INTRODUCTION
Pumpkin (Cucurbita moschata Duchesne), a member of the Cucurbitaceae family, is widely grown in tropical regions (Fig. 1a). Pumpkin flesh is cooked and consumed in many ways, such as cakes, candies, bread (Kim et al., 2012), jams, purées, juices, and alcoholic-derived beverages (Jiao et al., 2014). Pumpkin seeds can be considered as a potential source for obtaining some bioactive compounds with health benefits including tocopherol, phenolics, carotenoids, and sterols (Jiao et al., 2014; Naki? et al., 2006; Rezig et al., 2012, Rezig et al., 2018; Stevenson et al., 2007). The oil extracted from pumpkin seed, known as pumpkin seed oil (PSO), contains main fatty acid (FA) components including palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2), and linolenic (C18:3) acids (Perez Gutierrez, 2016). In certain countries, including Argentina, Brazil, China, India, Korea, and Mexico, PSOs have been exploited as traditional medicine in folk remedies.
In recent years, PSO has received considerable attention because of its protective effects on human health (Murkovic et al., 2004). Numerous researches have reported about the beneficial effects of PSO (Rohman and Irnawati, 2020), such as improving urinary disorders in human overactive bladder (Nishimura et al., 2014), reducing postprandial glycemia (Cândido et al., 2018), protective effect against cytotoxicity and genotoxicity (Elfiky et al., 2012), cancer cell growth inhibition (Medjakovic et al., 2016), antidiabetic activity (Bharti et al., 2013), and antioxidant activity (Jiao et al., 2014; Stevenson et al., 2007).
In industrial production, PSO is produced by hydraulic cold pressing (Salgin and Korkmaz, 2011). Numerous researches have reported about the physical and chemical characterization of PSO extracted by cold pressing (Kulaitien? et al., 2018; Rezig et al., 2012), aqueous enzymatic extraction assisted by microwave (Jiao et al., 2014), Soxhlet (Montesano et al., 2018), and supercritical CO2 (Koubaa et al., 2017). The temperature during the extraction process could affect the oil properties and minor constituents with some biological activities which are useful for maintaining human health conditions (Khoddami et al., 2011). Rezig et al. (2018) have compared the physicochemical properties of PSO extracted using two different methods, namely solvent extraction and cold pressing. However, the comparison of physicochemical properties of PSO as affected by three extraction methods has not been reported. The objective of this research was to compare the physical and chemical characterization, the profiles of FAs, and the antioxidant activities of PSO obtained from different origins and extraction methods.
MATERIALS AND MEHTODS
Materials
The pumpkin fruits were collected from 10 regions in Central Java and Yogyakarta, Indonesia. The seeds were separated from the flesh, cleaned, washed, and air-dried at room temperature (3 × 24 hours) and then stored at −20°C until used for analysis and extraction.
Moisture content
The moisture content was determined based on the Indonesian Pharmacopeia (The Ministry of Health, 1979).
Microwave pretreatment
The pumpkin seed was previously subjected to pretreatment as mentioned in Irnawati et al. (2021). Inside the microwave model MR-5750 (Hitachi, Japan), 100 g of pumpkin seed samples was placed in an even layer in Pyrex petri dishes. The samples were subjected to microwave extraction by treating those for 240 seconds at a power of 50%.
Oil extraction
During the extraction of PSO, three extraction techniques (Soxhlet, hot pressing, and ultrasound-assisted) were employed. Using the hot pressing method, pumpkin seeds previously treated with microwave were extracted using mechanical pressing (Maksindo MKS-J03) in hot conditions (Dang and Bui, 2019). The obtained oil was then separated from its sediment using a centrifuge (Thermo Scientific, USA) at 2,500 rpm for 10 minutes and stowed in a freezer at a temperature of −20°C for further analysis. For Soxhlet extraction, pumpkin seed powder (33 g) was continuously extracted with 500 ml of n-hexane for eight cycles at a temperature of 80°C in a set of Soxhlet extraction apparatuses. Once the Soxhlet extraction was complete, n-hexane was evaporated at 50°C using a rotary evaporator (IKA-Werke KRV06-ML, Germany). The oil obtained was weighed to calculate the yield of oil extraction. In ultrasound-assisted extraction (UAE), PSO was extracted using ultrasonic cleaning (ELMA, T760/DH) according to the method described by Irnawati et al. (2020). Briefly, 50 g of pumpkin seed powder was mixed with 500 ml n-hexane and sonicated at 150 W for 15 minutes at a temperature lower than 40°C. The solvent was later separated from the sediment using a centrifuge (Thermo Scientific, USA) at 2,500 rpm for 10 minutes. After that, the samples were treated as previously reported using Soxhlet extraction.
Physical and chemical characterization of PSO
Determination of some values (acid, saponification, peroxide, and iodine) of PSO was carried out according to the methods of the AOAC (2000).
Evaluation of FA profiles
The FA profiles of PSO were analyzed according to Rezig et al. (2018) using gas chromatography (GC) with slight modifications. The nonvolatile FAs were subjected to derivatization by converting them into FA methyl esters (FAMEs). This derivatization was carried out by adding 0.4 g PSO with 1.5 ml of methanolic potassium hydroxide and 2 ml of boron trifluoride-methanol. The FAME in heptane was analyzed by GC (Agilent Technologies, 7890B) equipped with a capillary column of DB-WAX (30 m × 250 × 0.25 µm). The temperatures of the injection inlet and detector (flame ionization detector) were set at 250°C and 280°C, respectively. The initial temperature of the column was set at 50°C for 1 minute and then was increased to 200°C at the rate of 25°C/minute and increased to 230°C at the rate of 3°C/minute with 18 minutes hold time. 1 µl solution containing FAME was injected. The flow rate of H2 was set at 40 ml/minute with a split ratio of 50:1. FA identification was carried out by comparing the retention times of FAMEs in PSO samples with those in FAME standards consisting of 37 FAMEs (CRM47885 FAME Mix 37 Supelco).
Determination of antioxidant activity
The antioxidant activities in vitro of PSO samples were assessed using ABTS•+ assay, DPPH assay, and bleaching assay of β-carotene.
Determination of antioxidant using DPPH assay
Antiradical activity using DPPH was carried out according to Brand-Williams et al. (1995) and Casoni et al. (2019). PSO samples (80 mg) were added to 1.0 ml of DPPH ethanolic solution at a concentration of 0.4 mM and 3.9 ml of ethanol. The absorbance of evaluated samples was measured at 515 nm after an operational time of 30 minutes in darkness at ambient temperature. The absorbance measurement of evaluated samples was corrected with blank absorbance containing ethanol and the studied PSO sample. The scavenging activity (%) of the DPPH radical was calculated using
Determination of antioxidant using ABTS assay
ABTS assay was carried out according to Ortega-Ortega et al.’s (2017) study, with slight modification. ABTS•+ radical cation was produced by reacting ABTS 7 mM with potassium persulfate 2.45 mM in darkness at an ambient temperature for 16 hours. The ABTS•+ solution was diluted with deionized water to get the absorbance value of around 0.7 (0.63–0.77) at wavelength 753 nm. An aliquot of 40 mg of sample was added with 4950 µl of diluted ABTS•+, and the absorbance was measured after 7 minutes in darkness at an ambient temperature at 753 nm (spectrophotometer, model U-2900). The percentage (%) of radical scavenging of ABTS was calculated as follows:
β-Carotene bleaching assay
The assay of β-carotene bleaching was carried out as mentioned by Hsouna et al. (2013) and Takada et al. (2006). The mixture of β-carotene and linoleic acid was made by adding 25 mg of β-carotene with 10 ml chloroform, 250 µl of linoleic acid, and 2.0 g Tween-20. After that, chloroform was evaporated and added with 1.0 l of distilled water (oxygen saturated), and the mixture was shaken vigorously. 4.0 ml of this mixture was dispensed into the test tube and added with 10 µl PSO. The emulsion was subjected to incubation for 2 hours at a temperature 50°C. After that, the absorbance was measured at wavelength 459 nm. The antioxidant based on bleaching of β-carotene was computed as follows:
in which degradation rate was calculated as follows:
degradation rate (degr) = (Lu[A0/At]), (4)
where A0 is the absorbance value of the PSO sample at zero times and At is the absorbance of the sample after 2 hours of incubation.
Determination of total phenolic content
The total phenolic content of PSO was determined by using Folin–Ciocalteu’s reagent methods as mentioned in Delfan-Hosseini et al.’s (2017) study with slight modification. 0.4 g PSO was dissolved in 1 ml n-hexane and the extraction procedure was carried out using 1 ml methanol:water (80%:20% v/v). 200 µl of methanol:water phase was mixed with 0.4 ml of Folin–Ciocalteu’s reagent and 4.0 ml Na2CO3 solution in a 10 ml volumetric flash and then was adjusted to volume with Aquadest. The measurement of absorbance was made at 750 nm after 60 minutes. The reference standard of gallic acid at 0.0625–4 µg/ml in methanol was applied for preparing the calibration curve.
RESULTS AND DISCUSSION
The moisture contents and extraction yield
Moisture analysis of pumpkin seed showed that the contents of water and volatile matter were in the range of 6.72%–8.88% (Table 1). The low moisture of the samples before being subjected to microwave treatment can make them more brittle. As a consequence, the yield of PSO during extraction can be increased (Uquiche et al., 2008). The yield of PSO extraction from different origins and extraction methods was expressed as the % of PSO obtained, as compiled in Table 2. The highest yield was obtained using the Soxhlet method compared to that obtained using the UAE and hot pressing methods. The high yield of PSO obtained by the Soxhlet method could be attributed to extended contact and repeated washing to eight cycles at high temperature (Ortega-Ortega et al., 2017). The obtained results were in line with those reported for black seed oil (Khoddami et al., 2011), grape seed oil (Da Porto et al., 2013), and purslane seed oil (Delfan-Hosseini et al., 2017).
Physicochemical properties of PSO
Table 2 shows the physicochemical properties of PSO from different origins extracted using different methods. The acid value (AV) and peroxide value (PV) were used as the most important characteristics for seed oil. Meanwhile, the saponification value (SV) and iodine value (IV) were used as quality parameters for seed oil. The AV represents the amount of free FAs in the oil. AVs of the PSO were within the range of 4.37–8.63 mg Potassium Hydroxide (KOH)/g oil. The similar results in AVs of PSO were reported by Rezig et al. (2012) in which the AV value obtained was 7.54 mg KOH/g oil and by Jiao et al. (2014) (6.97–7.08 mg KOH/g oil), but the reported AVs of PSO in this study were higher than that reported by Habib et al. (2015) (0.516 mg KOH/g oil). The difference was attributed to the sources of PSO used, namely unrefined oil (this study) and refined oil (Habib et al., 2015). Based on the ANOVA and Tukey’s test, the hot press extracted oil had the lowest AVs but there is no significant difference between ultrasound-assisted extracted oil and that of Soxhlet (p > 0.05) and there are no significant effects on the different origins of PSO (p > 0.05).
The PV indicated the degree of oxidation of the oil. The low value of PV indicated the high resistance to oxidation (Haile et al., 2019). PVs of PSO from different origins and different extraction methods were found to be in the range of 3.15–5.61 meq/kg. PVs of PSO showed a significant difference between extraction methods (p < 0.05). The highest PVs were found in PSOs extracted by the Soxhlet method which is attributed to the high temperature used during the extraction. The SV is an indicator of chain length and molecular weight of FAs composed of fats and oils. SVs of the PSO from different origins and extraction methods ranged from 145.28 to 195.27 mg KOH/g oil. SVs of PSO were also reported by Rezig et al. (2012) to be 175 mg KOH/g oil, Jiao et al. (2014) to be 183.37–184.41 mg KOH/g oil, and Habib et al. (2015) to be 193.73 mg KOH/g oil. The difference of SVs in this study and those reported by other researches may be attributable to different origins, cultivation age of pumpkin, and environmental conditions. SVs of PSOs showed a significant difference between extraction methods (p < 0.05). The IVs indicated the unsaturated bond present in fats and oils. The higher the IV, the greater the number of C = C double bonds (Aremu et al., 2006; Lixia et al., 2018). IVs of PSO from different extractions and different origins in this study were in the range of 81.47–113.61 g I2/g oil. The IV of PSO extracted using hot pressing is higher than those of other oils (p < 0.05). These results were similar to those reported by Habib et al. (2015) (114.33 g I2/g oil), Badr et al. (2011) (109 g I2/g oil), and El-Adawy and Taha (2001) (109 g I2/g oil).
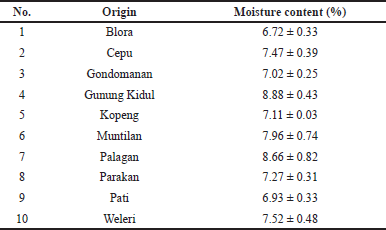 | Table 1. Moisture content of PSO obtained from different origins. [Click here to view] |
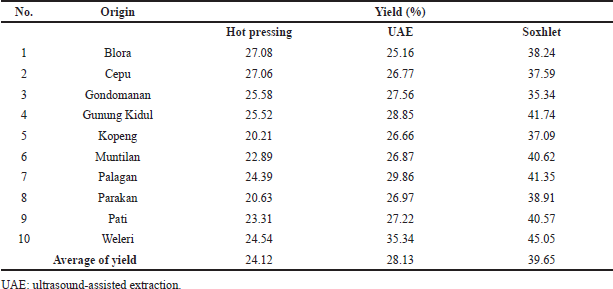 | Table 2. Yield (%) of PSO obtained from different origins and extraction methods. [Click here to view] |
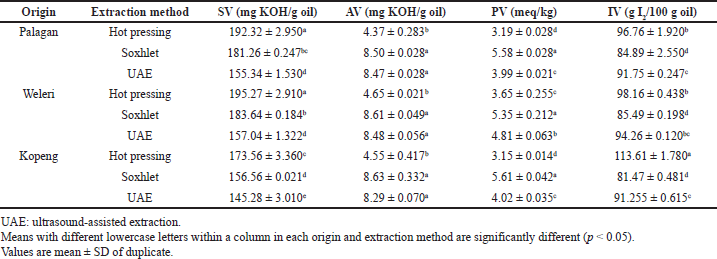 | Table 3. Physicochemical properties of PSO from different regions and extraction methods. [Click here to view] |
FA composition of PSO
The FA composition of PSOs from different origins extracted using the different methods is shown in Table 4. Four FAs were identified as dominant FAs in PSO, including palmitate, stearate, oleic, and linoleate (Fig. 1b). These findings are in agreement with those reported by Kulaitien? et al. (2018) and Rezig et al. (2018). These results obtained also indicated that the different extraction methods have no effect on the FA composition of PSO (p > 0.05).
Antioxidant activity of PSO
The antioxidant activities of PSOs as evaluated by the DPPH radical scavenging assay, ABTS assay, and β-carotene bleaching are shown in Table 5. The highest antioxidant activity was found in PSO from Gunung Kidul extracted by hot pressing on both DPPH radical assay (98.71%) and ABTS assay (88.08%) with total phenolic content of 142.61 µg gallic acid equivalent/g oil. Meanwhile, the highest β-carotene bleaching was found in PSO from Pati, Central Java, extracted by the Soxhlet method. Figure 1c shows the relationship between antioxidant activities and total phenolic content of PSO as evaluated using the loading plot of principal component analysis.
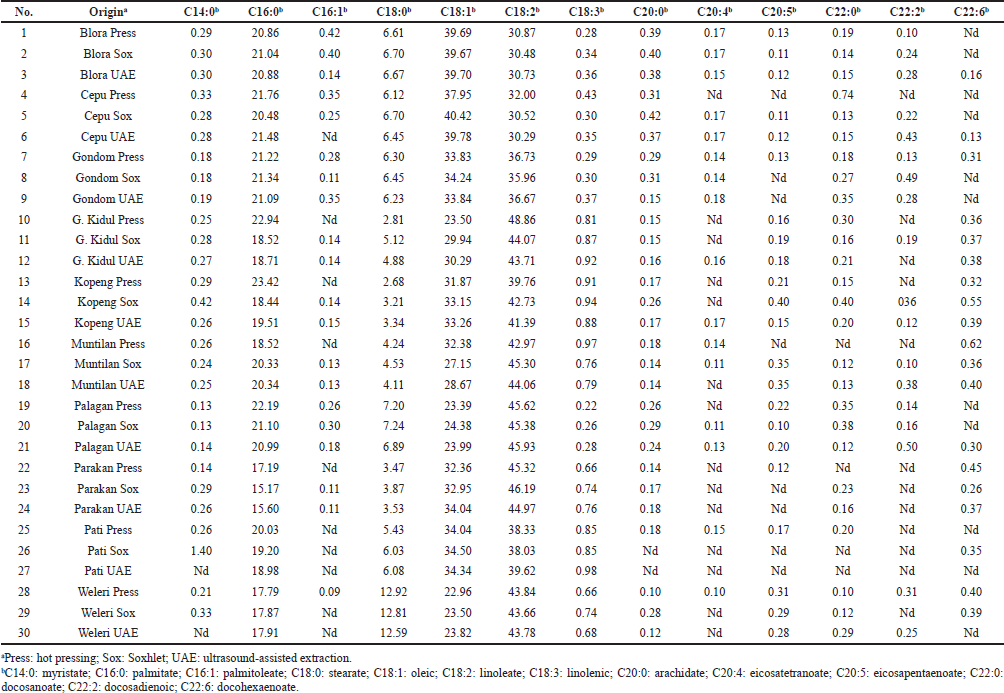 | Table 4. FA compositions of PSO from different origins and extraction methods as determined using GC with flame ionization detector**. [Click here to view] |
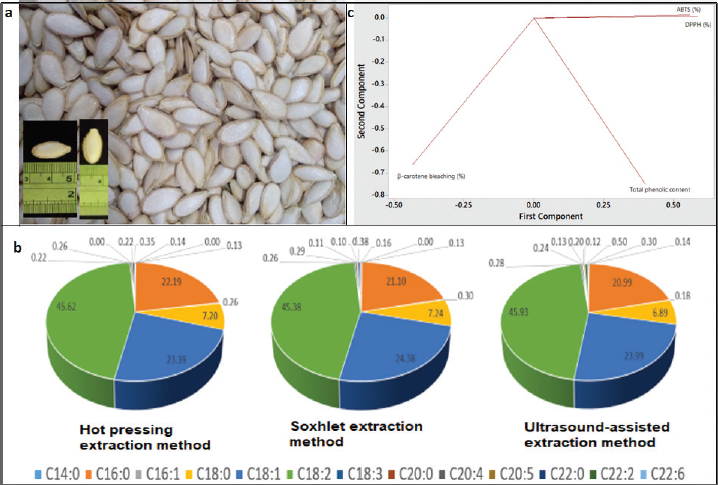 | Figure 1. (a) Example of pumpkin seeds (C. moschata Duchesne) used in this study. (b) FA composition of PSO from Palagan region, Yogyakarta. (c) Loading plot of principal component analysis for evaluating the correlation between antioxidant activities and phenolic compounds. [Click here to view] |
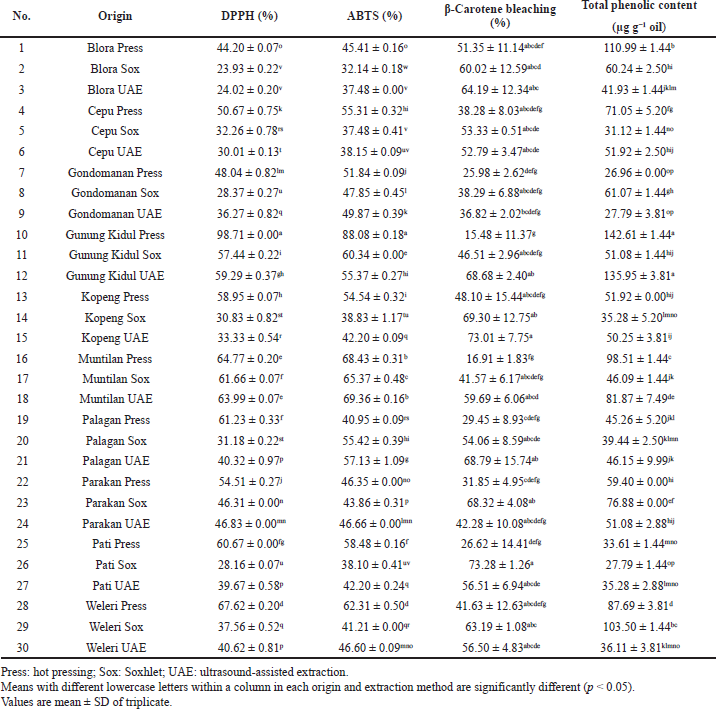 | Table 5. Antioxidant activity of PSO from different origins and extraction methods. [Click here to view] |