Rodriguez-Martinez JM, Poirel L, Pascual A, Nordmann P. Plasmid-mediated quinolone resistance in Australia. Microb Drug Resist, 2006; 12:99–102. CrossRef
Ruiz J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation, and DNA gyrase protection. J Antimicrob Chemother, 2003; 51:1109–17. CrossRef
Saksena R, Gaind R, Sinha A, Kothari C, Chellani H, Deb M. High prevalence of fluoroquinolone resistance amongst commensal flora of antibiotic naïve neonates: a study from India. J Med Microbiol, 2018; 67:481–8. CrossRef
Saleem Z, Hassali MA, Hashmi FK, Godman B, Saleem F. Antimicrobial dispensing practices and determinants of antimicrobial resistance: a qualitative study among community pharmacists in Pakistan. Fam Med Commun Health, 2019a; 7. CrossRef
Saleem Z, Saeed H, Hassali MA, Godman B, Asif U, Yousaf M, Ahmed Z, Riaz H, Raza SA. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: a longitudinal surveillance and implications. Antimicrob Resist Infect Control, 2019b; 8:1–7. CrossRef
Santiso R, Tamayo M, Fernández JL, del Carmen Fernández M, Molina F, Villanueva R, Gosálvez J, Bou G. Rapid and simple determination of ciprofloxacin resistance in clinical strains of Escherichia coli. J Clin Microbiol, 2009; 47:2593–5. CrossRef
Sedighi I, Arabestani MR, Rahimbakhsh A, Karimitabar Z, Alikhani MY. Dissemination of extended-spectrum β-lactamases and quinolone resistance genes among clinical isolates of uropathogenic Escherichia coli in children. Jundishapur J Microbiol, 2015; 8:e19184. CrossRef
Shamsudeen SM, Priya RS, Sujatha G, Muruganandhan J, Manikandan K. Self-medication with antibiotics: A knowledge, attitude, and practice appraisal of 610 dental patients in Chennai, India, from 2016 to 2017. J Educ Health Promot, 2018; 7:66. CrossRef
Shetty SS, Deekshit VK, Jazeela K, Vittal R, Rohit A, Chakraborty A, Karunasagar I. Plasmid-mediated fluoroquinolone resistance associated with extra-intestinal Escherichia coli isolates from hospital samples. Indian J Med Res, 2019; 149:92. CrossRef
Shrestha JTM, Kushwaha DK, Tiwari S, Bhattarai P. study of self-medication among first and seventh semester medical and dental undergraduate students of tertiary care teaching hospital in nepal: a descriptive cross-sectional study. JNMA J Nepal Med Assoc, 2021; 59:55. CrossRef
Singh T, Singh PK, Dar SA, Haque S, Akhter N, Das S. Changing paradigm of antibiotic resistance amongst Escherichia coli isolates in Indian pediatric population. PloS One, 2019; 14:e0213850. CrossRef
Smith AL, Brown J, Wyman JF, Berry A, Newman DK, Stapleton AE. Treatment and prevention of recurrent lower urinary tract infections in women: a rapid review with practice recommendations. J Urol, 2018;200:1174–91. CrossRef
Stapleton AE, Wagenlehner FME, Mulgirigama A, Twynholm M. Escherichia coli resistance to fluoroquinolones in community-acquired uncomplicated urinary tract infection in women: a systematic review. Antimicrob Agents Chemother, 2020; 64:e00862–20. CrossRef
Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev, 2009; 22:664–89. CrossRef
Takiff H, Guerrero E. Current prospects for the fluoroquinolones as first-line tuberculosis therapy. Antimicrob Agents Chemother, 2011; 55:5421. CrossRef
Tamang MD, Nam HM, Chae MH, Kim SR, Gurung M, Jang GC, Jung SC, Lim SK. Prevalence of plasmid-mediated quinolone resistance determinants among Escherichia coli isolated from food animals in Korea. Foodborne Pathog Dis, 2012; 9:1057–63. CrossRef
Tchesnokova V, Radey M, Chattopadhyay S, Larson L, Weaver JL, Kisiela D, Sokurenko EV. Pandemic fluoroquinolone-resistant Escherichia coli clone ST1193 emerged via simultaneous homologous recombinations in 11 gene loci. Proc Natl Acad Sci U S A, 2019; 116:14740–8. CrossRef
Terahara F, Nishiura H. Fluoroquinolone consumption and Escherichia coli resistance in Japan: an ecological study. BMC Public Health, 2019; 19:1–8. CrossRef
Tran JH, Jacoby GA. Mechanism of plasmid-mediated quinolone resistance. Proc Natl Acad Sci U S A, 2002; 99:5638–42. CrossRef
Tran JH, Jacoby GA, Hooper DC. Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob Agents Chemother, 2005; 49:118–25. CrossRef
Tran PT, Winterstein AG, Wang X, Rhew K, Antonelli PJ. Appropriateness of otic quinolone use among privately insured us patients. Otolaryngol Head Neck Surg, 2020; 162:102–7. CrossRef
Urmi UL, Jahan N, Nahar S, Rana M, Sultana F, Hossain B, Iqbal S, Hossain M, Mosaddek AS, Islam S. Gram-positive uropathogens: empirical treatment and emerging antimicrobial resistance. Biomed Res Clin Pract, 2019; 4:1–4. CrossRef
Urmi UL, Nahar S, Rana M, Sultana F, Jahan N, Hossain B, Alam MS, Mosaddek ASM, McKimm J, Rahman NAA, Islam S, Haque M. Genotypic to phenotypic resistance discrepancies identified involving β-lactamase genes, blaKPC, blaIMP, blaNDM-1, and blaVIM in uropathogenic Klebsiella pneumoniae. Infect Drug Resist, 2020; 13:2863. CrossRef
UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res, 2019; 47:D506–15. CrossRef
Vanden Broeck A, Lotz C, Ortiz J, Lamour V. Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex. Nat Commun, 2019; 10:1–12. CrossRef
van der Zee A, Roorda L, Bosman G, Ossewaarde JM. Molecular diagnosis of urinary tract infections by semi-quantitative detection of uropathogens in a routine clinical hospital setting. PloS One, 2016; 11:e0150755. CrossRef
Varughese LR, Rajpoot M, Goyal S, Mehra R, Chhokar V, Beniwal V. Analytical profiling of mutations in quinolone resistance determining region of gyrA gene among UPEC. PloS One, 2018; 13:e0190729. CrossRef
Vetting MW, Hegde SS, Fajardo JE, Fiser A, Roderick SL, Takiff HE, Blanchard JS. Pentapeptide repeat proteins. Biochemistry, 2006; 45:1–10. CrossRef
Vila J. Fluoroquinolone resistance. Frontiers in antimicrobial resistance: a tribute to Stuart B. Levy, 2005; 41–52. CrossRef
Wang M, Tran JH, Jacoby GA, Zhang Y, Wang F, Hooper DC. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob Agents Chemother, 2003; 47:2242–8. CrossRef
Weinstein MP, Lewis JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol, 2020; 58: e01864–19. CrossRef
Woodford N, Ellington MJ. The emergence of antibiotic resistance by mutation. Clin Microbiol Infect, 2007; 13:5–18.
Xiao YH, Wang J, Li Y; MOH National Antimicrobial Resistance Investigation Net. Bacterial resistance surveillance in China: a report from Mohnarin 2004–2005. European Journal of Clinical Microbiology & Infectious Diseases, 2008; 27:697–708. CrossRef
Xu P, Li X, Zhao M, Gui X, DeRiemer K, Gagneux S, Mei J, Gao Q. Prevalence of fluoroquinolone resistance among tuberculosis patients in Shanghai, China. Antimicrob Agents Chemother, 2009; 53:3170. CrossRef
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat Methods, 2015; 12:7–8. CrossRef
Zhanel GG, Walkty A, Vercaigne L, Karlowsky JA, Embil J, Gin AS, Hoban DJ. The new fluoroquinolones: a critical review. Can J Infect Dis, 1999; 10:207–38. CrossRef
Zou MX, Xia ZD, Liang XH. Antibiotic susceptibility of Neisseria gonorrhoeae epidemic strains in Changsha. Hunan yi ke da xue bao= Hunan yike daxue xuebao= Bulletin of Hunan Medical University, 2003; 28:53–5.
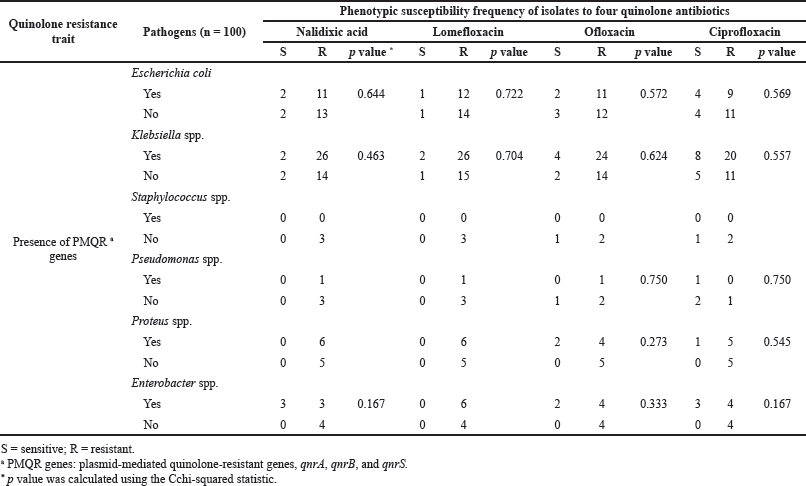 | Supplementary Table 1. Dispersal of PMQR genes and phenotypic quinolone susceptibilities of UTI pathogens. [Click here to view] |
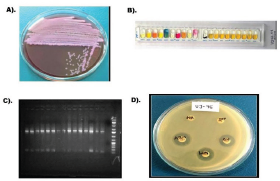 | Supplementary Figure 1. Uropathogen isolation, identification and antibiogram. A) Urine specimens were inoculated on MacConkey agar medium and incubated overnight at 37°C. Cultural characteristics of a positive-UTI were shown. B) Isolates were identified by API 20E test kits according to the manufactures’ instructions. C) Amplification of 16s rDNA gene for confirmed identification. D) Phenotypic susceptibilty analysis of quinolone antibitics to the UTI pathogenes. [Click here to view] |
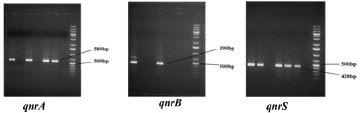 | Supplementary Figure 2. Detection of plasmid mediated quinolone resistant genes. [Click here to view] |
 | Supplementary Figure 2. Escherichia coli gyrase complex bound to inhibitor YacG (PDB ID:4TMA). [Click here to view] |