INTRODUCTION
Drug delivery systems designed using nanotechnology deal with a large variety of nanosized materials that have certain valuable characteristics that are dependent on their nano-size. Their ability to boost intracellular drug delivery and their subcellular targeting ability are some examples of these characteristics. They are also able to access certain inaccessible sites of the body due to their capability to overcome barriers to the anatomy and physiology of living beings (Banerjee, 2018). Targeted drug delivery to specific sites by nanocarriers reduces off-target effects or side effects. Fluctuations in the drug level in plasma are reduced, dosage frequency is lowered, the solubility of insoluble drugs is enhanced, and an overall increase in patient comfort is seen when nano-formulations are used for therapy (Wanigasekara et al., 2016). Due to this, a substantial number of studies are undertaken to design different types of drug nanocarriers. Nanogels are an example of one such nanocarrier.
Nanogels are hydrogel nanoparticles consisting of cross-linked polymeric networks, with a tunable size of less than 100 nm. Their biocompatibility, sustainability, and versatility make them biomedically useful (Rahdar et al., 2019). The integration of the properties of hydrogels with those of nanoparticles endows nanogels with the ability to deliver drugs in a targeted and time-controlled manner. They act as ideal vehicles for drug delivery because of their superior drug loading efficiency, stability, and responsiveness to stimuli like pH, temperature, ionic strength, etc (Zhang et al., 2016).
Stimuli-responsive nanogels have the potential to conduct controlled and site-specific drug delivery. They are also known as smart or intelligent nanogels. Chemical triggers like pH, enzymes, and ionic change, and physical triggers like temperature, pressure, and magnetic field, alter the nanogel’s swelling behavior or lead to the breakdown of its polymeric network. This affects drug release from the nanogel (Ahmed et al., 2020).
A variety of synthetic methods like polymerization and cross-linking are available for the fabrication of these nanogels (Liu et al., 2014). The drug loading mechanism must be selected carefully to ensure that the large surface area provided by the nanogel network is utilized and the maximum amount of the drug is loaded (Kendre et al., 2019). Physical entrapment, covalent conjugation, and self-assembly are some of the methods by which the drug is loaded into the nanogel (Zarekar et al., 2017). The molecular weight of the polymer, the thickness of the cross-linked network of the gel, the degradation rate of the polymer, and the interaction of the drug and polymeric chains in the gel influence the drug release characteristics of the system (Suhail et al., 2019). List of polymers used as a temperature and pH-responsive nanogels as intelligent drug delivery system are listed in Table 1.
Smart nanogels are used to deliver drugs in chemotherapy, diabetes, and pulmonary diseases, to name a few. They are also used as optical imaging agents and as thermo-chemotherapeutic agents (Vicario-de-la-Torre et al., 2017). Some of the recent research based on the biomedical applications of single, dual, and multi-stimuli-responsive nanogels are listed in Table 2.
Temperature (a physical trigger) and pH (a chemical trigger) are two extremely significant environmental conditions in biological and biochemical systems (Hassanpour et al., 2017). Therefore, nanogels responsive to these pH and temperature are of great interest, and research-based on these two formulations are on the rise as depicted in the citation report generated by Web of Science, depicted in Figure 1.
Nanogel pros
Because of their high-water content, nanogels are extremely biocompatible and biodegradable formulations that function in the system like real tissues. The drug loading capacity of Nanogel is usually high in nature. The nanogel’s sustained release can be regulated by crosslinking a polymeric network, and these polymeric networks are responsible for the nanogel’s tiny size, which improves permeation in biological membranes. The most significant advantage of nanogel is that it can incorporate both hydrophilic and hydrophobic drugs, with very little premature drug leakage from the solution. It is readily delivered by parenteral and mucosal routes, and it can reach the tiniest capillary capillaries due to their small volume, as well as infiltrate tissues via the paracellular or transcellular pathways.
Nanogel cons
While nanogels have many advantages, they do have certain drawbacks that may restrict their usage in particular instances. The following are some of the limitations of nanogels. (a) Complete removal of the solvent and surfactants necessitates the use of costly methods. (b) Surfactant or monomer traces may remain and cause toxicity. (c) Manufacturing variation, in which the usual qualities of nanogels are only feasible within a narrow range of sizes. (d) In some cases, a strong interaction between drug and polymer reduces the hydrophilicity of the nanogels and causes the structure to collapse, entrapping the drug molecules irreversibly and increasing the hydrophilicity of the nanogel matrix.
This review takes a closer look at nanogels which are singly responsive to pH and temperature as well as dual pH/temperature-responsive nanogels. The synthetic methods, evaluation techniques, and applications of both single pH and temperature-responsive nanogels as well as dual pH/temperature-responsive nanogels are considered.
pH-Responsive Nanogels
pH-responsive materials can deliver drugs in a targeted manner by taking advantage of the differences in pH in the different regions of the body. Healthy tissues have a pH of 7.4, the stomach has a pH ranging from 1.0 to 3.0 while tumor tissues have a pH range of 6.5 to 7.0. Nanogels show a greater degree of stability in physiological environments in comparison to other nano vehicles. Therefore, nanogels sensitive to such pH changes may act as ideal carriers of drugs to precise locations in the body (Hajebi et al., 2019). The pH-responsive behavior of these nanogels enhances the release of the drug while minimizing its loss in off-target sites (Sim et al., 2017).
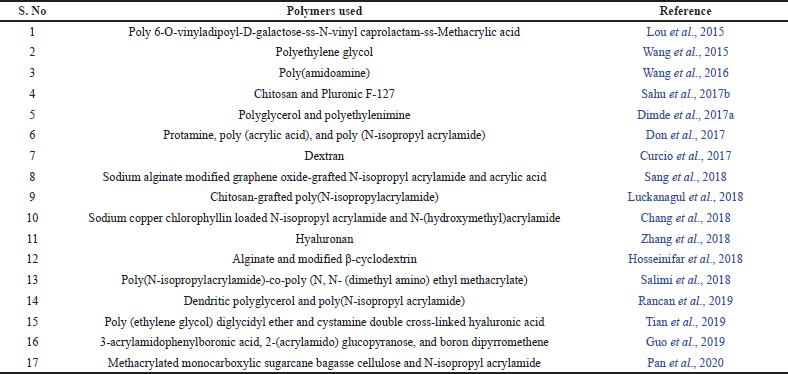 | Table 1. List of polymers used as a temperature and pH-responsive nanogels as intelligent drug delivery system. [Click here to view] |
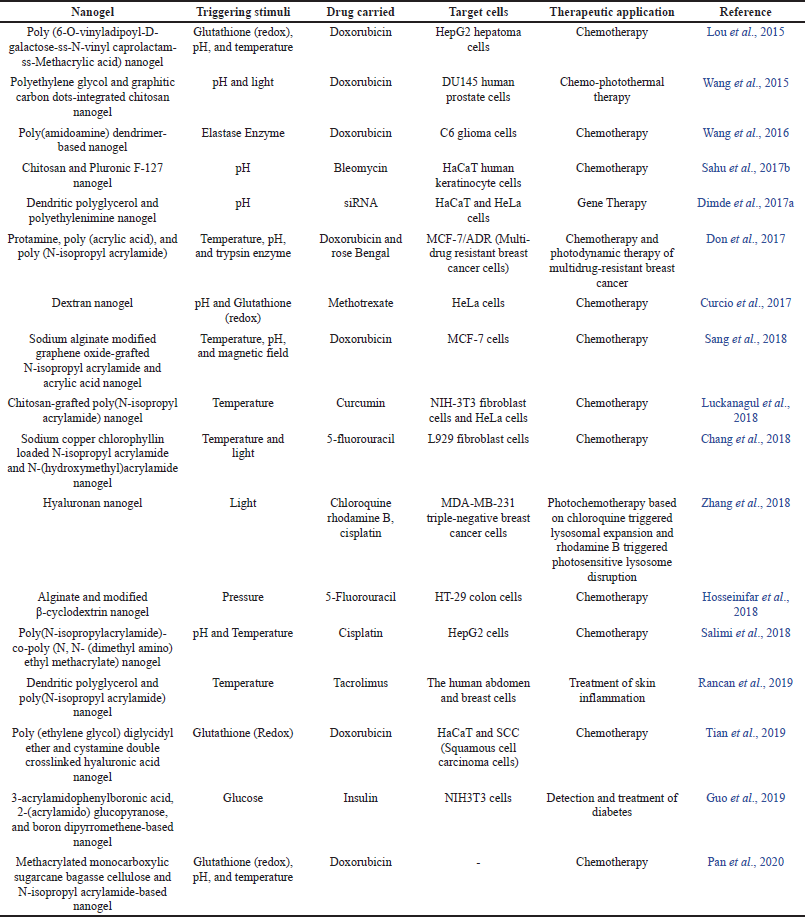 | Table 2. Smart nanogels and their biomedical applications. [Click here to view] |
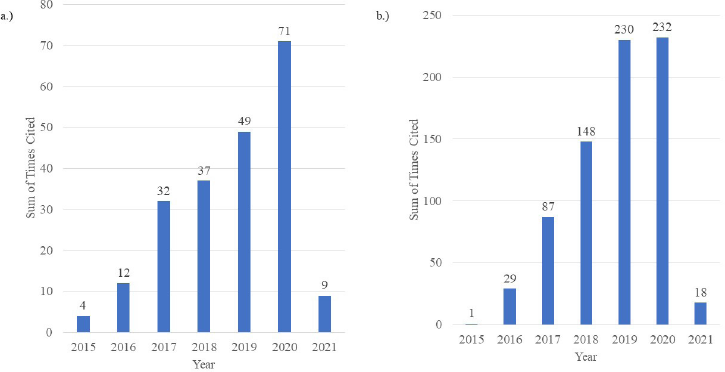 | Figure 1. Web of Science citation reports depicting the sum of citations per year (2015–2020) for the search terms. (a) “pH-responsive nanogels” and (b) “thermoresponsive nanogels” OR “temperature-responsive nanogels” (Data as of February 2021). [Click here to view] |
pH-responsive nanogels may swell or collapse depending on their ionization state due to the addition or removal of protons from the positively charged or negatively charged moieties in the nanogel system. Anionic nanogels having groups like carboxylic acid show a high degree of swelling when their environmental pH is high due to the removal of protons from the anionic groups. However, cationic nanogels consisting of amine groups are protonated at low or acidic pH values leading to the enhancement of the swelling rate of the nanogel (Gonzalez-Urias et al., 2019).
The pH-responsive behavior of nanogels may be utilized not only for drug release but also for drug loading (Qureshi et al., 2019). Both synthetic and organic polymers may be used in the fabrication of pH-triggered nanogels (Karimi et al., 2016).
Synthesis
A variety of techniques may be used to synthesize pH-responsive nanogels. Some of them are discussed in this section.
Inverse emulsion polymerization
The inverse emulsion technique is a commonly used synthetic technique for the preparation of pH-responsive nanogels. This process needs no complex apparatus and the size range of the nanogel formed may be controlled with ease. In the first step, the monomer or precursor polymer solution is emulsified in a continuous apolar phase. A suitable oil-soluble surfactant is used for this. In the next step, chemical crosslinking of the precursor polymer (which is enclosed in the droplets of the emulsion) is conducted. The monomers may also be directly polymerized. A suitable crosslinker or functional group of the polymer chain may be reacted to achieve crosslinking (Krisch et al., 2017).
In a study performed by Zhong et al. (2018), inverse emulsion polymerization was used to produce a nanogel comprising of bisacrylamide-crosslinked acrylamide along with methacrylic acid. The co-monomers were first dissolved in an aqueous solution having the bisacrylamide crosslinker and then they were added to an organic phase containing hexane, Brij-30, and dioctyl sulfosuccinate. The nanogels were polymerized via free radical polymerization inside the aqueous droplets suspended in hexane. This allowed the control of both aggregation and particle size. The synthesis technique produced uniform and biocompatible, pH-responsive (due to methacrylic acid) nanogel.
Click chemistry crosslinking polymerization
Click chemistry is a highly reactive and selective process. The yield of nanogel from this process is also sufficiently high (Buwalda et al., 2017).
For example, pH-responsive nanogels for cancer treatment were prepared in a study performed by Wang et al. (2017) using thiol-ene click chemistry. Ortho ester diacrylamide (OEAM), methoxy polyethylene glycol acrylate (mPEGA), and pentaerythritol tetra (3-mercapto propionate) (PT) were used as the reactants. PT and OEAM formed the 3D gel network of the formulation and the removal of free mercapto groups was done using mPEGA. This method was extremely efficient and free of by-products. Doxorubicin loaded into the nanogels was released at the pH of the tumor microenvironment.
Inverse nanoprecipitation
In the inverse nanoprecipitation method, hydrophilic nanogels are obtained by precipitating macromonomers in solvents such as acetone. The technique does not require surfactants or ultrasound so the processing conditions are considered to be very mild (Steinhilber et al., 2013).
A study performed to fabricate pH-degradable nanogel used the inverse nanoprecipitation method in combination with inverse electron demand Diels–Alder crosslinking chemistry. Dendritic polyglycerol was utilized as a biocompatible, synthetic polymer to functionalize the macromonomers tetrazine, norbornene, and bicyclo [6.1.0] nonyne. Benzacetal and tetrahydropyran-based acetals were incorporated into the macromonomers to endow the nanogel with pH-degradability. The process was uncomplicated and required no toxic catalysts or expensive synthetic precursors. The macromonomer solution in water (a solvent) was injected into a suitable non-solvent (acetone was used in this study). Water was found to disperse within acetone while the macromonomers were found to precipitate out of the solution due to their insolubility. Small aggregates were formed initially which eventually formed larger conglomerates. Since the local macromonomer concentration was high inside the aggregates, the reaction between the dienophile and methyl tetrazine was found to proceed rapidly and this resulted in the formation of crosslinks between the aggregates. Thus, a hydrophilic, pH-degradable nanogel was formed. Over time, smaller gel networks were found to form crosslinks among themselves until all the macromonomers were depleted. This resulted in the formation of a stable nanogel (Oehrl et al., 2020).
Evaluation of responsiveness
pH-responsive nanogels are first evaluated to figure out their particle size and morphology using techniques such as scanning electron microscopy, transmission electron microscopy, and dynamic light scattering. Zeta potential and polydispersity index of the nanogel are other instrumentally evaluated parameters. The gelation time and temperature as well as the rheological profile of the nanogel, similarly, require evaluation. The efficiency of entrapment of the therapeutic agent or drug within the nanogel is also evaluated. Apart from this, other application-specific ex vivo and in vivo studies are performed (Sahu et al., 2019).
However, to ensure that the nanogel formed is truly stimuli-responsive, specific tests must be performed to confirm its responsive behavior. Two of the in vitro tests for this purpose are swelling analysis and in vitro drug release studies. In both tests, the nanogel is exposed to buffer solutions of different pH values for specific periods (Sahu et al., 2017b).
In a study, pH-responsive, naproxen sodium-loaded nanogels were developed for gastro-protective drug delivery. The swelling behavior of this nanogel was figured out by exposing the nanogel to two buffer solutions having a pH of 1.2 and 6.8, respectively, for a specific interval of time. The degree of swelling was computed as a ratio of the mass of swollen particles to the mass of dried particles after immersion. At pH 1.2, the nanogel had remained unionized and therefore a lower degree of swelling was seen. However, at pH 6.8, a remarkable degree of swelling was seen because of the ionization of the carboxylic acid groups of the nanogel. This proved that the nanogel was responsive to the environmental pH and was able to protect the drug from the gastric pH. United States of Pharmacopoeia (USP) type-2 dissolution apparatus was used to perform in vitro drug release studies. The nanogel was added to buffer solutions of pH 1.2 and 6.8 in the dissolution apparatus and at specific time intervals, samples were withdrawn, and the amount of drug released was determined using an ultraviolet (UV) spectrophotometer. A higher drug release was seen at pH 6.8 as compared to pH 1.2. Further proof of the pH-dependent behavior of the formulated nanogel was, therefore, obtained (Sarfraz et al., 2019).
Applications
Smart nanogels facilitating drug release in response to environmental pH may conduct site-specific drug delivery. A few of the major applications of this intelligent drug delivery system are enlisted in this section.
Delivery of chemotherapeutic drugs
The pH of the tumor microenvironment is more acidic (ranges from pH 5 to 6.8) than normal tissues. This is due to the accumulation of compounds that are acidic within the tumor tissues (e.g., lactic acid). pH-sensitive polymers are used to fabricate pH-responsive nanogels that are capable of differentiating between the pH of normal cells and tumor cells, to deliver chemotherapeutic drugs specifically into the tumor tissue (Ahmadi et al., 2020).
Poly (N-isopropyl acrylamide-co-acrylic acid) nanogels were formulated specially to carry the chemotherapeutic drug doxorubicin. The nanogel showed superior drug loading capabilities due to electrostatic interactions between its anionic groups and the cationic groups of the drug. It was seen that a minimal amount of drug was delivered in a plasma simulated medium (pH 7.4, 0.14 M NaCl) while a stimulus-responsive release occurred in a lysosomal simulated medium (pH 5, 0.14 M NaCl), as depicted in Figure 2. Apart from this, the nanogels displayed low cytotoxicity [confirmed by 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay]. The gel was nanosized, so it accumulated in the tumor tissue via enhanced permeation and retention (EPR) effect. Thus, this smart nanogel was considered as an excellent candidate as a nanocarrier for doxorubicin, displaying pH-responsive drug release after endocytosis into tumor cells (Cuggino et al., 2016).
However, targeting may be inefficient if the nanogels rely only on the EPR effect to accumulate in the tumor. Therefore, they could be coupled with targeting ligands to achieve active targeting of tumors. A pH-responsive, hyaluronic acid-functionalized smart nanogel was formulated wherein methacrylate hyaluronic acid was copolymerized with a cross-linker having acid-degradable ortho-ester groups. Doxorubicin was loaded into the nanogel. Cellular uptake studies showed that the nanogel specifically targeted tumor cells. Evaluation tests using 3D tumor spheroids showed that the formulated nanogel was able to completely penetrate the tumor spheroids. Thus, greater inhibition of tumor cells was seen. The nanogels showed enhanced stability and minimal premature release of doxorubicin at neutral pH and maximal drug delivery at the acidic pH of the tumor microenvironment. Thus, targeted cancer therapy was achieved with this formulation (Yang et al., 2017).
pH-dependent, interpenetrating polymeric network nanogel carrying curcumin was also prepared using natural, non-immunogenic gelatin and acrylamidoglycolic acid. The nanogel was able to effectively encapsulate curcumin. The nanogel showed slow swelling in solutions of pH 1.2–5.0. However, it was seen to swiftly swell in solutions of pH 5.0–8.5. The formulation was also biocompatible with the fibroblast cell culture and released curcumin in a controlled fashion. It had enhanced dispersibility in aqueous solutions and improved chemotherapeutic properties in colorectal tumor cells in comparison to free curcumin. Thus, the bioavailability and site-specificity of the drug were enhanced by using smart nanogels (Madhusudana Rao et al., 2015).
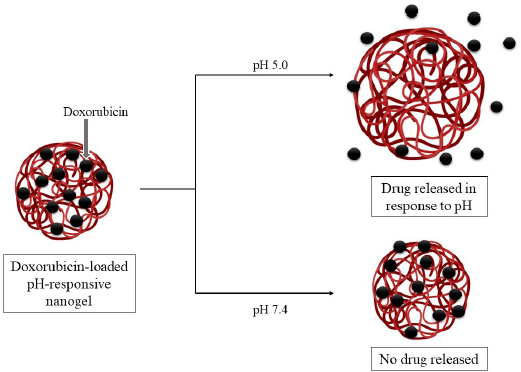 | Figure 2. Doxorubicin-loaded pH-responsive nanogels releasing the drug specifically at acidic pH (pH 5.0). Drug release does not take place at pH 7.4. [Click here to view] |
 | Table 3. Currently marketed nanogel formulations. [Click here to view] |
A polymer nanogel comprising of a pH-responsive core made of poly (2-diisopropylaminoethyl methacrylate) and a pH-sheddable shell made of polyethylene glycol (PEG) was fabricated to enhance cellular association. At the low tumor microenvironment pH, the PEG shell was cleaved and the charge on the surface of the smart nanogel was reversed. This enhanced the cellular association of the nanogel. Thus, such nanogels can be effectively used as carriers of chemotherapeutic drugs (Sui et al., 2019).
A biomimetic, temozolomide-loaded chitosan nanogel whose surface was modified with a penetration enhancer named transcutol was investigated as a possible nanocarrier system for the topical therapy of skin papilloma. The system offered pH-triggered release at the pH of the papilloma microenvironment. The formulation was stable, and a sustained, site-specific drug release took place in the tumor cells. Thus, the system could act as an alternative to needle-based chemotherapeutic systems for the treatment of skin cancer (Sahu et al., 2020).
Gene therapy
Small-sized (less than 100 nm) cationic nanogels were seen to be useful for ribonucleic acid (RNA) interference-based gene silencing. Larger gels were found to accumulate in lysosomal compartments and thus in vitro transfection failed (Nuhn et al., 2014). Cationic, pH-sensitive nanogels were investigated as carriers of genetic material. pH-cleavable dendritic polyglycerol and low molecular weight polyethylenimine-acrylamide were combined using the thiol-Michael nanoprecipitation technique. The method of synthesis was mild and did not require a catalyst, so sensitive small interfering ribonucleic acid (siRNA) could be encapsulated within the nanogel during the process of synthesis. This minimized the loss and degradation of siRNA. The nanogels formed had pH-responsive benzacetal bonds. The nanogel displayed gene silencing effects like modification-free polyethylenimine. However, the cytotoxicity of the formulated nanogel was significantly lower. It showed an enhanced transfection efficiency and controlled release of the genetic material. Thus, well-defined and user-friendly nano vehicles were formulated for gene therapy (Dimde et al., 2017a).
Gemcitabine, a chemotherapeutic drug, has a structural similarity with the deoxycytidine nucleotides of deoxyribonucleic acid (DNA). So, it could replace the nucleotides when designed as a gemcitabine-loaded DNA nanogel. The formulated nano vehicles could respond to changes in pH due to the formation of an i-motif-like quadruplex. The nanosize of the formulation enabled quick uptake by cells. The nanogel was seen to rapidly disassemble in response to pH and intracellular nuclease-mediated drug delivery was achieved. Thus, superior chemotherapeutic activity was obtained through the use of these intelligent nanostructures (Pan et al., 2019).
pH-sensitive hybrid nanogel particles were formulated via an oil-in-water emulsion process using fluorescently doped nanoparticles of silica encapsulated inside a positively charged poly (2-diethylamino ethyl methacrylate) hydrogel. siRNA loaded into the nanoformulation displayed acidic pH-triggered release. The formulation and the genetic materials were able to avoid sequestration by endosomes and degradation by enzymes. The nanogel was found to decrease the expression of the membrane receptor protein CXCR4 in MDA-MB-231 or human breast cancer cell lines. The formulation was preferentially accumulated in the tumor tissue and thus a potent chemotherapeutic effect was obtained (Khaled et al., 2016).
Miscellaneous applications
A core–shell nanogel formulated using polyethylene glycol-polyaspartic acid proved pH-sensitive swelling behavior. The nanogel was found to be hydrophilic and was able to encapsulate recombinant human insulin. Despite the generous size of the protein drug, loading efficiency was high as enhanced swelling of the nanogel took place when water was present. A low or acidic pH lowered the release of the drug due to the deswelling of the smart nanogel. Thus, the nanogel could protect the protein from acid degradation. At a pH of 7.4, 100% drug release was observed. The nanogel was also biocompatible and biodegradable. Thus, pH-responsive nanogels may be used to deliver protein drugs such as insulin orally (Park et al., 2013).
The oral route is non-invasive. Therefore, it is the route of choice for the administration of drugs. Thus, mannan-modified poly (2-hydroxyethyl methacrylate-co-methacrylic acid) nanogel was formulated as a pH-responsive oral vaccine nanocarrier. The nanogel was able to encapsulate and protect the vaccine at acidic pH and intestinal or basic pH was found to trigger the release of the vaccine. The presence of mannan further improved the internalization of the released protein by macrophages and the expression of required co-stimulatory moieties. Thus, the formulated intelligent nanogel system could be used for efficacious oral vaccine delivery (Durán-Lobato et al., 2014).
In another study, Carbopol and pH-sensitive methacrylic acid were used to formulate nanogels. Ketoprofen, a gastro-irritant, was loaded into the nanogel and it was seen that the nanogel was able to shield ketoprofen from the low (acidic) pH of the stomach and released it at a higher or basic pH of the intestine. So, pH-responsive nanogels may carry out gastro-protective drug delivery of irritant drugs (Sarfraz et al., 2020).
Dendritic polyglycerol was used to formulate a pH-responsive nanogel which was then used to measure pH values inside hair follicles. A porcine ear model was used for the ex vivo evaluation of the formulated nanogel. The nanogel was able to penetrate the skin at a great depth using the follicular pathway and the pH was found to increase from pH of 6.5 at the surface of the skin to pH 7.4 in deeper regions of the hair follicle. Additionally, the nanogel created was non-toxic. Thus, the system behaved as a pH-sensitive nanosensor and could be developed further as a smart delivery system for the intrafollicular delivery of drugs (Dimde et al., 2017b).
Temperature-Responsive Nanogels
The temperature may function as a trigger for intelligent drug delivery to inflamed areas and tumor tissue when they have elevated temperatures between 40°C and 45°C. They display superior stability during circulation and faster drug release kinetics in comparison to hydrogels (Hajebi et al., 2019). Temperature-responsive nanogels show shrinking-swelling behavior triggered by environmental temperature. This facilitates controlled drug release from the smart nanogel (Yin et al., 2020).
When heating alters the hydrophobic/hydrophilic ratio of polymers and reduces their solubility, they are characterized according to a value termed as lower critical solution temperature (LCST). Above LCST, these polymers become insoluble and the formulated nanogel collapses/shrinks, whereas below LCST the nanogel swells. Some polymers, on the other hand, become more soluble on being heated. They are characterized according to the upper critical solution temperature (UCST). Below UCST, these polymers become insoluble and the nanogel formed collapses, whereas above UCST, swelling of the nanogel occurs. A specific temperature known as volume phase transition temperature (VPTT) is correlated to the LCST and UCST values of the polymer used to create the nanogel. When the temperature is below the VPTT of the polymer, polar–polar interactions between hydrophilic portions of the polymer and water are predominant as compared to nonpolar–nonpolar interaction of the hydrophobic regions of the nanogels with each other. The degree of swelling of the gel is enhanced by improving the ratio of solvation of the chains of the polymer. Above VPTT, nonpolar–nonpolar interactions are predominant and water molecules are released into the solution phase. A deswelled polymer network is left behind (Ahmadi et al., 2020).
An example of a polymer used to create thermoresponsive, smart drug delivery vehicles is poly(N-isopropyl acrylamide) (PNIPAM) (Wang et al., 2019). It exhibits temperature-responsive behavior below LCST, at a temperature near 32°C (Lang et al., 2018). Below LCST, the polymer is hydrophilic, while above LCST the polymer aggregates and starts showing hydrophobic characteristics. The system is thus found to shrink above LCST. The LCST of the polymer may also be controlled by modifying the polymer chain (Yin et al., 2020).
Synthesis
Of the substantial number of synthetic techniques used to fabricate thermoresponsive nanogels, a few are discussed in this section.
Reversible addition-fragmentation chain transfer (RAFT) polymerization
Reversible addition-fragmentation chain transfer or RAFT polymerization is a flexible and popular method for imparting living characteristics to radical polymerization. It applies to a large number of monomers and can withstand not only different reaction conditions but also different solvents. The reaction does not require any metal catalysts (Peng et al., 2016b). So, it can be used to synthesize substances that have biomedical applications. Nanogels formulated using the RAFT polymerization technique can be controlled to a great extent concerning their structure and characteristics (Rajan et al., 2017).
A thermoresponsive nanogel was formulated by RAFT polymerization using the monomer 3-dimethyl(methacryloyloxyethyl)ammonium propane sulfonate. Its polymer was found to display UCST behavior in the presence of water. The experiment was conducted in a mixture of ethanol and water, at a temperature of 70°C. Poly (poly (ethylene glycol) methyl ether methacrylate) was used as a macro-chain transfer agent (CTA) to polymerize the monomer. A difunctional monomer was used as a cross-linker. Hairy nanogels were formed at a high concentration of ethanol. The smart nanogel displayed reversible swelling (induced by heating) and shrinking (induced by cooling). This was found to be by UCST behavior that was hypothesized. The nanogel also possessed a tunable volume changing the ratio, size, and transition temperature based on the concentration of ethanol in the ethanol–water mixture, molar ratio of monomer: macro-CTA, and quantity of crosslinker (Fu et al., 2017).
Inverse miniemulsion polymerization
Miniemulsion polymerization methods involve stable mixtures consisting of hydrophilic polymer-surface active agent assemblies in continuous organic mediums (Chiriac et al., 2019).
Inverse miniemulsion was used to fabricate thermoresponsive nanogels using N-vinyl caprolactam (NVCL) and 2-methoxyethyl acrylate (MEA) as monomers. Both monomers owned favorable biocompatibility. Poly (ethylene glycol) dimethacrylate (PEGDMA) functioned as a crosslinker, enhancing the thermoresponsive nature of the nanogel. An emulsifier solution of low polarity was made using the surfactants Span 80 and Tween 60. NVCL, MEA, PEGDMA, and sodium tetrafluoroborate were dissolved in water, resulting in a polar solution. Mixing and pre-emulsification were done, resulting in a crude emulsion. It was then sonicated and a monomer miniemulsion was formed. An initiator was added to it and nitrogen gas was used to purge the formulation. Polymerization was then conducted in an oil bath under magnetic stirring. The resultant smart nanogel displayed reversible temperature-responsive transition behavior (Gao et al., 2019).
Precipitation polymerization
Emulsion polymerization may be a versatile synthetic method, but it suffers from some drawbacks such as a difficult surfactant removal process and poor control over the polymetric network of the nanogel due to self-crosslinking at high temperatures. To overcome this, a facile synthetic process free of heat and surfactants was investigated. In this process, the thermosensitive PNIPAM nanogel was prepared by precipitation polymerization. A co-initiator system consisting of potassium persulfate (KPS) and N, N, N?, N″-tetramethyl ethylenediamine (TMEDA) was used and N, N?-methylene bisacrylamide (MBA) was used as a crosslinker. The mixture of N-isopropyl acrylamide (NIPAM), TMEDA, MBA, and deionized water was first taken in a three-neck round-bottomed flask. After the reagents were entirely dissolved, polymerization of NIPAM was initiated adding KPS to the solution dropwise. Purification of the nanogel obtained was done by dialysis. TMEDA functioned as a catalyst, speeding up KPS release, and the formation of free radicals. This led to the initiation of polymerization of NIPAM in the presence of an MBA. The nanogel displayed distinct thermoresponsive properties (Wei et al., 2018).
Evaluation of responsiveness
Similar to pH-responsive nanogels, before evaluation of the thermoresponsive behavior of the formulated nanogel, parameters such as zeta potential, size, and size distribution are determined using techniques such as dynamic light scattering (Gerecke et al., 2017). FT-IR and protein nuclear magnetic resonance studies may be performed to study the formation of the nanogel. Differential scanning calorimetry may be used to determine the thermal properties of the nanogel (Morelli et al., 2018).
Other evaluation tests to characterize thermoresponsive nanogels, such as the determination of the degree of polymerization of the nanogel by gel permeation chromatography studies, are also done. Transmission electron microscopy studies produce the average radius of the formulation. In a study, the thermoresponsiveness of the system was confirmed via its swelling and shrinking behavior on being exposed to successive cycles of heating and cooling. Five cycles of heating from 10°C to 60°C and cooling from 60°C to10°C were carried out and the hydrodynamic radius was measured to prove that the nanogel was sensitive to alterations in temperature (Ohshio et al., 2019).
Thermoresponsiveness of glycerol-based nanogels could also be determined using UV-Vis spectroscopy. The phase transition behavior of the nanogel could be estimated by measuring its cloud point temperature (Giulbudagian et al., 2014).
Apart from this, tests to determine the therapeutic efficacy and safety of the formulation may also be performed (Luckanagul et al., 2018).
Applications
Like pH-responsive nanogels, thermoresponsive nanogels can also conduct site-specific, stimulus-responsive drug release. Some of the biomedical applications of the formulation are elaborated in this section.
Treatment of skin inflammation
Thermoresponsive nanogels may be designed using the natural properties of the skin. An increase in skin temperature from 32°C to 37°C is seen with increasing depth. So, drug-loaded nanogels possessing a transition temperature of 35°C could utilize this temperature gradient to release the drug (Cuggino et al., 2019). Temperature triggers a reversible change in the volume of the nanogel when the VPTT is crossed. This allows controlled drug release by the nanogel (Tiwari et al., 2019).
In inflammatory skin conditions like psoriasis, the skin may be deficient in a stratum corneum barrier. Thermoresponsive nanogels loaded with etanercept were designed to treat such skin conditions. Release of the drug from the formulation was seen to take place in response to temperature, after topical application. The drug was distributed throughout the stratum corneum and the viable epidermis and an enhanced anti-inflammatory activity was seen (Giulbudagian et al., 2018b).
Polyglycerol-based thermoresponsive nanogels were also used for topical drug delivery across skin with both intact and disrupted barriers. The thermal trigger applied via infrared radiation enabled the formulation to penetrate the stratum corneum to a greater depth. The formulation also reached the epidermis and dermis of barrier-free skin in significant amounts. Thus, the nanogel could be used for treating inflammatory skin disorders (Rancan et al., 2017).
Thermoresponsive nanogels for topical administration, comprising of dendritic polyglycerol and thermoresponsive glycerol, were used as nanocarriers for dexamethasone. β-cyclodextrin was used as a penetration enhancer in the formulation. The drug was found to be localized in the hydrophobic cavity of cyclodextrin. Thus, effective delivery of the drug to the epidermis and dermis of the skin was seen and this nanotechnology-based therapeutic system could be used to treat inflammatory skin diseases. It proved to be superior to marketed dexamethasone creams (Giulbudagian et al., 2018a).
When gold nanoparticles were combined with thermoresponsive nanogels made of Pluronic F-127 and hydroxypropyl methylcellulose and investigated for its antibacterial and burn-induced wound healing capabilities, an enhancement in bioavailability, skin permeation, anti-bacterial, and anti-inflammatory activity was seen. The nanogel displayed prolonged and sustained effects (Arafa et al., 2018; Zafar et al., 2014).
The immunotoxicology of various thermoresponsive nanogels was studied by Edlich et al. (2017) Despite superior cellular uptake, no cytotoxic or genotoxic effects were observed. No reactive oxygen species were induced. Thus, thermoresponsive nanogels were found to be safe for topical use.
Penetration into the hair follicle
Nanoparticles can penetrate the hair follicle but are unable to overcome the follicular barrier. So, they cannot reach the viable cells or release the drug they carry. Alternatively, small-sized drugs cannot penetrate the hair follicle to a great depth. Thus, studies showed that the most effective method to deliver drugs via the follicular route was to use nanocarriers capable of releasing the drug in response to a stimulus, in the vicinity of the structure being targeted. This could be achieved using thermoresponsive nanogels (Sahle et al., 2017).
Thermoresponsive nanogels labeled with indodicarbocyanine, owning a cloud point of 34°C, were evaluated for their ability to penetrate the hair follicle. The results of the studies performed proved that the smart nanogel may be useful for temperature-triggered penetration into the follicles of the hair under physiological conditions (Jung et al., 2018).
Delivery of chemotherapeutic drugs
The onset of hyperthermia leads to increased vascular perfusion and tumor tissue permeability in cancer cells. Based on this property of cancer cells, thermoresponsive nanogels may be useful as site-specific, “on-demand” delivery vehicles for chemotherapeutic agents (Indulekha et al., 2017).
Poly(L-lactide)-g-pullulan copolymer-based nanogels were studied as nanocarriers for the delivery of chemotherapeutic drugs. A doxorubicin-loaded nanogel was prepared and an increase in drug release was seen to take place with increasing temperatures for 50 hours. Thus, the drug-loaded nanogel displayed effective long-term drug release and chemotherapeutic activity at elevated temperatures. The thermoresponsive behavior of the smart nanogel was investigated via the MTT assay. In vitro cytotoxicity was studied at 37°C and 42°C. Results showed that the nanogel was biocompatible. The cytotoxic effect of the nanogel at the higher temperature was 1.6 times more than that at the lower temperature as the amount of drug released at 42°C was more than the drug released at 37°C. Higher temperature led to higher hydrophobicity inside the drug-loaded nanocarrier. This allowed the nanogel to be internalized into the tumor cells easily (Seo et al., 2012).
5-fluorouracil and megestrol acetate were loaded into a fibrinogen-graft-poly (N-vinyl caprolactam) nanogel to obtain a biocompatible, temperature-sensitive formulation. The formulation was directed towards specific receptors present on breast cancer cells. The drug release was found to be significant above LCST rather than below it. The formulation displayed improved toxicity, apoptosis, and cellular uptake when studied using the MCF-7 cell line. The drug combination was released in a sustained manner in vivo. Thus, smart nanogels may effectively deliver chemotherapeutic drugs to breast cancer cells (Rejinold et al., 2015).
Hybrid core–shell nanogels consisting of NIPAM and vinyl-modified silica were used for thermoresponsive drug delivery. Doxorubicin-loaded nanogels were prepared and the drug release at various temperatures was observed via UV-vis spectroscopy. The release of the drug was found to be higher when the temperature was lower than the temperature of the gel collapse of the polymer. The smart nanogel was found to be biocompatible and could release the drug in a controlled manner in tumor tissues, as confirmed by cytotoxicity studies (Hajebi et al., 2020).
Nowadays, thermoresponsive nanogels are often combined with photosensitive materials (Strozyk et al., 2017). In a study, a temperature-sensitive poly (N-isopropyl acrylamide-co-acrylic acid) nanogel was used to encapsulate a heat shock protein 70 inhibitor, 2-phenylethynesulphonamide (PES). A photothermal coupling agent named poly (3,4-ethylene dioxythiophene) was also loaded in the nanogel. When irradiated with near-infrared (NIR) radiation, a temperature-triggered phase transition was found to take place and PES was released from the nanosystem. PES lowered the tumor cells’ tolerance to heat. So, tumor tissues could be ablated at a greater depth with a small amount of NIR radiation (Liu et al., 2016).
Gene delivery
Thermoresponsive nanogels have been studied as potential carriers of genes for the treatment of cancer and inflammatory diseases as a result of their capacity to transport the gene to specific regions in response to temperature changes (Wang et al., 2015).
PNIPAM was integrated into the side chain of polyethyleneimine to obtain a thermosensitive nanogel. TRP53, a tumor suppressor gene that plays a crucial role in the regulation of the cell cycle and programmed cell death, was loaded in the nanogel. The nanogel showed significant transfection efficiency, in vivo accumulation, and tumor inhibition. When the temperature of PNIPAM was increased above its VPTT, the cellular uptake of the nanogel was enhanced. Thus, the tumor was passively targeted and inhibited (Cao et al., 2015).
Genetic skin diseases which lead to the reduction of the synthesis of proteins, require the substitution of the specific proteins locally. The physicochemical properties of proteins exempt them from topical delivery. However, to prove the efficacy of intelligent nanogels for topical gene delivery, the protein transglutaminase 1-loaded nanogels consisting of temperature-responsive poly (N-isopropyl acrylamide)-polyglycerol were designed. It had a thermal trigger point of 35°C and this was suitable for cutaneous delivery based on the natural physiological temperature gradient of skin. When the temperature was greater than or equal to 35 C, particle size was seen to reduce, protein release was seen to increase. No alterations in the structure or the activity of the protein were seen. The formulation was also capable of penetrating the skin significantly (particularly in barrier-deficient skin). Transglutaminase 1 was efficiently delivered to skin models that lacked the protein and the skin model’s barrier function was found to be restored. Thus, this Nano formulation could be used to topically deliver genes for the treatment of genetic skin conditions (Witting et al., 2015).
Miscellaneous applications
Local anesthetics have poor percutaneous absorption across the skin. This makes its topical delivery complex. To overcome this problem, a thermoresponsive mixed micellar nanogel loaded with lidocaine and prilocaine was fabricated. When a temperature change took place, the system changed its phase (sol to gel). This property of temperature-induced phase transition made the nanogel suitable for topical application. The formulation was found to be compatible with the skin when applied topically and an enhanced anesthetic effect was observed when in vivo studies were performed. Thus, the smart nanotherapeutic system could be utilized for topical anesthesia (Sharma et al., 2017).
Thermoresponsive nanogels were also studied as potential ophthalmic drug delivery vehicles. A hydrophobic drug named muscone was loaded in the nanogel and the formulation exhibited phase transition at physiological temperature. The nanogel was also capable of resisting clearance because of blinking, leading to enhanced retention time on the surface of the cornea. It could permeate into the ocular surface more effectively. Additionally, it was found to have improved biocompatibility and bioavailability. Thus, such thermoresponsive systems could effectively deliver hydrophobic drugs to the eye (Wang et al., 2016).
The process of transporting antigens across the stratum corneum is complex and thus transdermal immunization is difficult. A poly (N-vinyl caprolactam)-based nanogel was studied as a potential thermoresponsive, antigen delivery vehicle. The temperature of the transition of the nanogel was found to be close to the physiological skin temperature. The nanogel was loaded with ovalbumin. In vitro evaluation tests proved that the formulation was cytocompatible and capable of loading and delivering the protein in a temperature-triggered fashion. Ex vivo skin penetration studies were performed using a human skin model. Enhanced skin permeation was seen after the topical application of the nanogel as compared to free ovalbumin. Thus, intelligent, temperature-responsive nanogels have great promise as topical vaccination systems (Sonzogni et al., 2018).
Smart nanogels comprising of poly[N-(2-hydroxypropyl) methacrylamide] and thermoresponsive poly[N(2,2-difluoroethyl) acrylamide] were developed as biocompatible, thermosensitive nanostructures. They possessed significant amounts of magnetically equivalent atoms of fluorine and could be used for 19F magnetic resonance imaging (Kolouchova et al., 2018).
4. DUAL pH/Thermoresponsive Nanogels
Single stimulus-responsive nanogels are unable to effectively release the drug they carry in a controlled manner. Dual stimuli-responsive nanogels have been suggested as an alternative to overcome this problem (Yin et al., 2020). Dual pH and temperature-responsive nanogels combine the advantages of both pH-responsive and temperature-responsive nanogels.
Pathological conditions such as cancer are associated with an increase in temperature locally (2°C–5°C increase) and a local decrease in pH (by 1–2.5 units of pH). Smart nanogels dually sensitive to pH as well as temperature may be used for site-specific therapy of such conditions, as depicted in Figure 3 (Salehi et al., 2015). Dual pH/redox-responsive (Yang et al., 2016), pH/glucose-responsive (Si et al., 2019), redox/thermal-responsive (Peng et al., 2016), photothermoresponsive nanogels (Chang et al., 2018) are some of the other dual stimuli-responsive formulations that have been investigated.
An example of a dual pH and temperature-responsive polymer is poly (N, N-diethylamino ethyl methacrylate) (PDEAEM). The tertiary amino moieties of the polymer could be easily changed to quaternary amino groups and this gave the polymer a positive charge. The pKa of the polymer was found to be close to 7 and the polymer displayed a physiologically significant volume phase transition pH ranging from 5.0 to 7.4. The smart polymer was also observed to have a pH-dependent temperature of transition (Manzanares-Guevara et al., 2018). Alternatively, acidic monomers such as acrylic acid may be copolymerized with thermoresponsive substances like PNIPAM to obtain dual responsive nanogels (Bardajee et al., 2017).
Synthesis
Some of the synthetic methods reported in the literature for the formulation of dual pH/thermoresponsive nanogels are detailed in this section.
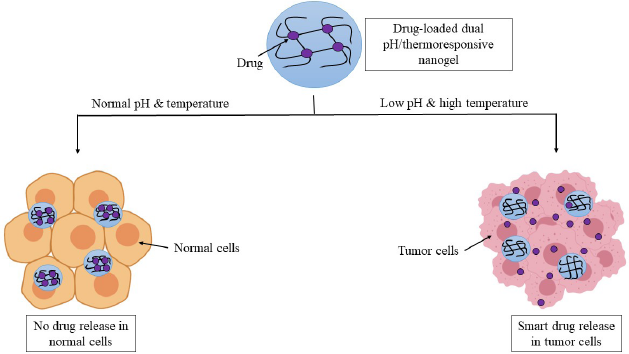 | Figure 3. Drug-loaded dual pH/thermoresponsive nanogel releases the drug at the specific pH and temperature of the tumor microenvironment. Side effects are decreased as no drug is released at the physiological pH and temperature of normal cells. [Click here to view] |
Free radical polymerization
Free radical polymerization could be used for the formulation of nanogels comprising PNIPAM and chitosan. MBA was used as the crosslinking agent and N, N, N?-ammonium persulfate (APS) functioned as the initiator. The monomers were converted into free persulfate radicals by the initiator. This resulted in the induction of polymerization. The initiator and catalyst (N?-tetramethyl ethylenediamine) were first dissolved in distilled water and the solution was heated to a temperature of 70°C. Degassing was done by using nitrogen. The mixture was stirred continuously. Sodium dodecyl sulfate was used to stabilize precursor particles and this led to the reduction of the particles to the nano-range. Reactant solutions consisting of NIPAAM, MBA, and sodium dodecyl sulfate were dissolved in distilled water. Glacial acetic acid was used to dissolve chitosan. The reactant solutions were then added dropwise to the mixture of initiator and catalyst and polymerization took place on constant stirring (Štular et al., 2018).
Free radical polymerization was also used to synthesize monodispersed nanogels consisting of oligo (ethylene glycol) methacrylate. Ultrasonication was used to assist the process as it lowered the reaction time and the desired nanoformulation could be obtained in the absence of an inert atmosphere (Macchione et al., 2019).
Emulsion polymerization
Acrylic acid and butyl acrylate were used as comonomers in the fabrication of dual responsive nanogels by emulsion polymerization in a study performed by Ashrafizadeh et al. (2019) Acrylic acid functioned as a highly hydrophilic, pH-sensitive, cost-effective, and biocompatible substance. Hydrophobic butyl acrylate, owning a low glass transition temperature, improved the chain flexibility and stretching ability of acrylic acid. The hydrophobicity of butyl acrylate also enhanced the strength of the crosslinking of the monomers. The presence of both hydrophilic and hydrophobic components in the nanogel allowed the loading and delivery of a larger variety of drugs. Chemical crosslinking was done using a bifunctional comonomer, ethylene glycol dimethacrylate (EGDMA) which also prevented the nanogel from disintegrating. Sodium persulfate functioned as the initiator. The reactants were fed into the reaction vessel and heating and continuous stirring led to the initiation of nucleation. Cooling resulted in the formation of a dispersion. It was filtered and purified by ultrafiltration to obtain the nanogel. Direct emulsion polymerization provided an easy synthetic route for the fabrication of the nanogel, free of multiple synthetic steps and costly, toxic solvents (Li et al., 2018).
To avoid using toxic surface-active agents, a soap-less or surfactant-free emulsion polymerization was adopted to fabricate nanogels comprising of a PDEAEM core and PEG shell (An et al., 2015). This is a “green” method of synthesis because the process is conducted in the water and no solvent-based purification stages are involved. A simple dialysis method is sufficient for purification. The method is scalable and uniformly sized nanogels are obtained. Diethylaminoethyl methacrylate (the main monomer), polyethylene glycol methyl ether methacrylate (stabilizer), and EGDMA (crosslinker) were mixed and dissolved in deionized water (Lipowska-Kur et al., 2020). The process of polymerization took place at a temperature of 85°C under continuous stirring. Potassium persulfate (initiator) functioned as the initiator of polymerization. The process was halted through cooling and the resultant dispersion was purified by dialysis to obtain the desired dual responsive nanogel (Manzanares-Guevara et al., 2018).
Evaluation of responsiveness
The nanogels must be evaluated to ensure that they are responsive to both pH and temperature. To that end, dual responsiveness of the smart nanogels comprising of crosslinked poly [itaconic anhydride-co-3,9-divinyl-2,4,8,10-tetraoxaspiro (5.5) undecane] and 1,12-dodecandiol was evaluated using dynamic light scattering technique. The effect of temperature on zeta potential and hydrodynamic radius of the nanogel was investigated using a Peltier device. The nanogel was exposed to a pH range of 4–11 and the effect of pH on the zeta potential and radius of the nanogel was studied to prove the pH responsiveness of the nanogel (Nita et al., 2016).
NIPAM nanogels fabricated via differential microemulsion polymerization were evaluated for their ability to respond to pH by using acid-base solutions and phosphate buffer solutions. The particle size was studied after exposure of the nanogels to different acidic and alkaline pH values. Similarly, acid-base and buffer solutions of certain fixed pH values were taken and the effect of increasing solution temperatures on the size of the nanogel particles was investigated to prove thermoresponsive behavior (Pruettiphap et al., 2017).
In another study, dual temperature and pH-responsive, 5-fluorouracil-loaded nanogels formulated using poly (N-vinyl caprolactam) were evaluated for their responsiveness to both stimuli by performing in vitro drug release studies using a tablet dissolution tester. The formulation was exposed to 0.1 M HCl solution of pH 1.2 and phosphate buffer solution of pH 7.4 at two different temperatures, 25°C and 37°C. Samples withdrawn at regular intervals were evaluated for the drug content using a UV spectrophotometer. The nanogels were fully swollen at the lower temperature (below its LCST) and they were present in a collapsed state at the higher temperature (above LCST). So, drug release was more at 25°C than 37°C. This confirmed the thermoresponsive behavior of the nanogel. The release of the drug was also higher at intestinal pH or pH 7.4 rather than at gastric pH (pH 1.2) due to greater swelling of the polymer network at higher pH. Thus, the nanogels were proved to be pH-responsive as well (Madhusudana Rao et al., 2013).
Applications
The advantages of pH and temperature-responsive nanogels are combined to obtain dual pH/temperature-responsive nanogels. These novel nanoformulations have a variety of biomedical applications, some of which are discussed in this section.
Delivery of chemotherapeutic drugs
Certain malignant cancers give rise to alterations in both pHs and the temperature around the tumor simultaneously. So, a small rise in local temperature and a slight lowering of extracellular pH (pH 6–7) are observed. The pH is found to decrease further within the cells, primarily within endosomes (pH 5–6) as well as lysosomes (pH 4–5.0). So, based on this property of the tumor, dual pH/thermoresponsive nanogels can be formulated. These nanogels can intelligently differentiate between tumor cells and normal, healthy cells. Thus, the targetability and therapeutic efficacy of the formulation is enhanced (Salehi et al., 2015).
Additionally, both pH and temperature can be regulated with ease with the help of external triggers (Soni et al., 2016).
Nanogels consisting of thermoresponsive NIPAM and pH-responsive N, N-(dimethylamino) ethyl methacrylate crosslinked using MBA were loaded with cisplatin and magnetic nanoparticles made of Fe3O4. As per the results of the MTT assay, the drug-loaded magnetic nanogel displayed increased cytotoxicity on HepG2 cell lines as compared to the free drug. Cytotoxicity assay results also proved the biocompatibility of the nanogel. A greater drug release was seen at the pH (pH 5.7) and temperature (40°C) of the hepatocellular carcinoma microenvironment rather than at physiological pH and temperature. Thus, the novel, dual stimuli-responsive nanogel designed in this study could be used for the efficient treatment of hepatocellular carcinoma (Salimi et al., 2018).
Bionanogels responsive to dual stimuli were formulated using pH-responsive carboxymethyl cellulose grafted with thermoresponsive oligo (-ethylene oxide)-based copolymers. At low pH (pH 6.5 extracellularly and pH 5.0–6.5 inside endosomes and lysosomes of cancer cells) and high temperature (higher than LCST), doxorubicin carried by the nanogel was released. Thus, the drug-loaded, intelligent bionanogel possessed enhanced chemotherapeutic potential (Wen et al., 2015).
A dual pH/thermoresponsive nanogel comprising of poly (N-isopropyl acrylamide-co-2-acrylamido-2-methyl-1-propanesulfonate-co-1-propene-2-3-dicarboxylate) crosslinked by ethylene glycol dimethacrylate was designed to deliver doxorubicin specifically to tumor tissue by bypassing several physiological barriers. The nanogel was conjugated to N, O-carboxymethyl chitosan to improve its pH-responsive behavior. The nanocarrier exhibited high drug loading capabilities and the drug exhibited slow and sustained drug release at the temperature (37°C) and acidic pH (pH 5.5) of the tumor microenvironment. The nanogel was found to be taken up by MCF-7 cells rapidly and exhibited preferential cytotoxic behavior towards breast cancer cell lines (MCF-7 and MB231) as compared to normal human breast epithelial cell lines (MCF10A). Thus, targeted and controlled delivery of the entrapped chemotherapeutic drugs took place leading to an enhancement in the efficacy of treatment (Verma et al., 2016).
Treatment of diabetes
Controlled and prolonged insulin delivery systems are needed to reduce the frequency of injections taken by diabetic patients. This, in turn, will enhance patient compliance with therapy (Yao et al., 2014).
Hydroxypropyl methylcellulose crosslinked with methacrylic acid using poly (ethylene glycol) diacrylate as a crosslinking agent, was used to fabricate hybrid nanogels. At a pH of 6, the LCST of the smart nanogel was found to be near the normal temperature of the human body. Thus, sustained release of insulin may take place. The nanogel also had a high insulin loading capacity and entrapment efficiency. By adjusting the pH and temperature, control over drug release from the nanogel can be achieved. Thus, a controlled release of insulin from the nanogel can be achieved in patients suffering from diabetes (Zhao et al., 2016).
Miscellaneous applications
Poly-ε-caprolactone nanoparticles carrying triclosan (antimicrobial drug) and chitosan-based hydrogels carrying flurbiprofen (an anti-inflammatory drug) were entwined to obtain smart nanogels. The formulation displayed both pH-responsive and thermoresponsive behavior. The nanogel was also sufficiently bioadhesive and in vivo studies proved that the formulation exhibited enhanced antibacterial and anti-inflammatory activity. The smart nanocarrier was thus a suitable therapeutic system for the treatment of periodontitis using triclosan and flurbiprofen (Aminu et al., 2019).
Nanogels possessing PDEAEMA cores and poly (N-vinyl caprolactam) (PVCL) shells were fabricated for the delivery of genes. The nanogel formed complexes with siRNA via electrostatic interactions. Extensive evaluation tests proved the dual responsive behavior of the nanogel. The novel, intelligent, biodegradable, cationic nanogel provided greater sophistication to the therapeutic system and was capable of carrying out controlled delivery of genes (Aguirre et al., 2016).
There are several nanogel formulations on the market. The majority of the marketed formulations are esthetic treatments, many tooth paste formulations minimize tooth decay issues, and other formulations are personal care items that are extensively used for skin care concerns. Currently marketed nanogel formulations are listed in Table 3.
CONCLUSION AND FUTURE PERSPECTIVE
The changing face of healthcare is giving rise to a demand for more efficient therapeutic systems. Stimuli-responsive nanogels were studied extensively and results proved that they were suitable alternatives to conventional drug delivery vehicles. They could intelligently deliver drugs to specific targets in the body in response to different physical and chemical stimuli. Therefore, they reduced unwanted off-target effects. pH and temperature, being the main chemical and physical properties of a biochemical system, respectively, are two of the major stimuli used to trigger drug release from nanogels. Therefore, nanogels singly responsive to pH and temperature were investigated at length. The advantages of these two types of nanogels were combined and dual pH/temperature-responsive nanogels were also fabricated. These nanogels could be synthesized in several diverse ways. Each synthetic process owned its unique advantages. By conducting evaluation tests to confirm their responsiveness to specific stimuli, it was seen that the nanogels could swell or collapse in response to specific pH or temperature or a combination of both. Thus, they can be used to intelligently deliver drugs in several different conditions which are associated with a change in pH, temperature, or both. Further research on ways to enhance the biomedical value of nanoformulation lead to the design of multi responsive nanogels. These nanogels may respond not only to dual pH and temperature stimuli but also to other stimuli like glucose concentration, redox conditions, light, pressure, etc. Greater control over drug delivery can be obtained with multi responsive nanogels leading to an enhancement in the therapeutic efficacy of the formulation. Fine-tuning of these intelligent nanocarriers to reduce problems related to toxicity and premature drug delivery and to enhance its site-specificity, biocompatibility, and therapeutic efficacy is needed. Methods to upscale the production of these nanogels must also be investigated. Once these formulations are optimized to possess the greatest therapeutic potential with the least chances of toxicity, steps may be taken to introduce them into clinical settings. This will revolutionize the treatment of many different diseases like cancer, inflammatory disorders, diabetes, etc.
ACKNOWLEDGMENTS
The authors acknowledge the help of the Manipal College of Pharmaceutical Sciences and Manipal Academy of Higher Education for their support throughout the study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICT OF INTEREST
The authors (Adrija Jha, Annamalai Rama, Bhautik Ladani, Natasha Verma, Sivakumar Kannan, and Anup Naha) declare no conflict of interest, financial, or otherwise.
ETHICAL APPROVALS
Not Applicable
REFERENCES
Ahmed S, Alhareth K, Mignet N. Advancement in nanogel formulations provides controlled drug release. Int J Pharm, 2020; 584:119435. CrossRef
Ahmadi M, Madrakian T, Afkhami A. Smart nanogels in cancer therapy. In: Nguyen-Tri P, Do T-O, Nguyen TA (eds.). Smart nanocontainers. Elsevier, Amsterdam, The Netherlands, pp 179–93, 2020. CrossRef
Aguirre G, Ramos J, Forcada J. Advanced design of t and pH dual-responsive PDEAEMA–PVCL core–shell nanogels for siRNA delivery. J Polym Sci Part A Polym Chem, 2016; 54(19):3203–17. CrossRef
Aminu N, Chan S-Y, Yam M-F, Toh S-M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int J Pharm, 2019; 570:118659. CrossRef
An D, Zhao D, Li X, Lu X, Qiu G, Shea KJ. Synthesis of surfactant-free hydroxypropylcellulose nanogel and its dual-responsive properties. Carbohydr Polym, 2015; 134:385–9. CrossRef
Arafa MG, El-Kased RF, Elmazar MM. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci Rep, 2018; 8(1):1–16. CrossRef
Ashrafizadeh M, Tam KC, Javadi A, Abdollahi M, Sadeghnejad S, Bahramian A. Synthesis and physicochemical properties of dual-responsive acrylic acid/butyl acrylate cross-linked nanogel systems. J Colloid Interface Sci, 2019; 556:313–23. CrossRef
Banerjee R. Nanotechnology in drug delivery: present status and a glimpse into the future. Ther Deliv, 2018; 9(4):231–2. CrossRef
Bardajee GR, Hooshyar Z, Farsi M, Mobini A, Sang G. Synthesis of a novel thermo/pH sensitive nanogel based on salep modified graphene oxide for drug release. Mater Sci Eng, 2017; 72:558–65. CrossRef
Buwalda SJ, Vermonden T, Hennink WE. Hydrogels for therapeutic delivery: current developments and future directions. Biomacromolecules, 2017; 18(2):316–30. CrossRef
Cao P, Sun X, Liang Y, Gao X, Li X, Li W, Song Z, Li W, Liang G. Gene delivery by a cationic and thermosensitive nanogel promoted established tumor growth inhibition. Nanomedicine, 2015; 10(10):1585–97. CrossRef
Chang R, Tsai W-B. Fabrication of photothermo-responsive drug-loaded nanogel for synergetic cancer therapy. Polymers, 2018; 10(10):1098. CrossRef
Chiriac AL, Gavrila AM, Cursaru B, Spatarelu CP, Sandu T, Sarbu A, Teodorescu M, Perrin FX, Nicolescu T-V, Zaharia A. Poly(ethylene Glycol) diacrylate-nanogels synthesized by mini-emulsion polymerization. MatPlast, 2019;56(3):514–9. CrossRef
Cuggino JC, Blanco ERO, Gugliotta LM, Alvarez Igarzabal CI, Calderón M. Crossing biological barriers with nanogels to improve drug delivery performance. J Control Release, 2019; 307:221–46. CrossRef
Cuggino JC, Molina M, Wedepohl S, Igarzabal CIA, Calderón M, Gugliotta LM. Responsive nanogels for application as smart carriers in endocytic pH-triggered drug delivery systems. Eur Polym J, 2016; 78:14–24.
Curcio M, Diaz-Gomez L, Cirillo G, Concheiro A, Iemma F, Alvarez-Lorenzo C. pH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur J Pharm Biopharm, 2017; 117:324–32. CrossRef
Dimde M, Neumann F, Reisbeck F, Ehrmann S, Cuellar-Camacho JL, Steinhilber D, Ma N, Haag R. Defined pH-sensitive nanogels as gene delivery platform for siRNA mediated in vitro gene silencing. Biomater Sci, 2017a; 5(11):2328–36. CrossRef
Dimde M, Sahle FF, Wycisk V, Steinhilber D, Camacho LC, Licha K, Lademann J, Haag R. Synthesis and validation of functional nanogels as pH-sensors in the hair follicle. Macromol Biosci, 2017b; 17(10):1600505. CrossRef
Don T-M, Lu K-Y, Lin L-J, Hsu C-H, Wu J-Y, Mi F-L. Temperature/pH/Enzyme triple-responsive cationic protein/PAA-b-PNIPAAm nanogels for controlled anticancer drug and photosensitizer delivery against multidrug resistant breast cancer cells. Mol Pharm, 2017; 14(12):4648–60. CrossRef
Durán-Lobato M, Carrillo-Conde B, Khairandish Y, Peppas NA. Surface-modified P(HEMA-co-MAA) nanogel carriers for oral vaccine delivery: design, characterization, and in vitro targeting evaluation. Biomacromolecules, 2014; 15(7):2725–34. CrossRef
Edlich A, Gerecke C, Giulbudagian M, Neumann F, Hedtrich S, Schäfer-Korting M, Ma N, Calderon M, Kleuser B. Specific uptake mechanisms of well-tolerated thermoresponsive polyglycerol-based nanogels in antigen-presenting cells of the skin. Eur J Pharm Biopharm, 2017; 116:155–63. CrossRef
Fu W, Luo C, Morin EA, He W, Li Z, Zhao B. UCST-type thermosensitive hairy nanogels synthesized by RAFT polymerization-induced self-assembly. ACS Macro Lett, 2017; 6(2):127–33. CrossRef
Gao F, Mi Y, Wu X, Yao J, Qi Q, Cao Z. Preparation of thermoresponsive poly (N-vinylcaprolactam-co-2-methoxyethyl acrylate) nanogels via inverse miniemulsion polymerization. J Appl Polym Sci, 2019; 136(46):48237. CrossRef
Gerecke C, Edlich A, Giulbudagian M, Schumacher F, Zhang N, Said A, Yealland G, Lohan SB, Neumann F, Meinke MC, Ma N, Calderón M, Hedtrich S, Schäfer-Korting M, Kleuser B. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology, 2017; 11(2):267–77. CrossRef
Giulbudagian M, Asadian-Birjand M, Steinhilber D, Achazi K, Molina M, Calderón M. Fabrication of thermoresponsive nanogels by thermo-nanoprecipitation and in situ encapsulation of bioactives. Polym Chem, 2014; 5(24):6909–13. CrossRef
Giulbudagian M, Hönzke S, Bergueiro J, I??k D, Schumacher F, Saeidpour S, Lohan SB, Meinke MC, Teutloff C, Schäfer-Korting M, Yealland G, Kleuser B, Hedtrich S, Calderón M. Enhanced topical delivery of dexamethasone by β-cyclodextrin decorated thermoresponsive nanogels. Nanoscale, 2018a; 10(1):469–79. CrossRef
Giulbudagian M, Yealland G, Hönzke S, Edlich A, Geisendörfer B, Kleuser B, Hedtrich S, Calderon M. Breaking the barrier—potent anti-inflammatory activity following efficient topical delivery of etanercept using thermoresponsive nanogels. Theranostics, 2018b; 8(2):450–63. CrossRef
Gonzalez-Urias A, Zapata-Gonzalez I, Licea-Claverie A, Licea-Navarro AF, Bernaldez-Sarabia J, Cervantes-Luevano K. Cationic versus anionic core-shell nanogels for transport of cisplatin to lung cancer cells. Colloids Surf Biointerfaces, 2019; 182:110365. CrossRef
Guo Q, Zhang X. Synthesized of glucose-responsive nanogels labeled with fluorescence molecule based on phenylboronic acid by RAFT polymerization. J Biomater Sci Polym Ed, 2019; 30(10):815–31. CrossRef
Hajebi S, Abdollahi A, Roghani-Mamaqani H, Salami-Kalajahi M. Temperature-responsive poly (N-isopropylacrylamide) nanogels: the role of hollow cavities and different shell cross-linking densities on doxorubicin loading and release. Langmuir, 2020; 36(10):2683–94. CrossRef
Hajebi S, Rabiee N, Bagherzadeh M, Ahmadi S, Rabiee M, Roghani-Mamaqani H, Tahriri M, Tayebi L, Hamblin MR . Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater, 2019; 92:1–18. CrossRef
Hassanpour S, Bagheri M. Dual-responsive semi-IPN copolymer nanogels based on poly (itaconic acid) and hydroxypropyl cellulose as a carrier for controlled drug release. J Polym Res, 2017; 24(6):91. CrossRef
Hosseinifar T, Sheybani S, Abdouss M, Hassani Najafabadi SA, Shafiee Ardestani M. Pressure responsive nanogel base on Alginate-Cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J Biomed Mater Res Part A, 2018; 106(2):349–59. CrossRef
Indulekha S, Arunkumar P, Bahadur D, Srivastava R. Dual responsive magnetic composite nanogels for thermo-chemotherapy. Colloids Surf B Biointerfaces, 2017; 155:304–13. CrossRef
Jung S, Nagel G, Giulbudagian M, Calderón M, Patzelt A, Knorr F, Lademann J. Temperature-enhanced follicular penetration of thermoresponsive nanogels. Zeitschrift für Physikalische Chemie, 2018; 232(5–6):805. CrossRef
Karimi M, Eslami M, Sahandi-Zangabad P, Mirab F, Farajisafiloo N, Shafaei Z, Ghosh D, Bozorgomid M, Dashkhaneh F, Hamblin MR. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents: pH-sensitive nanocarriers for targeted delivery. WIREs Nanomed Nanobiotechnol, 2016; 8(5):696–716. CrossRef
Kendre PN, Satav TS. Current trends and concepts in the design and development of nanogel carrier systems. Polym Bull, 2019; 76(3):1595–617. CrossRef
Khaled SZ, Cevenini A, Yazdi IK, Parodi A, Evangelopoulos M, Corbo C, Scaria S, Hu Y, Haddix SG, Corradetti B, Salvatore F, Tasciotti E. One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials, 2016; 87:57–68. CrossRef
Kolouchova K, Sedlacek O, Jirak D, Babuka D, Blahut J, Kotek J, Vit M, Trousil J, Konefa? R, Janouskova O, Podhorska B, Slouf M, Hruby M. Self-assembled thermoresponsive polymeric nanogels for 19F MR imaging. Biomacromolecules, 2018; 19(8):3515–24. CrossRef
Krisch E, Gyarmati B, Szilágyi A. Preparation of pH-responsive poly (aspartic acid) nanogels in inverse emulsion. Period Polytech Chem Eng, 2017; 61(1):19–26. CrossRef
Lang X, Patrick AD, Hammouda B, Hore MJ. Chain terminal group leads to distinct thermoresponsive behaviors of linear PNIPAM and polymer analogs. Polymer, 2018; 145:137–47. CrossRef
Li Y, Bui QN, Duy LTM, Yang HY, Lee DS. One-Step preparation of pH-responsive polymeric nanogels as intelligent drug delivery systems for tumor therapy. Biomacromolecules, 2018; 19(6):2062–70. CrossRef
Liu G, An Z. Frontiers in the design and synthesis of advanced nanogels for nanomedicine. Polym Chem, 2014; 5(5):1559–65. CrossRef
Liu D, Ma L, An Y, Li Y, Liu Y, Wang L, Guo J, Wang J, Zhou J. Thermoresponsive nanogel-encapsulated pedot and hsp70 inhibitor for improving the depth of the photothermal therapeutic effect. Adv Funct Mater, 2016; 26(26):4749–59. CrossRef
Lipowska-Kur D, Otulakowski ?, Trzebicka B, Utrata-Weso?ek A, Dworak A. Thermoresponsive Nanogels of Modified Poly ((di (ethylene glycol) methyl ether methacrylate)-co-(2-aminoethyl methacrylate))s. Polymers, 2020; 12(8):1645. CrossRef
Lou S, Gao S, Wang W, Zhang M, Zhang J, Wang C, Li C, Kong D, Zhao Q. Galactose-functionalized multi-responsive nanogels for hepatoma-targeted drug delivery. Nanoscale, 2015; 7(7):3137–46. CrossRef
Luckanagul JA, Pitakchatwong C, Bhuket PRN, Muangnoi C, Rojsitthisak Pranee, Chirachanchai S, Wang Q, Rojsitthisak P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr Polym, 2018; 181:1119–27. CrossRef
Macchione MA, Sacarelli MF, Racca AC, Biglione C, Panzetta-Dutari GM, Strumia MC. Dual-responsive nanogels based on oligo(ethylene glycol) methacrylates and acidic co-monomers. Soft Matter, 2019; 15(47):9700–9. CrossRef
Madhusudana Rao K, Krishna Rao KSV, Ramanjaneyulu G, Ha C-S. Curcumin encapsulated pH sensitive gelatin based interpenetrating polymeric network nanogels for anti cancer drug delivery. Int J Pharm, 2015; 478(2):788–95. CrossRef
Madhusudana Rao K, Mallikarjuna B, Krishna Rao KSV, Siraj S, Chowdoji Rao K, Subha MCS. Novel thermo/pH sensitive nanogels composed from poly(N-vinylcaprolactam) for controlled release of an anticancer drug. Colloid Surf B Biointerfaces, 2013; 102:891–7. CrossRef
Manzanares-Guevara LA, Licea-Claverie A, Paraguay-Delgado F. Preparation of stimuli-responsive nanogels based on poly (N, N-diethylaminoethyl methacrylate) by a simple “surfactant-free” methodology. Soft Mater, 2018; 16(1):37–50. CrossRef
Morelli A, Puppi D, Cheptene V, Disgraziati D, Ruggeri G, Chiellini F. Design, preparation, and characterization of thermoresponsive hybrid nanogels using a novel Ulvan-Acrylate crosslinker as potential carriers for protein encapsulation. Macromol Chem Phys, 2018; 219(10):1700631. CrossRef
Nita LE, Chiriac AP, Diaconu A, Tudorachi N, Mititelu-Tartau L. Multifunctional nanogels with dual temperature and pH responsiveness. Int J Pharm, 2016; 515(1–2):165–75. CrossRef
Nuhn L, Tomcin S, Miyata K, Mailänder V, Landfester K, Kataoka K, Zentel R. Size-dependent knockdown potential of siRNA-loaded cationic nanohydrogel particles. Biomacromolecules, 2014; 15(11):4111–21. CrossRef
Oehrl A, Schötz S, Haag R. Synthesis of pH-degradable polyglycerol-based nanogels by iEDDA-mediated crosslinking for encapsulation of asparaginase using inverse nanoprecipitation. Colloid Polym Sci, 2020; 298(7):719–33. CrossRef
Ohshio M, Ishihara K, Maruyama A, Shimada N, Yusa S. Synthesis and properties of upper critical solution temperature responsive nanogels. Langmuir, 2019; 35(22):7261–7. CrossRef
Pan Y, Liu J, Yang K, Cai P, Xiao H. Novel multi-responsive and sugarcane bagasse cellulose-based nanogels for controllable release of doxorubicin hydrochloride. Mater Sci Eng C, 2021; 118:111357. CrossRef
Pan G, Mou Q, Ma Y, Ding F, Zhang J, Guo Y, Huang X, Li Q, Zhu X, Zhang C. pH-Responsive and Gemcitabine-Containing DNA Nanogel To Facilitate the Chemodrug Delivery. ACS Appl Mater Interface, 2019; 11(44):41082–90. CrossRef
Park CW, Yang H-M, Lee HJ, Kim J-D. Core–shell nanogel of PEG–poly(aspartic acid) and its pH-responsive release of rh-insulin. Soft Matter, 2013;9(6):1781-8. CrossRef
Peng H, Huang X, Oppermann A, Melle A, Weger L, Karperien M, Woll D, Pich A. A facile approach for thermal and reduction dual-responsive prodrug nanogels for intracellular doxorubicin delivery. J Mater Chem B, 2016a; 4(47):7572–83. CrossRef
Peng H, Xu W, Pich A. Temperature and pH dual-responsive poly(vinyl lactam) copolymers functionalized with amine side groups via RAFT polymerization. Polym Chem, 2016b; 7(31):5011–22. CrossRef
Pruettiphap M, Rempel GL, Pan Q, Kiatkamjornwong S. Morphology and drug release behavior of N-isopropylacrylamide/acrylic acid copolymer as stimuli-responsive nanogels. Iranian Polym J, 2017; 26(12):957–69. CrossRef
Qureshi MA, Khatoon F. Different types of smart nanogel for targeted delivery. J Sci Adv Mater Devices, 2019; 4(2):201–12. CrossRef
Rahdar A, Sayyadi K, Sayyadi J, Yaghobi Z. Nano-gels: A versatile nano-carrier platform for drug delivery systems: a mini review. Nanomed Res J, 2019; 4(1):1–9.
Rajan R, Matsumura K. Tunable dual-thermoresponsive core–shell nanogels exhibiting UCST and LCST behavior. Macromol Rapid Commun, 2017; 38(22):1700478. CrossRef
Rancan F, Giulbudagian M, Jurisch J, Blume-Peytavi U, Calderon M, Vogt A. Drug delivery across intact and disrupted skin barrier: identification of cell populations interacting with penetrated thermoresponsive nanogels. Eur J Pharm Biopharm, 2017; 116:4–11. CrossRef
Rancan F, Volkmann H, Giulbudagian M, Schumacher F, Stanko JI, Kleuser B, Blume-Peytavi U, Calderón M, Vogt A. Dermal delivery of the high-molecular-weight drug tacrolimus by means of polyglycerol-based nanogels. Pharmaceutics, 2019; 11(8):394. CrossRef
Rejinold NS, Baby T, Chennazhi KP, Jayakumar R. Multi drug loaded thermo-responsive fibrinogen-graft-poly (N-vinyl caprolactam) nanogels for breast cancer drug delivery. J Biomed Nanotechnol, 2015; 11(3):392–402. CrossRef
Sahle FF, Giulbudagian M, Bergueiro J, Lademann J, Calderón M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale, 2017; 9(1):172–82. CrossRef
Sahu P, Kashaw SK, Jain S, Sau S, Iyer AK. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: in vitro and ex vivo studies. J Control Release, 2017a; 253:122–36. CrossRef
Sahu P, Kashaw SK, Kushwah V, Sau S, Jain S, Iyer AK. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorgan Med Chem, 2017b; 25(17):4595–613. CrossRef
Sahu P, Kashaw SK, Sau S, Kushwah V, Jain S, Agrawal RK, Iyer AK. pH responsive 5-fluorouracil loaded biocompatible nanogels for topical chemotherapy of aggressive melanoma. Colloids Surf B Biointerfaces, 2019; 174:232–45. CrossRef
Sahu P, Kashaw SK, Sau S, Kushwah V, Jain S, Iyer AK. Discovering pH triggered charge rebound surface modulated topical nanotherapy against aggressive skin papilloma. Mater Sci Eng C, 2020; 107:110263. CrossRef
Salehi R, Rasouli S, Hamishehkar H. Smart thermo/pH responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. Int J Pharm, 2015; 487(1–2):274–84. CrossRef
Salimi F, Dilmaghani KA, Alizadeh E, Akbarzadeh A, Davaran S. Enhancing cisplatin delivery to hepatocellular carcinoma HepG2 cells using dual sensitive smart nanocomposite. Artif Cells Nanomed Biotechnol, 2018; 46(5):949–58. CrossRef
Sang G, Bardajee GR, Mirshokraie A, Didehban K. A thermo/pH/magnetic-responsive nanogel based on sodium alginate by modifying magnetic graphene oxide: preparation, characterization, and drug delivery. Iranian Polym J, 2018; 27(3):137–44. CrossRef
Sarfraz RM, Akram MR, Ali MR, Mahmood A, Khan MU, Ahmad H. Development and in-vitro evaluation of pH responsive polymeric nano hydrogel carrier system for gastro-protective delivery of naproxen sodium. Adv Polym Technol, 2019; 2019. CrossRef
Sarfraz RM, Khan MU, Mahmood A, Akram MR, Minhas MU, Qaisar MN. Synthesis of co-polymeric network of carbopol-g-methacrylic acid nanogels drug carrier system for gastro-protective delivery of ketoprofen and its evaluation. Polym Plastics Technol Mater, 2020; 59(10):1109–23. CrossRef
Seo S, Lee C-S, Jung Y-S, Na K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly(l-lactide) copolymers. Carbohydr Polym, 2012; 87(2):1105–11. CrossRef
Sharma G, Kamboj S, Thakur K, Negi P, Raza K, Katare OP. Delivery of thermoresponsive-tailored mixed micellar nanogel of lidocaine and prilocaine with improved dermatokinetic profile and therapeutic efficacy in topical anaesthesia. AAPS PharmSciTech, 2017; 18(3):790–802. CrossRef
Si X, Song W, Yang S, Ma L, Yang C, Tang Z. Glucose and pH dual-responsive nanogels for efficient protein delivery. Macromol Biosci, 2019; 19(9):1900148. CrossRef
Sim T, Lim C, Hoang NH, Oh KT. Recent advance of pH-sensitive nanocarriers targeting solid tumors. J Pharm Investig, 2017; 47(5):383–94. CrossRef
Soni G, Yadav KS. Nanogels as potential nanomedicine carrier for treatment of cancer: a mini review of the state of the art. Saudi Pharm J, 2016; 24(2):133–9. CrossRef
Sonzogni AS, Yealland G, Kar M, Wedepohl S, Gugliotta LM, Gonzalez VDG, Hedtrich S, Calderón M, Minari RJ. Effect of delivery platforms structure on the epidermal antigen transport for topical vaccination. Biomacromolecules, 2018; 19(12):4607–16. CrossRef
Steinhilber D, Witting M, Zhang X, Staegemann M, Paulus F, Friess W, Küchler S, Haag R. Surfactant free preparation of biodegradable dendritic polyglycerol nanogels by inverse nanoprecipitation for encapsulation and release of pharmaceutical biomacromolecules. J Control Release, 2013; 169(3):289–95. CrossRef
Strozyk MS, Carregal-Romero S, Henriksen-Lacey M, Brust M, Liz-Marzán LM. Biocompatible, multiresponsive nanogel composites for codelivery of antiangiogenic and chemotherapeutic agents. Chem Mater, 2017; 29(5):2303–13. CrossRef
Štular D, Tomši? B, Jerman I, Simon?i? B, Mihel?i? M, No? L, Ili? IG. Comparison of responsive behaviour of smart PLA fabrics applied with temperature and pH responsive microgel and nanogel. Prog Organ Coatings, 2018; 124:213–23. CrossRef
Sui H, Gao Z, Guo J, Wang Y, Yuan J, Hao J, Dong S, Cui J. Dual pH-responsive polymer nanogels with a core–shell structure for improved cell association. Langmuir, 2019; 35(51):16869–75. CrossRef
Suhail M, Rosenholm JM, Minhas MU, Badshah SF, Naeem A, Khan KU, Fahad M. Nanogels as drug-delivery systems: a comprehensive overview. Ther Deliv, 2019; 10(11):697–717. CrossRef
Tian Y, Tian R, Chen L, Jin R, Feng Y, Bai Y, Chen X. Redox-responsive nanogel with intracellular reconstruction and programmable drug release for targeted tumor therapy. Macromol Rapid Commun, 2019; 40(8):1800824. CrossRef
Tiwari N, Sonzogni AS, Calderón M. Can dermal delivery of therapeutics be improved using thermoresponsive nanogels? Nanomedicine, 2019; 14(22):2891–5. CrossRef
Verma NK, Purohit MP, Equbal D, Dhiman N, Singh A, Kar AK, Shankar J, Tehlan S, Patnaik S. Targeted smart pH and thermoresponsive N, O-carboxymethyl chitosan conjugated nanogels for enhanced therapeutic efficacy of doxorubicin in MCF-7 breast cancer cells. Bioconjug Chem, 2016; 27(11):2605–19. CrossRef
Vicario-de-la-Torre M, Forcada J. The potential of stimuli-responsive nanogels in drug and active molecule delivery for targeted therapy. Gels, 2017; 3(2):16. CrossRef
Wanigasekara J, Witharana C. Applications of nanotechnology in drug delivery and design-an insight. Curr Trends Biotechnol Pharmacy 2016;10(1):78-91.
Wang H, Di J, Sun Y, Fu J, Wei Z, Matsui H, Alonso AC, Zhou S. Biocompatible PEG-chitosan@ carbon dots hybrid nanogels for two-photon fluorescence imaging, near-infrared light/pH dual-responsive drug carrier, and synergistic therapy. Adv Funct Mater, 2015; 25(34):5537–47. CrossRef
Wang D, Huang H, Zhou M, Lu H, Chen J, Chang Y-T, Gao J, Chai Z, Hu Y. A thermoresponsive nanocarrier for mitochondria-targeted drug delivery. Chem Commun, 2019; 55(28):4051–4. CrossRef
Wang Y, Luo Y, Zhao Q, Wang Z, Xu Z, Jia X. An enzyme-responsive nanogel carrier based on PAMAM dendrimers for drug delivery. ACS Appl Mater Interfaces, 2016; 8(31):19899–906. CrossRef
Wang G, Nie Q, Zang C, Zhang B, Zhu Q, Luo G, Wang S. Self-assembled thermoresponsive nanogels prepared by reverse micelle → positive micelle method for ophthalmic delivery of muscone, a poorly water-soluble drug. J Pharm Sci, 2016; 105(9):2752–9. CrossRef
Wang J, Wang X, Yan G, Fu S, Tang R. pH-sensitive nanogels with ortho ester linkages prepared via thiol-ene click chemistry for efficient intracellular drug release. J Colloid Interface Sci, 2017; 508:282–90. CrossRef
Wang Y, Xu H, Ma L. Recent advances of thermally responsive nanogels for cancer therapy. Ther Deliv, 2015; 6(10):1157–69. CrossRef
Wei J, Yu H, Liu H, Du C, Zhou Z, Huang Q, Yao X Facile synthesis of thermo-responsive nanogels less than 50 nm in diameter via soap-and heat-free precipitation polymerization. J Mater Sci, 2018; 53(17):12056–64. CrossRef
Wen Y, Oh JK. Intracellular delivery cellulose-based bionanogels with dual temperature/pH-response for cancer therapy. Colloids Surf B Biointerfaces, 2015; 133:246–53. CrossRef
Witting M, Molina M, Obst K, Plank R, Eckl KM, Hennies HC, Calderón M, Friess W, Hedtrich S. Thermosensitive dendritic polyglycerol-based nanogels for cutaneous delivery of biomacromolecules. Nanomed Nanotechnol Biol Med, 2015; 11(5):1179–87. CrossRef
Yang G, Fu S, Yao W, Wang X, Zha Q, Tang R. Hyaluronic acid nanogels prepared via ortho ester linkages show pH-triggered behavior, enhanced penetration and antitumor efficacy in 3-D tumor spheroids. J Colloid Interface Sci, 2017; 504:25–38. CrossRef
Yang H, Wang Q, Huang S, Xiao A, Li F, Gan L, Yang X. Smart pH/redox dual-responsive nanogels for on-demand intracellular anticancer drug release. ACS Appl Mater Interfaces, 2016; 8(12):7729–38. CrossRef
Yao R-S, Zhang W, Yang X-Z, Liu J, Liu H-T. HPMC/PAA hybrid nanogels via aqueous-phase synthesis for controlled delivery of insulin. Biomater Sci, 2014; 2(12):1761–7. CrossRef
Yin Y, Hu B, Yuan X, Cai L, Gao H, Yang Q. Nanogel: a versatile nano-delivery system for biomedical applications. Pharmaceutics, 2020; 12(3):290. CrossRef
Zafar M, Shah T, Rawal A, Siores E. Preparation and characterisation of thermoresponsive nanogels for smart antibacterial fabrics. Mater Sci Eng C, 2014; 40:135–41. CrossRef
Zarekar NS, Lingayat VJ, Pande VV. Nanogel as a novel platform for smart drug delivery system. Nanosci Nanotechnol Res, 2017; 4(1):25–31.
Zhang W, Tung C-H. Lysosome enlargement enhanced photochemotherapy using a multifunctional nanogel. ACS Appl Mater Interf, 2018; 10(5):4343–8. CrossRef
Zhang H, Zhai Y, Wang J, Zhai G. New progress and prospects: the application of nanogel in drug delivery. Mater Sci Eng C, 2016; 60:560–8. CrossRef
Zhao D, Shi X, Liu T, Lu X, Qiu G, Shea KJ. Synthesis of surfactant-free hydroxypropyl methylcellulose nanogels for controlled release of insulin. Carbohydr Polym, 2016; 151:1006–11. CrossRef
Zhong JX, Clegg JR, Ander EW, Peppas NA. Tunable poly(methacrylic acid-co-acrylamide) nanoparticles through inverse emulsion polymerization. J Biomed Mater Res Part A, 2018; 106(6):1677. CrossRef