INTRODUCTION
Drug-mediated adverse effects and associated toxicities are some of the most frequently experienced problems during medication practice. Several drugs have great therapeutic advantages in the management of several disorders, but unfortunately, their pharmacological use is notably limited by their correlated adverse reactions (Gamal Helal and Said, 2020). Methotrexate (MTX) is an example of those drugs which is used as a chemotherapeutic agent for the treatment of cancer and as an immunosuppressant agent in the treatment of rheumatoid arthritis, ectopic pregnancies, and other diseases (Malaviya, 2016) MTX -mediated anticancer and immunosuppressive effects are owing to its function as an antagonist for folic acid (competitively inhibits dihydrofolate reductase, the enzyme that catalyzes the conversion of dihydrofolate to tetrahydrofolate), which in turn prevents cellular mitosis by inhibiting thymidylate, purines, and folic acid required for DNA synthesis (Gibson et al., 2019), preventing cells from dividing (Liu et al., 2019). However, acute MTX-associated vital organ toxicity, including kidney, liver, gastrointestinal mucosa, and bone marrow (Bhatnagar et al., 2015; Fridlington et al., 2011), is the rate-limiting factor for its clinical use (Malaviya, 2016). Decreasing MTX toxicities and, in turn, restricting the discontinuation of MTX therapy is a current therapeutic challenge.
Importantly, hepatotoxicity is a serious intricacy of chronic MTX therapy, which is accompanied by the elevation of liver enzymes (Sotoudehmanesh et al., 2010). Although the main mechanism(s) of MTX-mediated hepatic injury has not yet been fully elucidated, there are previous studies which hypothesized that the arising oxidative stress gives rise to the formation of a copious amount of free radicals with concomitant lipid peroxidation of biological membranes as one of the main reasons (Ekinci-Akdemir et al., 2018). Emerging evidence suggests that such oxygen-derived free radicals substantially contributed to the etiology of liver injury and other types of tissues’ destruction (Oyagbemi et al., 2016). Indeed, MTX induces oxidative stress via two pathways: first, excessive production of reactive oxygen species (ROS) through dysfunction of mitochondria and respiratory chain defects (Famurewa et al., 2017); second, through the depletion of reduced glutathione (GSH) in the attribution of inhibition of reduced nicotinamide adenine dinucleotide phosphate production (Al Maruf et al., 2018; Salimi et al., 2019). GSH is an important nonenzymatic antioxidant bioactive peptide that appears in the liver, which binds toxic substances, such as electrophilic radicals and ROS, originating from extensive oxidative effects (Chen et al., 2013). Nitric oxide (NO) is another highly interactive pro-oxidant produced by liver parenchymal and nonparenchymal cells which acts as an important mediator of several physiological and pathological events. The reactivity of NO with ROS indicates many biological cascades through which NO might either enhance or decrease oxidative stress-induced tissue injury (Ozcelik et al., 2014). The oxidative inflammatory consequence is a common feature of chronic diseases. During inflammation, the immune system cells are recruited at the injury site leading to sudden increase of cellular respiration and oxygen consumption, thus accumulating ROS at the injury site (Reuter et al., 2010). Previous studies proved that Nrf-2 contributes to the anti-inflammatory process by regulating gene expression through the antioxidant responsive elements (ARE) including heme oxygenase-1 (HO-1) and orchestrating the recruitment of inflammatory cells (Tu et al., 2019). Such inducible HO-1 catalyzes the degradation of heme (a cellular pro-oxidant toxicant) to biliverdin, carbon monoxide, and free iron. Biliverdin is subsequently metabolized to the antioxidant, bilirubin, that scavenges peroxy-radicals and attenuates lipid peroxidation (Ryter et al., 2002). In addition, HO-1 negatively regulates the inflammatory mediators, interleukin- (IL-) 6 Nuclear factor kappa B (NF-κB), and TNF-α (Aleksunes et al., 2010), as one of the proinflammatory cytokines which is increased after MTX treatment which causes neutrophil infiltration and strikes apoptotic cell death (Cure et al., 2015), in response to such oxidative stress and inflammation (Wang, 2015), with the regulation of the antiapoptotic Bcl-2 family proteins that prevent apoptosis by inhibiting the activity of proapoptotic Bcl-2 associated-X-proapoptotic protein (Bax) protein (Barclay et al., 2015).
In animals, saponins can antagonize the action of the inflammatory mediators (Sagesaka et al., 1996) and possess anticarcinogenic properties (Xu et al., 2016) due to their amphiphilic nature, which causes the destruction of cancer cells (Top et al., 2017). The bark derived from the South American soap tree, Quillaja saponaria Molina, represents the main source of Q. saponaria saponins that have been studied for more than four decades for their relevant biological activities with a wide range of applications. In traditional medicine, the Q. saponaria tree has been used orally to relieve cough/bronchitis, as well as topically to eliminate dandruff and to relieve scalp itching (Leung, 1980). The raw materials have an anti-inflammatory, hypocholesterolemic effect (Fleck et al., 2019), with strong immunostimulant activity that makes it an ideal vaccine adjuvant (Rajput et al., 2007). Historically, the powder of the inner bark has been used as a detergent (owing to the presence of a glycoside saponin) (Fleck et al., 2019). The saponin of the Quillaja bark is a triterpenoid saponin glycoside comprising a hydrophilic sugar moiety and hydrophobic aglycone backbone (sapogenin). The main sapogenin that is most commonly reported is a triterpene termed quillaic acid (Guo and Kenne, 2000; Guo et al., 1998; Osbourn et al., 2011). The Q. saponaria bark saponin (QBS) is structurally distinct from other triterpenoid saponins by exhibiting a fatty acid and an aldehyde group at C4 of the triterpene (Kensil et al., 1995). Although most known saponins cause hemolysis of blood cells (Top et al., 2017), the triterpenoid QBS was reported, interestingly, to be relatively nonhemolytic, with immunostimulant and antioxidant effects (Ahmed Abdel-Reheim et al., 2017). In addition, QBS has been demonstrated to have in vitro antimicrobial (Hassan et al., 2010) and anticancer (Hassan et al., 2013; Hu et al., 2010) activities. Moreover, quillaic acid demonstrated powerful topical in vivo anti-inflammatory efficacy in different mouse models (Rodríguez-Díaz et al., 2011).
The MTX-induced hepatic, hematologic, and immune complications along with the QBS-mediated anti-inflammatory-, antioxidant-, and immune-stimulant effects are observations which attract our attention to the possibility of using QBS as an adjuvant therapy with MTX. In fact, no studies, to date, have investigated the role of Q. saponaria bark extracts in alleviating MTX-mediated liver injury. Accordingly, we tried to build a rationale for using such saponin to counteract the deleterious effects of MTX, and the current study was designed to investigate the virtual hepatoprotective activity of the saponin derived from the Q. saponaria bark against MTX-mediated liver injury and to highlight some of the possible molecular mechanisms underlying these effects. To fulfill such aims, biochemical markers illustrating the loss of liver integrity, including serum aspartate aminotransferase (AST), Serum alanine aminotransferase (ALT), serum lactate dehydrogenase (LDH), and serum alkaline phosphatase (ALP), were assessed, and tissue redox biomarkers, MDA, GSH, and total nitric oxide (NOx) content, were determined. Furthermore, the levels of hepatic Bcl-2 and Bax mRNA expression were assessed for determining the level of apoptosis, and histological studies were executed to assess the extent of histopathological lesions associated with adopted treatments. Finally, histochemical immunoreactivity of Nrf-2/HO-1 proteins was utilized to validate the involvement of the inflammatory responses of the designated treatments.
MATERIALS AND METHODS
Ethics statement
All procedures in the current study were approved by the Research Ethics Committee of the Experimental Animals Use and Care, Faculty of Pharmacy, Beni-Suef University (REC-A-PhBSU). The experimental protocol of laboratory animals handling was in accordance with the approved guidelines of the Animal House Rules of the Dept. of Pharmacology and Toxicology, Section B, Faculty of Pharmacy, Beni-Suef University in 2009, that follows the conventional guidelines of the National Institutes of Health (NIH), Eighth Edition, The National Academies Press, Washington, DC, revised in 1985, for the care and use of laboratory animals.
Animals
Male Wistar rats (weight, 150–180 g; age, 6–8 weeks) were obtained from the National Research Center, Cairo, Egypt. The rats were kept in plastic cages and maintained in an animal care facility under standard conditions of humidity, temperature (25°C ± 1°C), and 12 hours light/dark cycles. Rats were allowed free food/water access and were left for 7 days before the treatment to acclimatize.
Drugs
Methotrexate
MTX was obtained from Baxter Company (Cairo, Egypt) and was singly injected into the animals (i.p., at a dose of 20 mg/kg on the fifth day) (Fouad et al., 2020).
Saponin from Q. saponaria Molina
QBS powder was purchased from Sigma-Aldrich (St. Louis, MO). After dissolving it in normal saline, the resultant solution was given orally to the animals (at a dose of 100 mg/kg/day, for 10 days) (Ahmed Abdel-Reheim et al., 2017). The main aglycone (sapogenin) that is most commonly reported in QBS is the triterpenoid, quillaic acid, a pentacyclic triterpene, a hydroxy monocarboxylic acid, and an aldehyde of predominantly 30-carbon atoms of the Δ12-oleanane type (C30H46O5), that is characterized chemically and using nuclear magnetic resonance spectroscopy and mass spectrometric methods (Guo et al., 1998). QBS is soluble in water with an average MW of 56,000. QBS solutions are stable for about 1 month when stored at 2°C–8°C, while QBS powder is stable for at least 1 year at room temperature when stored dry (Sigma Product No. S4521).
Preparation of the sapogenin
According to SIGMA Product Specifications, sapogenin devoid of any sugars is isolated from the bark of the South American soap tree, Q. saponaria Molina (Rosaceae family), by acid hydrolysis of saponins, and purified by ultrafiltration to reduce low-molecular-weight contaminants (Dalsgaard, 1978). The sapogenin content is ≈35%. The isolation, quantification, and quality control of the purified acylated triterpenoid saponin were reported in the Product Information Data Sheet (Sigma Product No. S4521).
Chemicals and kits
AST and ALT reagents kits were purchased from Diamond Diagnostics, Cairo, Egypt. ALP kits were obtained from Biodiagnostics, Cairo, Egypt. LDH kits were obtained from Biosystems (Barcelona, Spain). N-(1-Naphthyl) ethylenediamine dihydrochloride, Ellman’s reagent, MDA, reduced GSH, and thiobarbituric acid were obtained from Sigma-Aldrich (St. Louis, MO). 3,3?-Diaminobenzidine tetrahydrochloride (DAB) was obtained from Vector Laboratories Inc., Burlingame, CA. Nrf-2 and HO-1 rabbit polyclonal and monoclonal antibodies, respectively, were obtained from Abcam Biochemicals (Cambridge, MA). The GF-1 total RNA extraction kit was obtained from Vivantis Technologies Sdn Bhd (Malaysia). The complementary DNA (cDNA) synthesis kit was obtained from Bio-Rad, CA. All other used chemicals were of the highest standard analytical grade.
Experimental design
Thirty two rats were randomized into four groups, eight rats in each. Group I served as control rats and received oral 0.9% normal saline (vehicle of saponin; 0.2 ml/day, for 10 consecutive days), in addition to a single i.p. injection of 0.9% saline (vehicle of MTX) on day 5. Group II of rats (saponin alone-treated group, QBS) was administered oral QBS (100 mg/kg/day) dissolved in normal saline for 10 consecutive days, with an additional single i.p. injection of 0.9% saline on day 5 only. Group III of rats was administered oral 0.9% normal saline (for 10 consecutive days), in addition to a single i.p. injection of MTX (20 mg/kg) on the fifth day of the experiment. In group IV (MTX + QBS), the rats received the saponin (100 mg/kg/day, p.o., for 10 consecutive days) along with a single i.p. dose of MTX (20 mg/kg) on the fifth day of the experiment, 2 hours before saponin administration.
Twenty four hours after the last dose of the saponin treatment, animals were anesthetized using thiopental sodium (i.p., 75 mg/kg), and the blood samples were withdrawn using heparinized microcapillary tubes from the retroorbital plexus, collected in centrifuge tubes, left at room temperature to coagulate and put in a water bath at 37°C for 10 minutes and then centrifuged at 1,000 g for 20 minutes. After centrifugation, the separated serums were collected and stored at −20°C until the assessment of AST, ALT, ALP, and LDH levels (Kiran et al., 2012). After blood assembling, rats were sacrificed by cervical dislocation, and the livers were immediately dissected out and washed three times with ice-cooled normal saline (Kiran et al., 2012). Each liver was cut up into three sections. One section was fixed in 10% phosphate-buffered formalin for histopathological and immunohistochemical examinations of Nrf-2/HO-1. The second part was homogenized (1/5 w/v) in ice-cold Tris-HCl buffer (0.1 M, pH 7.4) for preparation of 20% tissue homogenates and was kept at −20°C for biochemical assays. The third part was shock-frozen in liquid nitrogen and was kept at −80°C for quantitative real-time-polymerase chain reaction (qRT-PCR) analysis for determination of Bcl-2 and Bax mRNA expression levels. This model was adopted after Mehrzadi et al. (2018) with slight modifications.
Biochemical assays
Serum biomarkers assessments
Serum biomarkers were determined using commercial kits; serum AST and serum ALT were determined according to the Reitman and Frankel (1957) method; serum ALP was determined according to the Belfield and Goldberg (1971) method; serum LDH was determined according to the Vassault (1983) method.
Oxidative stress parameters assessments
To prepare a 20% tissue homogenate, 1 g of each liver was homogenized with five volumes of ice-cooled phosphate-buffered saline (PBS) using an IKA homogenizer (Model T 25 ULTRA-TURRAX, Staufen, Germany). About 2 ml from each of the obtained homogenates was moved into a 2 ml Eppendorf tube and centrifuged (at 700× g), and the obtained supernatants were used for the determination of hepatic GSH, according to the Sedlak and Lindsay (1968) method; hepatic MDA, according to the Uchiyama and Mihara (1978) method; and NO content, according to the Miranda et al.’s (2001) method.
Histological investigations
Histopathological analysis
The isolated portions of the liver were washed using normal saline, kept in 10% phosphate-buffered formalin for 72 hours in well-sealed containers, dehydrated with a sequence of graded alcohol solutions, cleared in xylene, and infiltrated by Paraplast tissue embedding media (Sigma-Aldrich, St. Louis, MO). Paraffin-embedded tissue blocks were sectioned (using a sledge microtome), and 4 μ thick sections were put on glass slides, deparaffinized, stained with hematoxylin and eosin dye, and coded for blind assessment of microscopic analysis of liver histology under a light microscope (Leica Microsystems GmbH, Wetzlar, Germany). The liver injury was assessed by a hepatopathologist who was unaware of the treatments that had been received. For semiquantitative evaluation of common indices (e.g., degenerative hepatocytes, congestion of hepatic sinusoids/vasculature, inflammatory cell infiltration, and cellular apoptosis/necrosis), the scoring system was adopted as follows: 0> normal, 1> mild, 2> moderate, and 3> severe, and the total score was determined by averaging all scores of each group (Michael, 2008). The microphotomicrographs (×100 magnification) were encoded on 24 bits/pixel on three channels (blue, green, and red), and the representative-colored images were shown. All standard procedures for samples fixation and staining were according to Culling (1974).
Immunohistochemical determination of Nrf-2 and HO-1
Immunohistochemical staining was conducted as follows: Paraffin-embedded 4 μm thickness tissue sections of different groups were deparaffinized and rehydrated. The endogenous activity of peroxidase was occluded by adding 3% H2O2 for 20 minutes, followed by a 15 minutes wash in PBS. Antigens were retrieved by placing the sections in a 10 mM sodium citrate buffer (pH 6.0) and warmed up at 121°C in a hot oven for 30 minutes. Then, sections were cooled at room temperature for 20 minutes and rinsed in PBS. For blocking nonspecific protein binding, sections were incubated for 1 hour in Tris-buffered saline (containing 5% bovine serum albumin). Sections were then incubated overnight (at 4°C) with the primary rabbit polyclonal antibody against rat Nrf-2 from Abcam Co. (ab92946) or rabbit monoclonal antibody against rat HO-1 from Abcam Co. (ab13248) (dilution 1:2,000). The slides were then washed by PBS before incubation with the secondary antibody, HRP Envision Kit (DAKO), for 20 minutes. The slides were then washed with PBS. The positive immunoreactivity was developed and visualized with 0.05% DAB color reaction for 15 minutes. After washing with PBS, the sections were counterstained with hematoxylin for staining nuclei, dehydrated, and cleared in xylene. The cover-slipped tissue sections were observed for brown color formation (that was considered as an indicator of protein expression) under a light microscope. The stained slides were coded before examination by a specialist who was blinded to the treatment protocol. Morphological assessments and data analysis were attained using the Leica Application module for tissue sections analysis that is connected to a full HD microscopic imaging computer system (Leica Microsystems GmbH, Germany). Quantitative determinations of protein expression levels of Nrf-2 or HO-1 were done by estimation of mean area percentage of Nrf-2 or HO-1 immunoexpression using a microscopic camera attached to the Leica software that converts the measured units (pixels) given by the image-analyzer program into actual micrometer units used for immunoexpression analysis. Six nonoverlapping fields in the immunostained tissue sections were randomly selected and acquired from each sample for quantification of positive immunoexpression percentage levels of Nrf-2 and HO-1.
Real-time PCR and determination of hepatic mRNA levels of Bcl-2 and Bax
Total RNA was isolated from hepatic tissues using GF-1 total RNA extraction kit (GF-TR-050, Vivantis Technologies Sdn Bhd, Malaysia) according to the manufacturer’s instructions. To get rid of the contaminating DNA, the isolated RNAs were treated with a DNase I, RNase-free kit (Fermentas, MD). One microgram of the total RNA and random primers were used for cDNA synthesis by adopting the Script™ cDNA synthesis kit (Bio-Rad, CA). For qRT-PCR, the cDNA samples were run in triplicate, and the PCR reactions were carried out using Power SYBR® Green (Life Technologies, CA) and were attached to the Applied Biosystem, version 3.1 software (StepOne™) (CA). The typically adopted thermal profile was 95°C for 5 minutes (for denaturation), followed by 45 amplification cycles of 95°C for 30 seconds (denaturation step), 56°C for 30 seconds (annealing step), and 72°C for 30 seconds (elongation step). After PCR amplification, the values of ΔCt were calculated by subtraction of the β-actin Ct from the Ct of each sample, and the relative levels of gene expression were determined. β-actin is used as a reference gene. The used primers sequences are shown in Table 1.
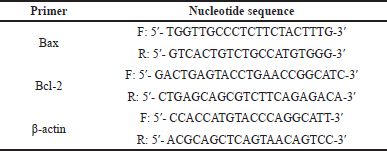 | Table 1. The sequences of the primers used in the current study. [Click here to view] |
Statistical analysis
Values were expressed as means ± SEM. Multiple comparisons between different treatment regimens were obtained using the one-way analysis of variance (ANOVA) test followed by the Tukey-Kramer comparison tests that were applied across the four groups or using the chi-squared (χ2) test, wherever indicated. The results were considered significantly different at p < 0.05. Data analysis was accomplished using the computer software GraphPad Prism and GraphPad InStat, San Diego, CA.
RESULTS
Treatment with QBS ameliorated MTX-induced hepatic damage and improved liver integrity profile
Alterations in serum levels of AST, ALT, LDH, and ALP might indicate liver dysfunction. As shown in Table 2 and Figure 1, MTX significantly elevated serum AST, ALT, ALP, and LDH levels in MTX-treated rats as compared to their corresponding levels in the normal rats from the control group. Pretreatment with QBS significantly attenuated MTX-induced elevations in serum levels of AST (by ~43%), ALT (by ~40%), ALP (by ~23%), and LDH (by ~46%), as compared to MTX-treated rats. Furthermore, rats administered QBS did not exhibit change in the levels of the indicated biomarkers when compared to the normal control group of rats. Such results propose the hepatoprotective activities of this saponin against MTX-mediated damage of liver cells.
 | Table 2. The effects of QBS treatment on the serum levels of AST, ALT, ALP, and LDH in MTX-treated rats. [Click here to view] |
 | Figure 1. The modulatory effects of QBS pretreatment on the serum levels of AST (A), ALT (B), ALP (C), and LDH (D) in MTX-treated rats. The depicted data describes the fold changes ± SEM of the levels of the demonstrated biomarkers in the serum of the rats from the indicated groups (N = 8). Statistical inspections were accomplished using ANOVA followed by Tukey’s multiple-comparison test; a p < 0.05, versus normal control animals; b p < 0.05, versus MTX-treated animals. [Click here to view] |
 | Table 3. The effects of QBS on the oxidative stress biomarkers (hepatic contents of GSH, MDA, and NOx) in MTX-treated rats. [Click here to view] |
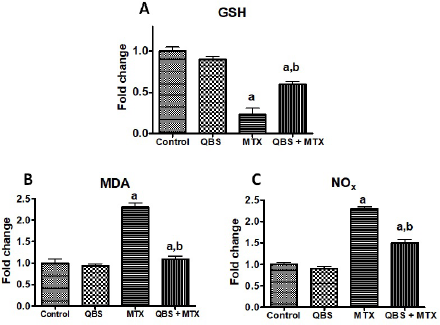 | Figure 2. The modulating activities of QBS pretreatment on the oxidative stress biomarkers [hepatic contents of GSH (A), MDA (B), and NOx (C)] in MTX-treated rats. The depicted data describes the fold changes ± SEM of the levels of the demonstrated oxidative stress biomarkers in the hepatic tissues of the rats in the indicated groups (N = 8). Statistical inspections were accomplished using ANOVA followed by Tukey’s multiple-comparison test; a p < 0.05, versus normal control animals; b p < 0.05, versus MTX-treated animals. [Click here to view] |
Treatment with QBS attenuated hepatic oxidative stress and suppressed hepatic lipid peroxidation
GSH is an important nonenzymatic antioxidant that maintains normal cell integrity by binding/inhibiting toxic electrophilic radicals and oxygen free radicals (Chen et al., 2013), most of which have a reactive nature with NO. NO is one of the important oxidant mediators which possesses prominent cytotoxicity and stress-induced cell injury (Ozcelik et al., 2014). Such a chain of oxygen free radicals cascade generates lipid peroxidation in several biological systems with the formation of the final product: malondialdehyde (MDA) (Tsikas, 2017). Hence, decreased hepatic GSH levels with elevated MDA (a measure of lipid peroxidation) and total NOx contents characterize the hepatic oxidative stress (Cruz et al., 2020). With regard to the current study, our data showed that MTX markedly decreased (~77%) liver content of GSH and outstandingly increased hepatic MDA and NOx contents in MTX-treated rats (by about 1.3-fold and 1.4-fold, respectively), in comparison with the untreated rats in the control group (Table 3 and Fig. 2). However, pretreatment with QBS showed a remarkable elevation in the hepatic GSH level (by ~57%) along with a notable decline in both MDA and NOx contents (by ~57% and 36%, respectively) as compared to MTX-treated rats (Table 3). The QBS-mediated improvement of the oxidant/antioxidant profile and the reduction in pro-oxidant load might be accountable for the hepatoprotective effects of the used saponin against liver injury induced by MTX.
QBS mitigated MTX-induced histopathological/inflammatory modifications in the liver tissues
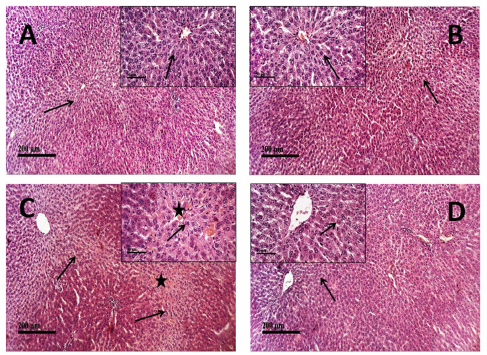
Hematoxylin-eosin (H and E) staining was employed in the current study to assess the pathologic alterations in hepatic tissues dissected from rats exposed to the indicated treatments (Fig. 3A–D). As indicated in Figure 3A, microscopic examinations of liver tissues from vehicle-treated control rats revealed normal histological structures of hepatic parenchyma with intact radiating hepatocytes in hepatic lobules showing large vesicular nuclei with prominent nucleoli (arrow), intact hepatic sinusoids with lining endothelial cells and normal resident Kupffer cells as well as intact vasculatures (score = 0). Rats treated with QBS alone (Fig. 3B) exhibited similar normal histological features of the hepatic lobule with intact hepatocytes (arrow) without abnormal morphological or structural changes (score = 0). Conversely, the liver tissues obtained from MTX-treated rats showed moderate centrilobular vacuolar degenerative changes (score = 2) of most of the hepatocytes (arrow) in the examined hepatic sections accompanied with obvious congested hepatic sinusoids and central veins (star) revealing sinusoidal leukocytosis with inflammatory cell infiltration (score = 3), along with sporadic hepatocyte apoptosis or necrosis (score = 2) (Fig. 3C). However, pretreatment with QBS extremely mitigated the severity of MTX-produced hepatic pathological conversions. The hepatic sections from rats treated with QBS/MTX showed better organized morphological architecture of hepatic lobules with significant reduction in the congested sinusoids and vasculature (score = 1), a few inflammatory cells infiltration, and only mild sporadic vacuolar degenerative changes (arrow) of hepatocytes (score = 1) (Fig. 3D). Taken together, such observations indicate that QBS treatment dramatically impaired MTX-induced liver injury in the examined rats.
Effects of QBS treatment on Nrf-2/HO-1 pathway in the livers of MTX-treated rats
Nrf-2/HO-1 is a defensive pathway whose activation is increased in various pathological conditions (Aleksunes et al., 2010) to counteract the inflammatory and apoptotic actions of the causative insults (Goodman et al., 2007). In the present work, we hypothesized that Nrf-2/HO-1 contributes in mediating the protective effect of QBS against MTX. To validate the role of the Nrf-2/HO-1 pathway, we further determined the expression of Nrf-2 and its target protein, HO-1, using immunostaining of the hepatic tissues from the treated animals. The present data revealed that the examined control hepatic sections (group I) showed moderate positive Nrf-2 immunoreaction (average mean area percent of 11.8%), which appeared in the cytoplasm and nuclei of the cells. As for group II (QBS group), marked widespread brown cytoplasmic and nucleic Nrf-2 immunoreactivity (up to 18% of mean area percent) was evident. On the other hand, MTX treatment (in group III) induced about 40% decrease (≈7.5% of mean area percent of Nrf-2 immunoexpression) in the hepatic sections of MTX-treated rats when compared to the basal Nrf-2 expression in the control group. QBS pretreatment, however, reversed such inhibition in the combined group (QBS + MTX) and recorded about 11.03% of the mean area percent of Nrf-2 immunoreaction (Fig. 4A–D, respectively). Likewise, the expression levels of the HO-1, the downstream protein of Nrf-2, exhibited comparable immunostaining alterations to those of Nrf-2. The greatest mean area percent of HO-1 immunoexpression was recorded in the QBS group (16.2%; Fig. 4F) that represents about 2-fold more than its level in the control group (5.1%; Fig. 4E). Conversely, MTX treatment demonstrated low HO-1 expression (1.9%; Fig. 4G). QBS pretreatment, however, increased the mean area percent of HO-1 immunoexpression in the combined group (QBS + MTX) up to 3.7% (≈1-fold more than its level in the MTX group) (Fig. 4H).
QBS inhibits MTX-induced apoptosis in liver tissues
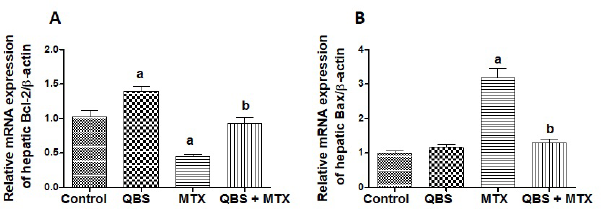
MTX intoxication is partially prompted via significant elevation of hepatic apoptosis (Gamal Helal and Said, 2020) that may likely be underlaid by the evoked oxidative stress (Herman et al., 2005). In the present study, Bcl-2 and Bax mRNA expressions were measured as antiapoptotic and proapoptotic markers, respectively, to further validate the obtained virulent histological alterations induced by MTX. Our data showed that MTX significantly decreased hepatic Bcl-2 expression level (by ~0.5-fold; Fig. 5A) and significantly increased hepatic Bax expression level (>2-fold; Fig. 5B), as compared to the respective control groups, suggesting the proapoptotic role of MTX in the investigated rats’ livers. However, QBS pretreatment protected against these proapoptotic events as shown by remarkable reversible actions of MTX effects. This is shown by prevention of MTX-induced decrease in hepatic Bcl-2 expression (Fig. 5A) and suppression of MTX-mediated induction of hepatic Bax expression (Fig. 5B), with consequent relative maintaining of normal hepatic Bcl-2 and Bax levels in the QBS/MTX group.