INTRODUCTION
Triamcinolone acetonide is a synthetic glucocorticosteroid (Mohamadi et al., 2017) has great immunosuppressive and anti-inflammatory activity. It is acetonide salt form of Triamcinolone. This medication works as intermediate to strong efficacy on various skin diseases like dry allergies, eczema, dermatitis, and rashes on the skin and rheumatic disorders (Decker, 1983). It is also used to prevent deterioration of asthma, Chronic obstructive pulmonary disease (COPD) (Vogelmeier et al., 2017). The intake of this medication by mouth, injection into a muscle and inhalation and ointment on surface use only. The prolonged intake of this results adverse effects like osteoporosis (Curry et al., 2018), cataract (Mohammadpour et al., 2019), most serious case leads to psychosis (Leucht et al., 2009), thrush and muscle weakness. Usage during the pregnancy is generally safe. It works by decreasing inflammation (Chiba et al., 2012) and the activity of the immune system (Kurosaki et al., 2015).
When this ointment was applied on affected area reduces the strength of swelling, itching, and redness at affected body part except underarms, face, and groin it cannot applied. The efficacy of this medication is depending on the form which we use on affected skin area. The pharmacologic activity of Triamcinolone acetonide alters gene expression it binds particular receptors of cytosolic glucocorticoid and glucocorticoids response on DNA results for the synthesis of anti-inflammatory proteins and inflammatory medications. This synthesis gave reduction in chronic inflammation, autoimmune reactions, and vital medicinal importance are the main reasons for studying quantification and validation. The structure of Triamcinolone is shown in Figure 1.
MATERIALS AND METHODS
Chemicals
Merk made analytical grade (A.R) of acetonitrile, ortho phosphoric acid (OPA), and octane sulphonic acid were procured from Merck India Pvt. Ltd, Worli, Mumbai, India. The active pharma ingredient (API) of Triamcinolone acetonide taken as a reference internal standard was procured from Dr. Reddy’s laboratory, Hyderabad. Carbonate free triple distilled water was used throughout the experiment.
Instrument
High sensitive with accuracy of Waters alliance make high performance liquid chromatography (HPLC) of model e-2,695 consisting of quaternary pump with Photodiode-Array Detection (PDA) detector-2996 and inbuilt chromatographic software Empower-2.0 was used for all chromatograms.
Buffer solution
Under the present experimental conditions, the Chromatography was optimized at pH-2.5 was maintained by using buffer solution. It was prepared by dissolving 2.5 g of octane sulphonic acid in to HPLC grade of 1 l distilled water by using 0.1 % OPA and the buffer solution was filtered through 0.45 μ filter paper.
Mobile phase solution
Analytical grade acetonitrile and buffer solutions are mixed in equal ratio (50:50) to get homogeneous after that filtered from 0.45 μ filter paper to discard insoluble impurities.
Standard aqueous Triamcinolone acetonide solution
Triamcinolone acetonide stock solution was prepared in 100 ml volumetric flask by dissolving 20 mg of Triamcinolone acetonide with 70 ml of diluent was added with 30 minutes sonication to get homogeneous. From this stock solution, we prepared required concentrations (5 into 50 ml volumetric flask) makeup with diluent.
 | Figure 1. Structure of Triamcinolone. [Click here to view] |
Validation Methods
The evolution and validation of any chemical compound by the prescribed analytical methods, and it is a drug-specific methods (validation of analytical procedure, 1996; CIPAC, 2003; ISO, 1986). The validation process consisting of several assessments parameters like appropriateness, linearity, precession, accuracy, robustness, stability, and forced degradation studies results from the accuracy and suitability of analytical parameters of the system (European Commission, 2010; Horwitz, 1982; ISO, 1996; Thompson et al., 2008).
RESULTS AND DISCUSSION
X-bridge phenyl column is an efficient separation column with significant dimensions like 150 × 4.6 mm with a pore size is 3.5 μ was used for the separation of Triamcinolone acetonide. This column has a flow rate of 1 ml/minute with operational inflow strength of 10 μl was cultivated at ambient temperature. The predicted drug was identified at wavelength region was fixed at 235 nm. The Photodiode Array Detector spectrum was operated under unique conditions shown in Table 1, and PDA spectrum of Triamcinolone acetonide was shown in Figure 2.
ACCURACY OF THE METHOD
In order to stabilize the HPLC system was balanced for about 1 hour to get standard baseline shown in Figure 3. There should be six trials were performed by injecting standard solutions into the chromatogram and the results are summarized in Table 2.
PRECESSION PARAMETERS OF THE PROPOSED APPROACH
Linearity
This analytical parameter is used to study the variations of several ingredients with respect to their strengths of analytes within specific range. This can be performed by injecting standard analyte solution by using suitable solvent for six significantly different strengths between 10% and 150% working limit. The graph is drawn between the strengths against the peak areas of Triamcinolone acetonide shown in Figure 4. From this graph, the linear trend was observed where the strengths are in between 2 and 30 μg/ml of Triamcinolone acetonide. The linearity results of Triamcinolone acetonide gave regression equation y = 43,591 × + 14,344 and correlation coefficient (R2) 0.9997 were calculated and the results are shown in Table 3.
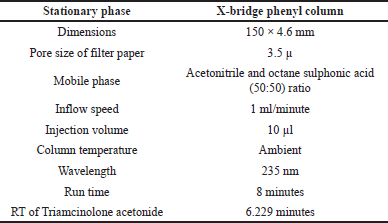 | Table 1. The improved chromatogram specifications. [Click here to view] |
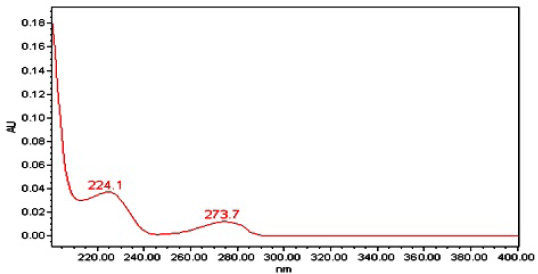 | Figure 2. PDA spectrum of Triamicinolone acetonide. [Click here to view] |
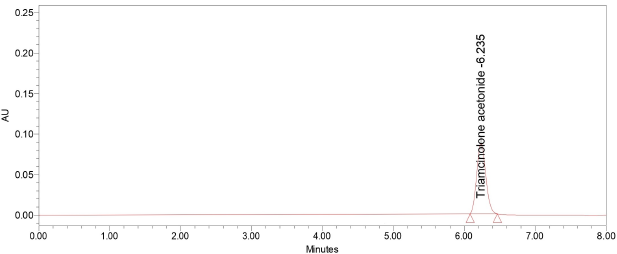 | Figure 3. Chromatogram of system precision. [Click here to view] |
 | Table 2. Results of the system precision. [Click here to view] |
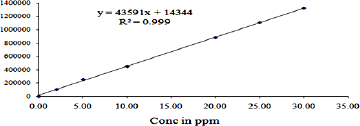 | Figure 4. Linearity calibration plots of Triamicincolon acetonide. [Click here to view] |
Method precision
Several Analytical parameters measures the degree of repeatability under optimum conditions, and it can be represented in % relative standard deviation (RSD) for specific samples which are studied according to the ICH guidelines. There should be three levels of precision, namely intermediate, repeatability, and reproducibility. Intermediate precision was performed by chromatographic pattern shows no significant variations are observed for different HPLC systems operated at different analysts with different columns. The reports of intermediate precision are % RSD is 0.24 for intra-day precision and 0.65 for inter-day precision supports the ruggedness of the proposed approach.
Accuracy
Accuracy is the comparable parameter between expected reference value and actual measured value shoes the correctness of the analytical value. To measure these three different strengths of 50%, 100%, and 150%, samples were injected for each cycle where the percentages of recovery should not less than 98% and not exceeded 99.6%. The results are shown in Table 4.
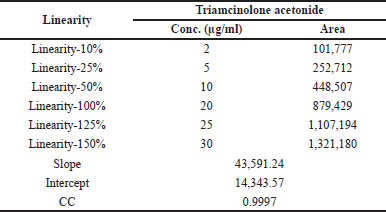 | Table 3. Linearity results. [Click here to view] |
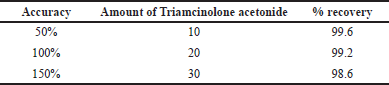 | Table 4. Accuracy of the method. [Click here to view] |
 | Table 5. Robustness of method. [Click here to view] |
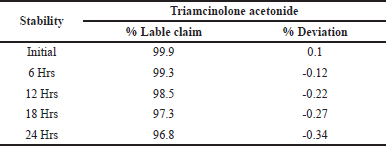 | Table 6. Results of solution stability. [Click here to view] |
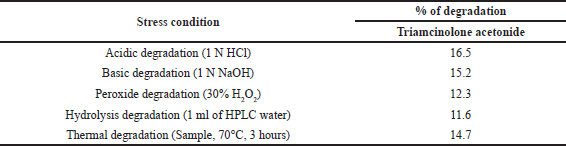 | Table 7. Results of forced degradation study. [Click here to view] |
Robustness
Robustness determines the appropriateness of the method by applied small variations like pH, ionic strength, % of solvent, and temperature on method results. The percentage of RSD indirectly assesses the robustness of the current new approach for neomycin, and it should be lower than 2%. The marginal variations are found in optimized parameters related to flow rate, it should be ± 20%, and another parameter like percentage of organic solvent in the movable phase is ± 10%. The results are shown in Table 5.
Solution stability test
To determine the standard solution stability by impurity barbed solution and control samples are studied for different intervals from 6 to 24 hours at relative wavelengths results from their percentage of RSD boundary is 2%. The reports are shown in Table 6. Under the present approach, there is no characteristic variations predicted for Triamcinolone acetonide in the standard solution stability test. From this study, the Triamcinolone acetonide solutions and sample solutions do not form any by-products during the experiment for about 24 hours.
Forced degradation study
This study, sometimes also known as stress testing, to know the stability of the API during this test drug was degraded by applying some external forces. Degradation study results from the formation of any impurities established during the storage under existed environmental conditions. The study was carried under the ICH guidelines, including various degradative reagents like acidic, basic, hydrogen peroxide, hydrolyzed, and thermal degradations are studied. The graphical representation shows that there are no impurities are formed under these conditions. Results are shown in Table 7, and the drug Triamcinolone acetonide was stable.
Advantages of the proposed method
The proposed approach has not at reported and it has great advantages like commonly available column (X-bridge phenyl), mobile phase is 1:1 acetonitrile with octane sulphonic acid, good percentage of recovery (99.6%) was achieved at a wavelength of 235 nm. This approach is comparatively quick, rugged, economic with green chemical process was adopted. The literature results (Abasi et al., 2020; Aquino et al., 2011; Kedor-Hackmann et al., 1997; Muralidharan et al., 2016; Van Heugten et al., 2018; Xiao et al., 2016) are shown in Table 8.
AGREE-Analytical GREEnness metric
AGREE is analytical tool (Pena-Pereira et al., 2020) and others (Gamal et al., 2021) for any analytical methods results show how best the proposed methods are environmental friendly and safety point of view toward humans. This tool was operated based on 12 parameters few of them are quantity of substances and reagents with toxicity, operational energy, number of run times, number of substances are identified in a single operation, etc. The proposed method was run under the AGREE-Analytical GREEness metric results 0.72 supports the approach is eco-friendly shown in Figure 5.
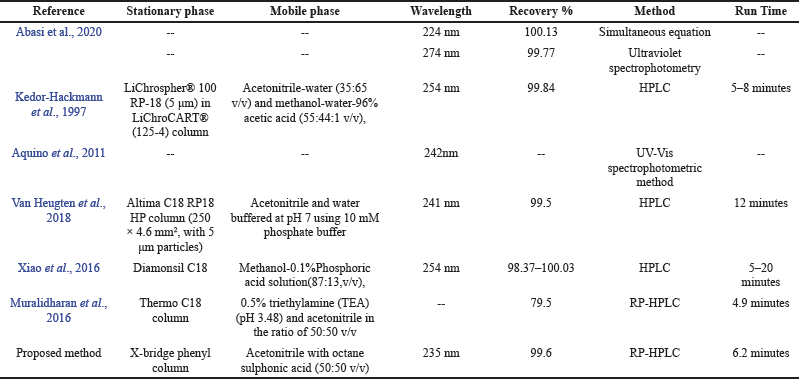 | Table 8. Comparison of the current approach with literature results. [Click here to view] |
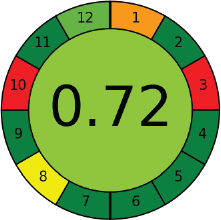 | Figure 5. Analytical GREEness. [Click here to view] |
CONCLUSIONS
The developed new approach for validation and quantification of Triamcinolone acetonide in medication shots by RP-HPLC method was validated for both specific and unspecific impurities from ICH guidelines. This green chemical method is very economical, quick response with high reliability. The Forced Degradation study shows it is unique and more stable approach. The Photodiode Array Detector spectrum and calibrated curves are unique and linear with correlation coefficient >0.99. The quantification and appropriateness of the method was studied by the various testing parameters like recovery test, fidelity, accuracy, robustness, and stability of the solution, one can understand the correctness of the approach.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abasi N, Sohrabi MR, Motiee F, Davallo M. Enhancement spectra resolution for spectrophotometric simultaneous determination of triamcinolone, neomycin, and nystatin based on continuous wavelet transform and first derivative transform in commercial ointment formulation. Optik, 2021; 226(1):165315. CrossRef
Aquino G, Stopilha R, Fernandes-Pedrosa M, Santos K, Egito S, Oliveira AG, Silva-Junior AA. Validation of quantitative analysis method for triamcinolone in ternary complexes by UV-Vis spectrophotometry. Rev Cienc Farm Basica Apl, 2011; 32(1):35–40.
Chiba T, Marusawa H, Ushijima T. Inflammation associated cancer development in digestive organs. Mechanisms and roles for genetic and epigenetic modulation. Gastroenterology, 2012; 143(3):550–63. CrossRef
CIPAC Document No. 3807. Guidelines on method validation to be performed in sup- port of analytical methods for agrochemical formulations. cipac.org, Harpenden, UK, 2003.
Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Kemper AR, Kubik M, Landefeld CS, Mangeone CM, Phipps MG, Pignone M, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for osteoporosis to prevent fractures, US preventive services task force recommendation statement. JAMA, 2018; 319(24):2521–31. CrossRef
Decker JL. American Rheumatism Association nomenclature and classification of arthritis and rheumatism. Arthritis Rheum, 1983; 26(8):1029–32. CrossRef
European Commission. Annex 15. EU guide to good manufacturing practice: qualification and validation. European Commission, Bruxelles, Brussel, BELGIQUE, pp 1–0, 2010,vol 4.
Gamal M, Naguib IA, Panda DS, Abdallah FF. Comparative study of four greenness assessment tools for selection of greenest analytical. Anal Methods, 2021; 13:369–80. CrossRef
Horwitz W. Evaluation of analytical methods used for regulation of foods and drugs, Anal Chem, 1982; 54:67A–76. CrossRef
International Standard ISO 5725. Precision of test methods—repeatability and reproducibility. International Standard ISO, Geneva, Switzerland, 1986.
ISO standard 11095. Linear calibration using reference material. ISO, Geneva, Switzerland, 1996.
Kedor-Hackmann ERM, Gianotto EAS, Santoro MIRM. Determination of triamcinolone acetonide in ointment by UV derivative spectrophotometry and high performance liquid chromatography. Anal Lett, 1997; 30(10):1861–71. CrossRef
Kurosaki T, Kometani K, Ise W. Memory B cells. Nature reviews. Immunol, 2015; 15(3):149–59. CrossRef
Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second generation antipsychotic drugs? A meta analysis of placebo controlled trials. Mol Psychiatry, 2009; 14(4):429–47. CrossRef
Mohamadi A, Chan JJ, Cleassen FM, Ring D, Chen NC. Corticosteroid injections give small and transient pain relief in rotator cuff Tendinosis, a meta analysis. Clin Orthop Relat Res, 2017; 475(1):232–43. CrossRef
Mohammadpour M, Shaabani A, Sahraian, Momenaei B, Tayebi F, Bayat R, Mirshahi R. Updates on managements of pediatric cataract. J Curr Opthalmol, 2019; 31(2):118–26. CrossRef
Muralidharan S, Venugopal V, Kumar J, Parasuraman S. Development and validation of a new RP-HPLC method for the analysis of triamcinolone in human polasma. Turkish J Pharm Sci, 2016; 13(1):9–16. CrossRef
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—Analytical GREEnness metric approach and software. Anal Chem, 2020; 92(14):10076–82. CrossRef
Thompson M, Ellison SLR, Wood R. Harmonised guidelines for single laboratory validation of method of analysis. Pure Appl Chem, 2008; 74:835–55. CrossRef
ICH. Validation of analytical procedure: methodology Q2B. In: ICH harmonized tripartite guidelines. Center for Drug Evaluation and Research & Center for Biologics Evaluation and Research, Geneva, Switzerland, 1996.
Van Heugten AJP, de Boer W, de Vries WS, Markesteijn CMA, Vromans H. Development and validation of a stability-indicating HPLCUV method for the determination of triamcinolone acetonide and its degradation products in an ointment formulation. J Pharm Biomed Anal, 2018; 149:265–70. CrossRef
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Bames PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, Varela MVL, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agusti A . Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Respirol, 2017; 22(3):575–601. CrossRef
Xiao L, Ding Z, Shigui XU, Guo Y, Wang Y, Luo M. Simultaneous determination of triamcinolone acetonide acetate and tretinoin in dimensional ointment by HPLC. China Pharm, 2016; 27(3):381–3.