INTRODUCTION
Medicines from natural origin have been used for centuries to treat human diseases since they contain chemicals of therapeutic value (Beutler, 2009; Nostro et al., 2000). The World Health Organization published a report in 2008 stating that over 80% of the world’s populace depends on traditional medicine for their primary healthcare needs (Vital and Rivera, 2009). So, traditional medicines serve as important sources of new drugs or drug candidates (Papia et al., 2016) and more than 80% of drug candidates are either directly obtained from natural products or developed from natural compounds (Lautié et al., 2020; Maridass and De Britto, 2008). In addition, about 50% of pharmaceuticals are derived from substances first identified or isolated from natural sources such as herbs/plants, organisms, animals, and insects (Krief et al., 2004).
Pain is manifested as a defensive response resulting from either cellular dysfunction or imbalance in its functions due to harmful stimulus. Pain is accompanied by human and animal diseases and has become the focus of global scientific research (Malarvizhi et al., 2020; Sarker et al., 2012). Many drugs are used to relieve pain, but none are devoid of side effects (Devaraj and Karpagam, 2011). Generally, compounds obtained from natural sources have little or no side effects, thus searching for new analgesic drugs from traditionally used plant species is still a rational strategy.
6-(3,4-Dihydroxyphenyl)-4-hydroxyhexa-3,5-dien-2-one (Hispolon) (Fig. 1) is a natural phenolic type bioactive compound first isolated from Inonotus hispidus in 1996 (Ali et al., 1996) then from Phellinus linteus (Chen et al., 2013; Toopmuang et al., 2014) and Phellinus igniarius (Mo et al., 2004). Hispolon possesses diverse biological activities, including antioxidant (Huang et al., 2011; Sarfraz et al., 2020), hepatoprotective (Huang et al., 2012), analgesic and anti-in?ammatory (Chang et al., 2011; Sarfraz et al., 2020), antiproliferative (Huang et al., 2011), antimetastatic (Huang et al., 2010), antidiabetic (Sarfraz et al., 2020) immunomodulatory, and antiviral effects (Ali et al., 1996, 2003). Hispolon also inhibits the chemiluminescent response of human mononuclear cells and downregulates nitrogen-induced proliferation of spleen lymphocytes of mice (Ali et al., 1996).
The analgesic action of hispolon is due to the inhibition of prostaglandin biosynthesis by cyclooxygenase (COX) enzyme (Chang et al., 2011), as it inhibits the nociception induced by acetic acid and formalin (late phase). Furthermore, hispolon demonstrated a significant inhibition of edema development induced by Carr in the third phase, indicating inhibition of prostaglandin synthesis.
Prostaglandins are biosynthesized by the COX enzyme (Goodman, 1996; Malarvizhi et al., 2020). COX-1 and COX-2 are isoforms of COX enzyme. The COX-1 is constitutively expressed in the stomach, kidneys, and platelets and plays a vital role in protection of the mucosa and functioning of platelets, whereas COX-2 is inducible and causes prostaglandin biosynthesis in inflammatory cells (Griswold and Adams, 1996; Malarvizhi et al., 2020). Recently, Wang et al. (2017) concluded that hispolon likely inhibits COX-2 and demonstrates analgesic and anti-in?ammatory action.
The literature survey revealed no report on the molecular mechanism of COX-2 inhibition by hispolon, which may open a window to develop new COX-2 inhibitors, i.e., analgesic and anti-in?ammatory drugs. Therefore, we have carried out a widely accepted virtual screening (molecular docking) of hispolon and a compound library containing hispolon skeleton curated from the PubChem compound database (https://pubchem.ncbi.nlm.nih.gov/search/search.cgi#) to explore the molecular mechanism of COX-2 inhibition and to find novel COX-2 inhibitors. Also, in silico pharmacokinetic studies such as absorption [cellular permeability and human intestinal absorption (HIA)] and distribution [plasma protein binding (PPB), brain to blood partitioning, and interaction with P-Glycoprotein] of hispolon and its top hits (H1–H7) were carried out with PreADMET (https://preadmet.bmdrc.kr/).
METHODOLOGY
Molecular docking study
Preparation of target protein
The ligand bound X-ray crystal structure of target protein is essential to carry out a molecular docking study since docking software searches compatible binding site(s) of ligands within the protein of interest (Khan et al., 2018). Hence, for a molecular docking study, the availability of ligand-bound structure is a prerequisite. Furthermore, the structure should have acceptable values for Root mean square error (RMS) deviation from ideality, Rwork/Rfree, and validation parameters such as rotamer outliers, Ramachandran outliers, bad bond, or bad angle count. In the current investigation, the crystal structure of mammalian COX-2 complexed with celecoxib (PDB ID: 3LN1) (Wang et al., 2010) has been selected since the structure was solved at 2.4 Å resolution with Rwork/Rfree of 0.232/0.264, and the validation parameters suggests good quality of the modeled structure. At first, the protein molecule was prepared by removing water, ligands, heteroatoms, and chains B, C, and D, and adding polar hydrogen to the macromolecule using PyMOL (Version 1.7.4.4, Schrödinger). The energy of the protein structure was minimized with YASARA Energy Minimization Server (http://www.yasara.org/minimizationserver.htm).
Preparation of ligands
The structures of hispolon (CID 53395354) and celecoxib (CID 2662) were downloaded from PubChem. Molecular geometry was optimized with MMFF94 level of theory available in Open Babel (O’Boyle et al., 2011). Appropriate charges were then assigned to the optimized structure of ligands.
Preparation of ligand library
The library was prepared by utilizing the structure of hispolon as a substructure. The simplified molecular-input line-entry system string of hispolon was used to search for similar structures in the PubChem compound database (https://pubchem.ncbi.nlm.nih.gov/search/search.cgi#) which yielded 1,699 structures. These structures were then optimized and prepared for docking by the method described above.
Protein–ligand docking
AutoDock Vina (Trott and Olson, 2010) implemented in PyRx 0.8 (Dallakyan and Olson, 2015) was used to conduct docking of target protein with the ligand. Docking was carried out with a box volume of 25.00 × 25.00 × 25.00 Å3 with a center of 30.7423, −22.209, and −15.738. Throughout the docking study, the flexible ligands searched its complementary site(s) within the search space of rigid macromolecule. Ligands with lowest binding affinity and promising binding pose were chosen as the best conformation. The interactions of protein residues with hispolon and other ligands were analyzed by PyMOL (DeLano, 2002).
Initially, celecoxib was re-docked into the binding pocket of COX-2 to validate the docking method. The root mean square deviation (RMSD) between the docked and experimental celecoxib was then calculated. The result revealed that the first pose of celecoxib nearly superimposes (RMSD < 1) with the experimental structure of celecoxib (Fig. 2). Thus, the docking method was reasonably accurate and reproducible.
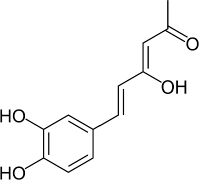 | Figure 1. Structure of hispolon. [Click here to view] |
Calculation of drug-like properties
Since the purpose of docking the ligand database is to find compounds with higher affinity than hispolon, we have selected those with binding affinity greater or equal to the binding affinity of hispolon. This list of compounds was further standardized by applying Lipinski’s (2004) rule of five and other drug-like properties in order to achieve compounds with good bioavailability (Veber et al., 2002), less adverse effects (Hughes et al., 2008), and lower risk of compound attrition (Ritchie and Macdonald, 2009). Lipinski (2004) stated that an orally active drug should have molecular weight below 500 Da, no more than 5 hydrogen bond donors, no more than 10 hydrogen bond acceptors, and partition coefficient less than or equal to 5. Good oral bioavailability of compounds was also observed in rats if they contain rotatable bonds (ROTB) and polar surface areas (PSAs) less than 10 and 140 Å2, respectively (Veber et al., 2002). Hughes et al. (2008) reported that compounds with logP less than 3 and PSA greater than 75 Å2 have 6 times less likely to exhibit adverse events in in-vivo tolerance studies. In addition, the number of aromatic rings influences the risk of compound attrition and it was found that aromatic rings greater than 3 significantly increase the risk of compound attrition (Ritchie and Macdonald, 2009). So, we have standardized the list by applying the following drug-like properties:
- molecular weight below 500 Da;
- no more than five hydrogen bond donors;
- no more than 10 hydrogen bond acceptors;
- logP less than 3;
- ROTB less than 10;
- PSA greater than 75 Å2 but less than 140 Å2;
- aromatic rings less than or equal to 3.
Pharmacokinetic study
The pharmacokinetic properties were assessed with PreADMET server (https://preadmet.bmdrc.kr/). Pharmacokinetic parameters such as HIA, Caco-2 cell permeability (PCaco-2), interaction with P-glycoprotein (Pgp), skin permeability (PSkin), penetration of blood–brain barrier, and PPB were calculated and predicted.
RESULTS AND DISCUSSION
Calculation of drug-like properties
Since hispolon (H) demonstrated a binding affinity of −7.6 Kcal/mol (Table 1), we sorted out the list of compounds having binding affinity greater than or equal to −7.6 Kcal/mol, which yielded 696 compounds. Further standardization based on drug-like properties provides a list of seven compounds (H1–H7) (Table 1 and Fig. 3). It is expected that in vitro investigation of these ligands would demonstrate good bioavailability, less adverse effects, and a lower risk of attrition.
Molecular docking study
The crystal structure of 3LN1 revealed that COX-2 enzyme appears as a dimer and each monomer consists of an N-terminal Epidermal Growth Factor (EGF)-like domain, a membrane binding domain (MBD), and a large catalytic domain with two active sites, the peroxidase site, and the COX site (Blobaum et al., 2015). The critical interactions between the inhibitors and residues at the active site of COX enzymes have been identified by X-ray crystallographic and site-directed mutagenesis studies (Kalgutkar et al., 2000; Picot et al., 1994; Rowlinson et al., 2003). The COX-2 inhibitors are thought to first enter through the four helical (A, B, C, and D) MBDs into an open area called the lobby, consisting of Val74, Leu78, Trp85, Ile98, Tyr101, and Val102 (Blobaum et al., 2015; Picot et al., 1994). The lobby is separated from the active site by a gate made of three conserved residues Arg106, Tyr341, and Glu510 (Blobaum et al., 2015). It has been established that the selectivity of celecoxib and rofecoxib for COX-2 is due to the ability of inserting the sulfonamide and methyl sulfone groups, respectively, into the side pocket of COX-2 constituting of His75, Arg499, Val509, and Glu510 (Blobaum et al., 2015). In addition, Val509 at the active site of COX-2 confers the selectivity of selective COX-2 inhibitors since mutation of this residue to Ile abolishes the selectivity (Gierse et al., 1996). It has been found that celecoxib also makes hydrophobic interaction with Val509. Therefore, molecules having the ability to insert a functional group into the side pocket of COX-2 and makes hydrophobic interaction with Val509 may act as selective COX-2 inhibitors.
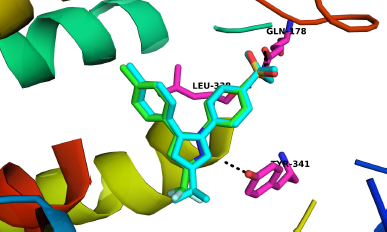
The binding affinity and interactions of H and top hits (H1, H2, H3, H4, H5, H6, and H7) are presented in Table 1 and 2 and Figures 4 and 5. H displays binding affinity of −7.6 Kcal/mol and forms hydrogen bonding with the side chains of TYR371, SER516, and with the backbone of PHE504. Furthermore, H associates via hydrophobic interaction with VAL509, the crucial residue involved in COX-2 selectivity. The aromatic ring of H is accommodated in the side pocket of COX-2 (Fig. 4); thereby, it is expected that H may inhibit the biosynthesis of prostaglandins by selectively inhibiting COX-2.
H1 interacts with a binding affinity of −8.9 Kcal/mol to the side chain of TYR341 and the backbone of LEU338 through hydrogen bond formation and the complex is further stabilized by the hydrophobic interactions with VAL335, LEU338, SER339, and VAL509. H2 shows a binding affinity of −8.6 Kcal/mol and forms only hydrophobic interactions with VAL335, VAL509, and ALA513. H3 makes hydrophobic interactions with SER339, VAL509, and ALA513 with a binding energy of −8.4 Kcal/mol. The compound H4 demonstrates a binding energy of −8 Kcal/mol and forms hydrogen bonding with GLN178, ILE503, and PHE504 which is further stabilized by the hydrophobic interactions with VAL335, LEU338, VAL509, GLY512, and ALA513. H5 forms hydrogen bond with the –OH of TYR341 and the complex is stabilized by hydrophobic interactions with VAL509 and ALA513 with a binding affinity of −8 Kcal/mol. H6 and H7 interact through hydrogen bond formations with TYR341 and ARG106, and TYR371 and PHE504 (with the backbone), and SER516 and GLN178 with the binding energy of −7.8 and −7.7, respectively. Both of them form hydrophobic interactions with VAL509.
 | Figure 2. The superposition of the best docking pose of celecoxib (PDB ID: 3LN1) with the X-ray structure. (?) Experimental celecoxib; (?) Docked celecoxib. [Click here to view] |
The analysis of binding mode reveals that ligands H1, H2, H3, H4, and H7 form hydrophobic interaction with VAL509 and insert their aromatic ring in the site pocket of COX-2; hence, it may be assumed that these ligands exert their inhibitory action on the biosynthesis of prostaglandins by selectively inhibiting COX-2.
Pharmacokinetic study
Pharmacokinetic studies, including absorption (HIA and cellular permeability) and distribution (PPB, brain to blood partitioning, and interaction with P-Glycoprotein) of H and its selected top hits (H1–H7) were performed with PreADMET server (https://preadmet.bmdrc.kr/). The predicted absorption and distribution parameters are presented in Table 3.
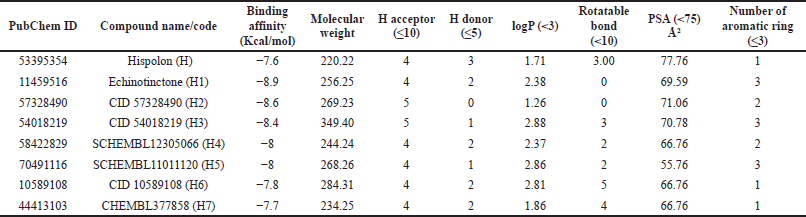 | Table 1. Binding affinity (Kcal/mol) and other drug-like properties of hispolon and its analogs. [Click here to view] |
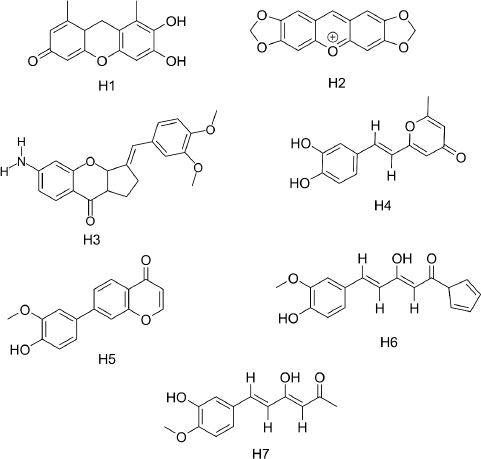 | Figure 3. Structure of hispolon derivatives. [Click here to view] |
The computed HIA of H and top hits (Table 3) ranged from 84.38%–98.34%, suggesting all the compounds are well absorbed through the intestinal cell (Yee, 1997). H and top hits were moderately permeable since values for absorption through Caco-2 cells (PCaco-2) were 9.76–56.89 nm/s (Yazdanian et al., 1998). PSkin is a crucial parameter for assessing drugs and chemicals requiring transdermal administration (Singh and Singh, 1993). All compounds were found to be impermeable through the skin since the calculated PSkin were −2.65 to −4.49.
The distribution properties of the compounds were evaluated based on the brain to blood partitioning (Cbrain/Cblood) and PPB values. Strongly bound compounds have a PPB value greater than 90%, whereas lower than 90% PPB indicates weakly bound chemicals (https://preadmet.bmdrc.kr/adme-prediction/). H1, H3, and H5 were considered strongly bound chemicals since they had PPB values of 99.5%, 91.4%, and 93.3%, respectively, whereas H, H2, H4, H6, and H7 were weakly bound since they had PPB values lower than 90%. Due to its strong binding nature, it could be inferred that H1, H3, and H5 would be less likely to be encountered in the systemic circulation.
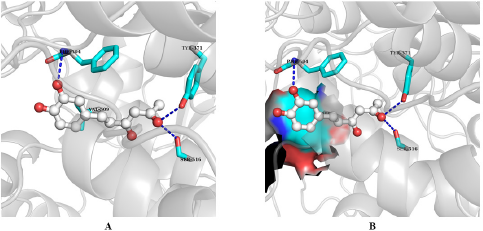 | Figure 4. Interactions of hispolon at the binding site of COX-2. A. Interactions of different residues with H. B. Aromatic side chain is at the side pocket of COX-2. Interacting residues are presented in stick model and ligands are in ball and stick model. Blue dash indicates hydrogen bonding. [Click here to view] |
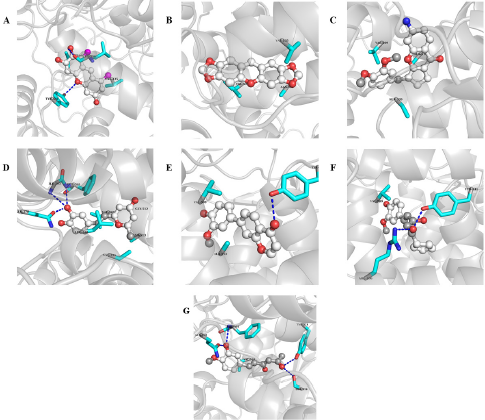 | Figure 5. Interactions between COX-2 and A) H1, B) H2, C) H3, D) H4, E) H5, F) H6, and G) H7. Interacting residues and ligands are presented in stick and ball and stick models. Blue dash indicates hydrogen bonding. [Click here to view] |
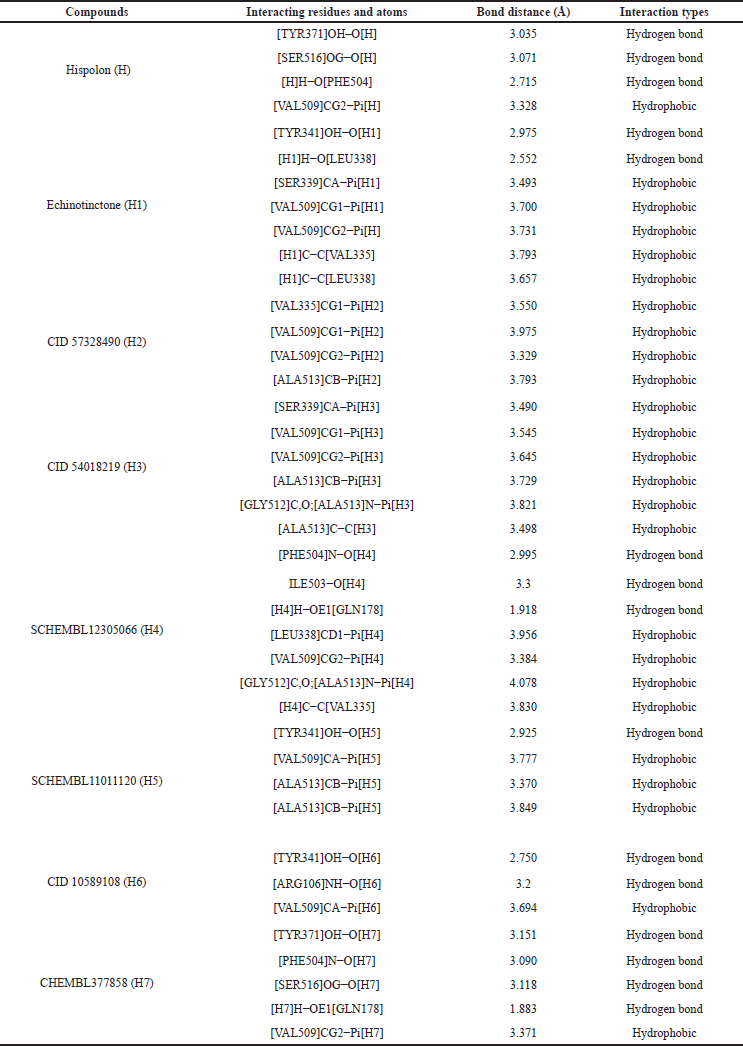 | Table 2. Interactions of hispolon (H) and its selected analogs (H1–H7) with COX-2 enzyme. [Click here to view] |
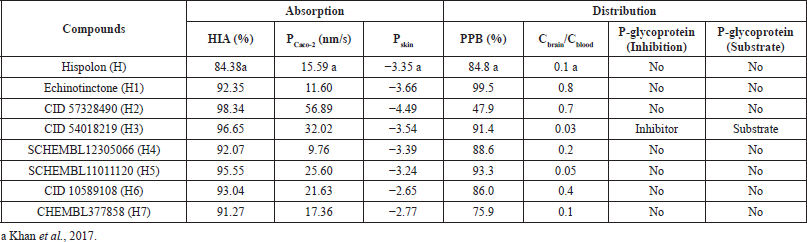 | Table 3. Absorption and distribution of hispolon and its analogs. [Click here to view] |
Chemicals can be classified into three groups based on their absorption to Central Nervous System (CNS). Compounds with Cbrain/Cblood values greater than 2.0 have high absorption to CNS, whereas compounds with Cbrain/Cblood values from 0.1 to 2.0 and less than 0.1 are considered to have moderate and low absorption to CNS, respectively. Based on these values, it can be inferred that all compounds except H3 and H5 have moderate absorptions to CNS. Both H3 and H5 have low absorption, and hence are likely to be present in the systemic circulation.
P-glycoprotein (PGP) is an adenosine triphosphate-dependent efflux transport protein encoded by the multidrug resistance gene. It has been shown to influence the absorption, distribution, and excretion of drugs due to its efflux action (Khan et al., 2018; Schinkel, 1999). Ineffective cancer and antibiotic chemotherapy is an outcome of increased expression of this gene (Cabrera et al., 2006; Kim et al., 1998). Compounds that act as substrates for PGP will have low efficacy; hence, substrates for PGP need to be identified and eliminated (de Cerqueira Lima et al., 2006; Wang et al., 2005). However, this is done through laborious in vitro and in vivo analyses (Joung et al., 2012). The likelihood of compounds to act as substrates for PGP can be predicted by in silico tools (Joung et al., 2012). The computer-aided screening demonstrated that only H3 is a dual inhibitor and substrate for PGP analogous to quinidine (Zhou, 2008). This phenomenon is due to the complex modulatory interaction of H3 with PGP, and hence H3 acts as both a substrate and an inhibitor (Zhou, 2008). Therefore, coadministration of H3 with a chemotherapeutic agent may demonstrate a beneficial effect in cancer chemotherapy.
CONCLUSION
In the current investigation, our virtual screening based on structure similarity search afforded 1,699 compounds which were further standardized based on binding affinity, binding poses, and drug-like properties. The screening and filtering parameters yielded a list of seven compounds (H1, H2, H3, H4, H5, H6, and H7). Therefore, these compounds likely demonstrate good bioavailability, less adverse effects, and lower risk of attrition.
The in silico pharmacokinetic study revealed that these compounds possess good human intestinal absorption and moderate permeability through Caco-2 cells. Furthermore, the Cbrain/Cblood ratio of these drugs indicates moderate penetrability of CNS except H3 and H5, which are low-absorbing agents for CNS and will predominately reside in the systemic circulation. Since H3 acts as both inhibitor and substrate for P glycoprotein due to complex modulatory interactions with the PGP (Zhou, 2008), it is unlikely that H3 would be pumped out of the cells completely and hence reduce the possibility of resistance mediated by the efflux pump. Therefore, the coadministration of H3 with a chemotherapeutic agent may demonstrate a beneficial effect in cancer chemotherapy.
Moreover, molecular docking of H and its selected top hits revealed that all the ligands cause an hydrophobic interaction with Val509, and the H, H1, H2, H3, H4, and H7 ligands can accommodate their aromatic ring inside the side pocket of COX-2. Therefore, these ligands likely exert their analgesic action by selectively blocking the biosynthesis of prostaglandins mediated by COX-2.
These COX-2 inhibitor candidates may be subjected to in vitro experiments, followed by in vivo experiments to assess the COX-2 inhibitory activity. The investigated pharmacokinetic properties and molecular docking will guide the synthesis of hispolon derivatives with better pharmacokinetic properties and COX-2 inhibitory activity.
ACKNOWLEDGMENT
The authors thank State University of Bangladesh for providing computational lab facility.
CONFLICTS OF INTERESTS
The authors declare that there is no conflicts of interests.
FUNDING
Not applicable.
CONSENT TO PARTICIPATE
Not applicable.
ETHICS APPROVAL
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Data and material are included in the article.
AUTHORS’ CONTRIBUTIONS
Conceived and designed the experiments: MFK and AR. Performed theoretical investigations: MM, MFK, and SMN. Analyzed the data: MM, MFK, RBR, and SMN. Wrote the manuscript: MM, MFK, RBR, and MAR. All authors read and approved the final manuscript for publication.
REFERENCES
Ali NA, Ludtke J, Pilgrim H, Lindequist U. Inhibition of chemiluminescence response of human mononuclear cells and suppression of mitogen-induced proliferation of spleen lymphocytes of mice by hispolon and hispidin. Pharmazie, 1996; 51:667–70.
Ali NA, Mothana R, Lesnau A, Pilgrim H, Lindequist U. Antiviral activity of Inonotus hispidus. Fitoterapia, 2003; 74:483–5. CrossRef
Beutler JA. Natural products as a foundation for drug discovery. Curr Protoc Pharmacol, 2009; 46:9–11. CrossRef
Blobaum AL, Xu S, Rowlinson SW, Duggan KC, Banerjee S, Kudalkar SN, Birmingham WR, Ghebreselasie K, Marnett LJ. Action at a distance mutations of peripheral residues transform rapid reversible inhibitors to slow, tight binders of cyclooxygenase-2. J Biol Chem, 2015; 290:12793–803. CrossRef
Cabrera MA, González I, Fernández C, Navarro C, Bermejo M. A topological substructural approach for the prediction of P-glycoprotein substrates. J Pharm Sci, 2006; 95:589–606. CrossRef
Chang HY, Sheu MJ, Yang CH, Lu TC, Chang YS, Peng WH, Huang SS, Huang GJ. Analgesic effects and the mechanisms of anti-inflammation of hispolon in mice. Evid Based Complement Altern Med, 2011; 2011:478246. CrossRef
Chen YC, Chang HY, Deng JS, Chen JJ, Huang SS, Lin IH, Kuo WL, Chao W, Huang GJ. Hispolon from Phellinus linteus induces G0/G1 cell cycle arrest and apoptosis in NB4 human leukaemia cells. Am J Chinese Med, 2013; 41:1439–57. CrossRef
Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Curr Protoc Chem Biol, 2015; 243–50. CrossRef
de Cerqueira Lima P, Golbraikh A, Oloff S, Xiao Y, Tropsha A. Combinatorial QSAR modeling of P-glycoprotein substrates. J Chem Inf Model, 2006; 46:1245–54. CrossRef
DeLano WL. The PyMOL user’s manual. DeLano Scientific, San Carlos, CA, 2002.
Devaraj A, Karpagam T. Evaluation of anti-inflammatory activity and analgesic effect of Aloe vera leaf extract in rats. Int Res J Pharm, 2011; 2:103–10.
Gierse JK, McDonald JJ, Hauser SD, Rangwala SH, Koboldt CM, Seibert K. A single amino acid difference between cyclooxygenase-1 (COX-1) and− 2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem, 1996; 271:15810–4. CrossRef
Goodman LS. Goodman and Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill; 1996.
Griswold DE, Adams JL. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med Res Rev, 1996; 16:181–206. CrossRef
Huang GJ, Deng JS, Chiu CS, Liao JC, Hsieh WT, Sheu MJ, Wu CH. Hispolon protects against acute liver damage in the rat by inhibiting lipid peroxidation, proinflammatory cytokine, and oxidative stress and downregulating the expressions of iNOS, COX-2, and MMP-9. Evid Based Complement Altern Med, 2012; 2012:480714. CrossRef
Huang GJ, Deng JS, Huang SS, Hu ML. Hispolon induces apoptosis and cell cycle arrest of human hepatocellular carcinoma Hep3B cells by modulating ERK phosphorylation. J Agric Food Chem, 2011; 59:7104–13. CrossRef
Huang GJ, Yang CM, Chang YS, Amagaya S, Wang HC, Hou WC, Huang SS, Hu ML. Hispolon suppresses SK-Hep1 human hepatoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen activator through the PI3K/Akt and ERK signaling pathways. J Agric Food Chem, 2010; 58:9468–75. CrossRef
Hughes JD, Blagg J, Price DA, Bailey S, DeCrescenzo GA, Devraj RV, Ellsworth E, Fobian YM, Gibbs ME, Gilles RW, Greene N. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg Med Chem Lett, 2008; 18:4872–5. CrossRef
Joung JY, Kim HJ, Kim HM, Ahn SK, Nam KY, No KT. Prediction models of p-glycoprotein substrates using simple 2d and 3d descriptors by a recursive partitioning approach. Bull Kor Chem Soc. 2012;33(4):1123–7. CrossRef
Kalgutkar AS, Crews BC, Rowlinson SW, Marnett AB, Kozak KR, Remmel RP, Marnett LJ. Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci USA, 2000; 97:925–30. CrossRef
Khan MF, Aktar S, Rashid RB, Rashid MA. In silico Investigation of physicochemical, pharmacokinetic and toxicological properties of hispolon. Der Pharm Chem, 2017, 9(19):9–13.
Khan MF, Nahar N, Rashid RB, Chowdhury A, Rashid MA. Computational investigations of physicochemical, pharmacokinetic, toxicological properties and molecular docking of betulinic acid, a constituent of Corypha taliera (Roxb.) with Phospholipase A2 (PLA2). BMC Complement Altern Med, 2018; 18:48. CrossRef
Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Investig, 1998; 101:289–94. CrossRef
Krief S, Martin MT, Grellier P, Kasenene J, Sévenet T. Novel antimalarial compounds isolated in a survey of self-medicative behavior of wild chimpanzees in Uganda. Antimicrob Agents Chemother, 2004; 48:3196–9. CrossRef
Lautié E, Russo O, Ducrot P, Boutin JA. Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol, 2020; 11:397. CrossRef
Goodman LS. Goodman and Gilman’s the pharmacological basis of therapeutics. McGraw-Hill, New York, NY, 1996.
Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discovery Today Tech, 2004; 1:337–41. CrossRef
Malarvizhi R, Sali V, Bhardwaj M, Mani S, Vasanthi H. Inhibition of cyclooxygenase enzyme by bioflavonoids in horsegram seeds alleviates pain and inflammation. Comb Chem High Throughput Screen, 2020; 23(9):931–8. CrossRef
Maridass M, De Britto AJ. Origins of plant derived medicines. Ethnobot Leafl, 2008; 2008:44.
Mo S, Wang S, Zhou G, Yang Y, Li Y, Chen X, Shi J. Phelligridins C-F: cytotoxic pyrano [4, 3-c][2] benzopyran-1, 6-dione and furo [3, 2-c] pyran-4-one derivatives from the fungus Phellinus igniarius. J Nat Prod, 2004; 67:823–8. CrossRef
Nostro A, Germano M, D’angelo V, Marino A, Cannatelli M. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol, 2000; 30:379–84. CrossRef
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminf, 2011; 3:33. CrossRef
Papia S, Rahman MM, Rahman MM, Adib M, Khan MF. In vitro membrane stabilizing and in vivo analgesic activities of Boehmeria glomerulifera Miq. in Swiss-albino mice model. Bangladesh Pharm J, 2016; 19:185–9. CrossRef
Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature, 1994; 367:243. CrossRef
Ritchie TJ, Macdonald SJ. The impact of aromatic ring count on compound developability-are too many aromatic rings a liability in drug design? Drug Discov Today, 2009; 14:1011–20. CrossRef
Rowlinson SW, Kiefer JR, Prusakiewicz JJ, Pawlitz JL, Kozak KR, Kalgutkar AS, Stallings WC, Kurumbail RG, Marnett LJ. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J Biol Chem, 2003; 278:45763–9. CrossRef
Sarfraz A, Rasul A, Sarfraz I, Shah MA, Hussain G, Shafiq N, Masood M, Adem ?, Sarker SD, Li X. Hispolon: a natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Environ Res, 2020; 190:110017. CrossRef
Sarker M, Das SC, Saha SK, Mahmud ZA, Bachar SC. Analgesic and Anti-inflammatory activities of flower extracts of Punica granatum Linn.(Punicaceae). J Appl Pharm Sci, 2012; 2(4):133. CrossRef
Schinkel AH. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Del Rev, 1999; 36:179–94. CrossRef
Singh S, Singh J. Transdermal drug delivery by passive diffusion and iontophoresis: a review. Med Res Rev, 1993; 13:569–621. CrossRef
Toopmuang P, Khamchum C, Punsuvon V. Detection and confirmation of hispolon in the mushroom Phellinus linteus. J Sci Asia, 2014; 40:141–4. CrossRef
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem, 2010; 31:455–61. CrossRef
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem, 2002; 45:2615–23. CrossRef
Vital PG, Rivera WL. Antimicrobial activity and cytotoxicity of Chromolaena odorata (L. f.) King and Robinson and Uncaria perrottetii (A. Rich) Merr. extracts. J. Med Plants, 2009; 3:511–8.
Wang J, Chen B, Hu F, Zou X, Yu H, Wang J, He H, Zhang H, Huang W. Effect of hispolon from Phellinus lonicerinus (Agaricomycetes) on estrogen receptors, aromatase, and cyclooxygenase II in MCF-7 breast cancer cells. Int J Med Mushrooms, 2017; 19(3):232–42. CrossRef
Wang JL, Limburg D, Graneto MJ, Springer J, Hamper JRB, Liao S, Pawlitz JL, Kurumbail RG, Maziasz T, Talley JJ, Kiefer JR. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorganic Med Chem Lett, 2010; 20:7159–63. CrossRef
Wang YH, Li Y, Yang SL, Yang L. Classification of substrates and inhibitors of P-glycoprotein using unsupervised machine learning approach. J Chem Inf Model, 2005; 45:750–7. CrossRef
Yazdanian M, Glynn SL, Wright JL, Hawi A. Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm Res, 1998; 15:1490–4. CrossRef
Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm Res, 1997; 14:763–6. CrossRef
Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab, 2008; 9:310–22. CrossRef