INTRODUCTION
Currently, edible vegetable oils are taken into account as major economic resources in certain nations. Vegetable oils are typically applied in some industries such as food, pharmaceuticals, cosmetics, and oleochemicals. In view of the nutritional aspects, vegetable oils also contain some bioactive compounds like essential fatty acids, fat-soluble vitamins, and phenolic compounds needed in human metabolism and energy sources (Zhang et al., 2012). Pumpkin seed oil (PSO), extracted from pumpkin (Cucurbita maxima) seeds, has been known as a functional oil due to its active components with certain biological activities (Rohman and Irnawati, 2020). PSO is reported to contain phenolic compounds, tocopherols, and carotenoids. These compounds are well known as a source of natural antioxidants (Al-Farsi et al., 2005). Several researches have reported the antioxidant activity of PSO using in vitro methods including 2,2′-diphenyl-1-picrylhydrazil (DPPH) radical scavenging assay and β-carotene/linoleic acid bleaching test and correlated these antioxidant activities with phenolics compounds (Jiao et al., 2014; KulaitienÄ— et al., 2018; Rezig et al., 2018; Siano et al., 2016). However, the determination of antioxidant activities using physicochemical tests involved reagents and several analytical steps; as a consequence, some fast techniques like the Fourier transform infrared (FTIR) spectroscopy method are continuously developed for measurement antioxidant activities.
Antioxidants are typically defined as any substance or any compound either synthetic or natural having the capability to significantly delay or prevent the oxidation reactions of the substrate when present at low levels compared with those of an oxidizable substrate (Rohman et al., 2020a). Due to some restrictions regarding the use of synthetic antioxidants, the natural antioxidants coming from plants are explored and developed, and among these are antioxidants from vegetable oils (Widodo et al., 2019). Some evaluations on antioxidant activities in vitro have existed in the scientific literature including 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay, Trolox equivalent antioxidant capacity method known as 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) radical cation decolorization assay, lipid peroxidation inhibition assay, beta-carotene bleaching method, metal chelating assay Ferric thiocyanate method, and cupric ion reducing antioxidant capacity method (Alam et al., 2013). DPPH radical scavenging assay is the most popular method applied for screening the antioxidant activities of edible fats and oils (Marina et al., 2009).
With the development of chemometrics or multivariate data analysis, some chemists tried to correlate the antioxidant with spectroscopic profiles for specific reasons including classification of edible oils or prediction of antioxidant activities using absorbance values of certain spectroscopic methods. Rohman et al. (2020b) have conducted a review on the application of FTIR spectra combined with chemometrics for authentication analysis of fats and oils. However, the application of FTIR spectroscopy combined with chemometrics for the prediction of antioxidant activities is very limited. Casoni et al. (2019) have successfully classified edible vegetable oils based on their UV-Vis spectra and the profiles of DPPH radical scavenging combined with chemometrics of pattern recognition of principal component analysis (PCA) and fuzzy-PCA. The combination of proton (1H)-NMR and proton (31P)-NMR spectroscopy and chemometrics of discriminant analysis were successful for the classification of 13 types of edible vegetable oils (Vigli et al., 2003). The other spectroscopic techniques combined with chemometrics applied in the classification of oils in different states are FTIR spectroscopy (Lu and Rasco, 2012; Valasi et al., 2020) and fluorescence spectroscopy (Cao et al., 2017). However, using literature investigation, there is no reported publication on the application of FTIR spectra for the classification of PSO. Therefore, the objectives of this study were (1) to classify PSO from different origins based on FTIR spectra and chemometrics of PCA and cluster analysis (CA) and (2) to predict DPPH radical scavenging activities based on FTIR spectra combined with the multivariate calibration of partial least square.
MATERIALS AND METHODS
Materials
Pumpkin seeds were obtained from several locations around Yogyakarta and Central Java, Indonesia. The pumpkin fruit was authenticated in the Laboratory of Pharmacognosy, Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada, under the supervision of Dr. Djoko Santosa. DPPH was purchased from Sigma (Aldrich, St. Louis, MO). The other reagents and solvents used for analysis were of analytical grade obtained from E. Merck (Darmstadt, Germany).
Microwave pretreatment
Microwave pretreatment of pumpkin seeds was carried out according to the method described by Azadmard-Damirchi et al. (2010). Pumpkin seed samples (100 g) were placed in an even layer in Pyrex Petri dishes inside the microwave (Hitachi, model MR-5750). Samples were microwave treated at a power of 50% for 240 seconds.
Oil extraction by pressing
PSO was extracted from the microwave-treated pumpkin seed samples by mechanical hot pressing (Maksindo MKS-J03) according to the method described by Dang and Bui (2019). The oil was later separated from the sediment by centrifugation at 2,500 rpm (Thermo Scientific) for 10 minutes and stored in a freezer at −20°C for subsequent analysis.
DPPH radical scavenging activity (RSA) assay
DPPH radical scavenging assay was carried out according to Casoni et al. (2019). PSO samples were diluted with ethanol in a 10 ml volumetric flask. For each of the analyzed PSO samples, total radical scavenging capacity was determined by measuring the absorbance of DPPH solution 0.4 mM at 515 nm (Abs control) and the absorbance of DPPH solution added with the evaluated PSO samples after a reaction time of 30 minutes (Abs sample). All absorbance measurements were corrected with the absorbance of blank containing solvent and the studied PSO samples. For the calculation of RSA percentage (%), the following formula was used:
FTIR spectra measurement
The FTIR spectra of studied PSO samples were carried out using the conditions as previously reported by Irnawati et al. (2020). The spectra were scanned using an FTIR spectrophotometer (Thermo Scientific Nicolet iS10, Madison, WI), controlled with the operating Omnic software. The measurements were carried out in a mid-infrared region of 4,000–650 cm−1 with a scanning number of 32 and a resolution of 8 cm−1. The used sampling accessory was horizontal attenuated total reflectance composed of ZnSe crystal. All FTIR spectra were corrected against the FTIR spectrum of air as background. After every scan, a new reference air background spectrum was taken. These spectra recorded as absorbance values at each data point in triplicate were used for making the correlation between antiradical activity and FTIR spectra.
Chemometrics analysis
The absorbance values of FTIR spectra were used as variables during the classification of PSO samples from different regions with the aid of chemometrics of PCA and CA. Furthermore, the prediction of DPPH RSA using a variable of absorbance values of FTIR spectra was assisted with the multivariate calibration of partial least square. All chemometric analyses [PCA, CA, and partial least square (PLS)] were carried out using Minitab® version 17 (Minitab Inc., State College, PA).
RESULTS AND DISCUSSION
In this study, the antioxidant activities of PSO from different regions around Yogyakarta and Central Java, Indonesia, were evaluated by DPPH radical scavenging activities. This technique was based on the capability of chemical compounds present in PSO to reduce DPPH radicals into nonradicals (Rohman et al., 2006; Widyastuti et al., 2021). Table 1 shows the percentage of DPPH radical scavenging activities of PSO with a concentration of PSOs of 20 μl/ml. The RSA of PSOs from Gunung Kidul and Tawangmangu had the highest RSA among others, accounting for 98.71% ± 0.02% and 83.57% ± 0.13%, respectively.
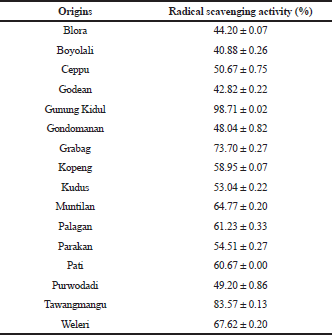 | Table 1. Radical scavenging activities of pumpkin seed oils (PSOs) from different origins at concentration levels of 100 μl/5 ml. [Click here to view] |
In order to classify PSOs from different origins, two chemometric classifications, namely, PCA and CA, were used. PCA and CA are unsupervised pattern recognition in chemometrics analysis (Che Man et al., 2011). PCA is a feature for the reduction of the amount of data when there is a correlation present (Miller and Miller, 2010). In this study, PCA was accomplished using FTIR-ATR spectra absorbance of 16 PSO samples at 14 wavenumber and radical scavenging activities, as shown in Table 2. Each peak or shoulder is associated with structural or functional group information in PSO (Saucedo-Hernández et al., 2011). The functional group and vibration mode for the absorption of peak and shoulder in PSO are presented in Table 3. The functional groups represented in FTIR spectra are correlating with chemical compounds present in PSO. Edible fats and oils including PSO are mainly composed of triacylglycerols (TAG) in approximately 95%–98%; as a consequence, the functional groups present in TAG dominate (Rohman et al., 2020c). The functional groups of methyl, methylene, ether, carbonyl, and backbone of fatty acids are represented in FTIR spectra. In addition, the functional groups which are representative for the minor components like functional group of −OH phenolics disappear due to the low levels; therefore, they are not detected in FTIR spectra. Figure 1 shows the score plot of PCA of 16 PSOs from different origins representing the projection of samples defined by the first principal component (PC1) and the second principal component (PC2). An eigenvalue of about 99.3% was achieved using six PCs (Fig. 2). The variance (69.7%) is being described by PC1 and PC2. PC1 accounted for 49.2% of the variation, while PC2 described 20.4% of the variation. Figure 3 shows the loading plot for the determination of wavenumbers contributing to the separation of the PSO from different origins. Based on the loading plot, the wavenumbers at 2,953, 1,460, and 1,160 cm−1 made a large contribution to the PCA model.
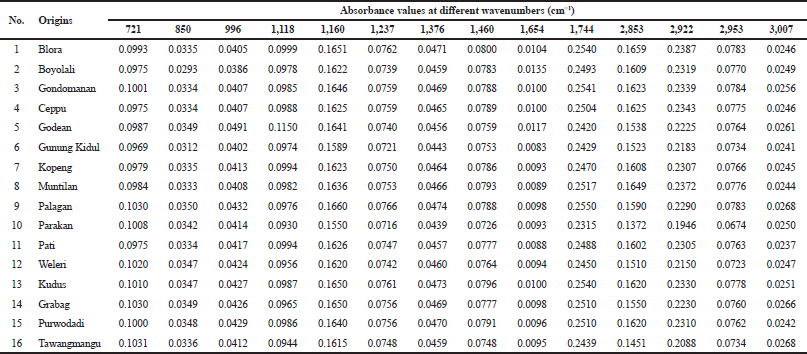 | Table 2. Peak absorbances for each wavenumbers and radical scavenging activity of PSO from different origins. [Click here to view] |
 | Table 3. Functional groups responsible for absorption of peaks and shoulders in PSO. [Click here to view] |
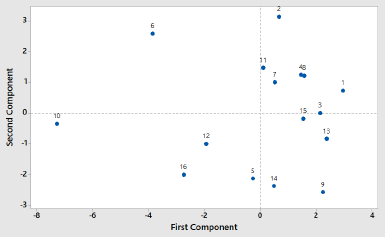 | Figure 1. The PCA score plot of pumpkin seed oils (PSOs) from different origins: (1) Blora; (2) Boyolali; (3) Gondomanan; (4) Ceppu; (5) Godean; (6) Gunung Kidul; (7) Kopeng; (8) Muntilan; (9) Palagan; (10) Parakan; (11) Pati; (12) Weleri; (13) Kudus; (14) Grabag; (15) Purwodadi; (16) Tawangmangu. [Click here to view] |
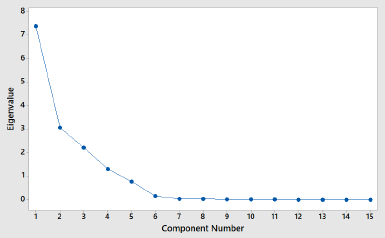 | Figure 2. Eigenvalue of PCA of PSO from different origins. [Click here to view] |
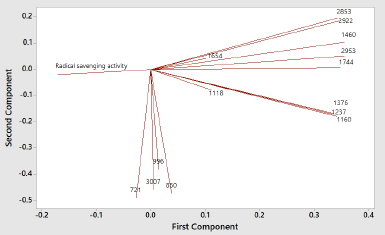 | Figure 3. The loading plot of PCA models of PSO from different origins. [Click here to view] |
CA is a hierarchical method in which successive partition of the data set results in a cluster represented as a dendrogram or tree (Djuris et al., 2013). In addition, CA can provide numerical values of similarity among the objects evaluated and therefore obtained more objective information (Che Man et al., 2011). We divided a group of PSOs from different origins into classes so that similar characteristics of PSO are in the same class. The classification stages of PSO can be seen in Table 4. It can be stated that the first joined are PSOs from Palagan and Pati, followed by PSOs from Gondomanan and Purwodadi with distance levels being 0.5607 and 0.1643, respectively, and so on until all PSOs from different origins are grouped into one class. Figure 4 showed a dendrogram using the single linkage method. This analysis suggests that the evaluated PSOs from different origins fall into five groups. Group 1 contained PSO from Palagan, Pati, Kopeng, Muntilan, and Weleri; PSOs from Gondomanan, Purwodadi, Ceppu, Parakan, and Kudus formed group 2; PSOs from Blora, Godean, and Boyolali formed group 3; PSOs from Grabag and Tawangmangu formed group 4. Meanwhile, PSO from Gunung Kidul formed a separate group (group 5).
The multivariate calibration of PLSR was used for the prediction of antioxidant activities through DPPH radical scavenging activities using absorbance values of FTIR spectra as variables during modeling. The variation in FTIR spectra of PSO was used to build a calibration model for the prediction of radical scavenging activities in PSO. In this study, to obtain the best prediction model, the wavenumber regions (3,900–650, 3,500–650, 1,700–650, 1,130–700, and combined wavenumbers of 3,050–2,800 and 1,790–650 cm−1) and FTIR spectral treatment (normal, first derivative, and second derivative) were optimized. Spectra derivatization is able to produce better resolution (Windarsih et al., 2020). Table 5 shows the optimization of the calibration model using the PLS calibration model. The selection of optimum conditions was based on the highest coefficient correlation and the lowest root mean square error of calibration (RMSEC) and the root mean square error of prediction (RMSEP) (Irnawati et al., 2019). Finally, the first derivative FTIR spectra at wavenumber region at 3,500–650 cm−1 were selected for the prediction of RSA in PSO with R2 value of 0.9996 and 0.9418 in calibration and validation models, while RMSEC and RMSEP were 0.425% and 4.93 %, respectively (Fig. 5a). Figure 5b shows the residual analysis between actual and predicted value. It is clear that all point differences are located above and below zero value; therefore, the systematic error during PLS modeling between actual values of antioxidant activities and FTIR predicted values could be negligible. The capability of FTIR spectroscopy combined with chemometrics to predict the antioxidant activities is not surprising because FTIR spectra are fingerprints in nature so that the analyst can select the peaks in FTIR spectra which correlated with the antioxidant activities to be predicted with antioxidant activities. Because of its benefits, in the future, it is hoped that FTIR spectra combined with chemometrics techniques of multivariate calibration can be used as an alternative method for determining the antioxidant activity of PSO in vitro.
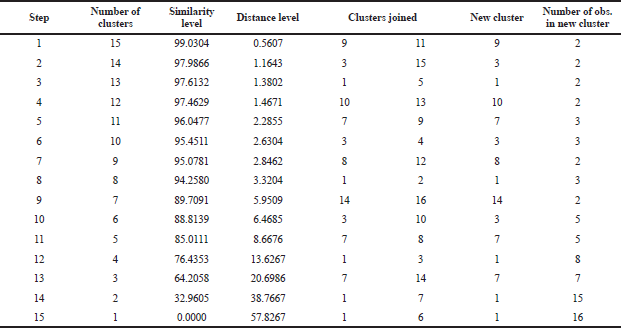 | Table 4. The classification stages of PSO from different origins. [Click here to view] |
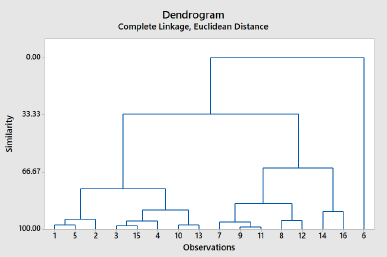 | Figure 4. The dendrogram of PSOs from different origins: (1) Blora; (2) Boyolali; (3) Gondomanan; (4) Ceppu; (5) Godean; (6) Gunung Kidul; (7) Kopeng; (8) Muntilan; (9) Palagan; (10) Parakan; (11) Pati; (12) Weleri; (13) Kudus; (14) Grabag; (15) Purwodadi; (16) Tawangmangu. [Click here to view] |
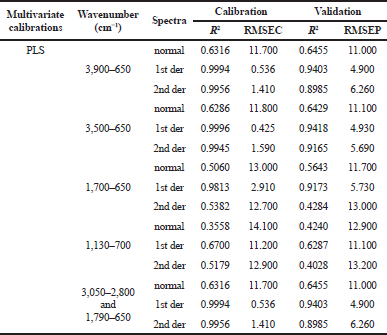 | Table 5. Multivariate calibration of PLSmodel for the prediction of radical scavenging activity in PSO. [Click here to view] |
 | Figure 5. PLS calibration model (a) and residual factor (b) for prediction of radical scavenging activity in PSO. The x-axis showed the actual radical scavenging activity in PSO; the y-axis showed the calculated radical scavenging activity of PSO. [Click here to view] |
CONCLUSION
FTIR spectra combined with chemometrics techniques are effective means for the classification of PSO from different origins and for the prediction of antioxidant activities. PCA and CA were successfully applied for the classification of PSOs from different origins as indicated by clear separation among the evaluated samples. In addition, PLS regression was also accurate and precise for predicting the antioxidant activities as indicated by a high coefficient of determination (R2) and low errors. The developed method is considered a green analytical technique due to the use of less solvent and with minimum sample preparation; therefore, the method could be considered an alternative method for determining antioxidant activities.
ACKNOWLEDGEMENTS
The authors acknowledge the Ministry of Research, Technology and Higher Education, Republic of Indonesia, for financial support during this study through Hibah Penelitian Dasar Unggulan Perguruan Tinggi (PUPT) 2020 with contract number 1761/UN1.DITLIT/DIT-LIT/LT/2020.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J, 2013; 21(2):143–52. CrossRef
Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem, 2005; 53(19):7592–9. CrossRef
Azadmard-Damirchi S, Habibi-Nodeh F, Hesari J, Nemati M, Achachlouei BF. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem, 2010; 121(4):1211–5. CrossRef
Cao J, Li C, Liu R, Liu XR, Fan Y, Deng ZY. Combined application of fluorescence spectroscopy and chemometrics analysis in oxidative deterioration of edible oils. Food Anal Methods, 2017; 10(3):649–58. CrossRef
Casoni D, Simion IM, Sârbu C. A comprehensive classification of edible oils according to their radical scavenging spectral profile evaluated by advanced chemometrics. Spectrochim Acta A Mol Biomol Spectrosc, 2019; 213:204–9. CrossRef
Che Man YB, Rohman A, Mansor TST. Differentiation of lard from other edible fats and oils by means of Fourier transform infrared spectroscopy and chemometrics. J Am Oil Chem Soc, 2011; 88(2):187–92. CrossRef
Dang TQ, Bui HQH. Effect of roasting and microwave heating on the yield, quality, total phenolics and antioxidant capacity of oil from red pumpkin seed. EC Nutr, 2019; 8:588–96.
Djuris J, Ibric S, Djuric Z. Chemometric methods application in pharmaceutical products and processes analysis and control. In: Djuris J (ed.). Computer-aided applications in pharmaceutical technology. Woodhead Publishing Limited, Cambridge, UK, pp 57–90, 2013. CrossRef
Irnawati, Riyanto S, Martono S, Rohman A. Analysis of palm oil as oil adulterant in olive and pumpkin seed oils in ternary mixture systems using ftir spectroscopy and chemometrics. Int J Appl Pharm, 2019; 11(5):210–5. CrossRef
Irnawati I, Riyanto S, Martono S, Rohman A. The employment of FTIR spectroscopy and chemometrics for authentication of pumpkin seed oil sesame oil. Food Res, 2020; 4(1):42–8. CrossRef
Jiao J, Li ZG, Gai QY, Li XJ, Wei FY, Fu YJ, Ma W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chem, 2014; 147:17–24. CrossRef
KulaitienÄ— J, ÄŒerniauskienÄ— J, JarienÄ— E, DanilÄenko H, LevickienÄ— D. Antioxidant activity and other quality parameters of cold pressing pumpkin seed oil. Not Bot Hortic Agrobo, 2018; 46(1):161–6. CrossRef
Lu X, Rasco BA. Determination of antioxidant content and antioxidant activity in foods using infrared spectroscopy and chemometrics: a review. Crit Rev Food Sci Nutr, 2012; 52(10):853–75. CrossRef
Marina AM, Che Man YB, Nazimah SAH, Amin I. Chemical properties of virgin coconut oil. J Am Oil Chem Soc, 2009; 86(4):301–7. CrossRef
Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. 6th edition, Prentice Hall, Harlow, UK, p 278, 2010.
Rezig L, Chouaibi M, Ojeda-Amador RM, Gomez-Alonso S, Salvador MD, Fregapane G, Hamdi S. Cucurbita maxima pumpkin seed oil: From the chemical properties to the different extracting techniques. Not Bot Hortic Agrobotanici Cluj-Napoca, 2018; 46(2):663–9. CrossRef
Rohman A, Ghazali MAB, Windarsih A, Irnawati, Riyanto S, Yusof FM, Mustafa S. Comprehensive review on application of FTIR spectroscopy coupled with chemometrics for authentication analysis of fats and oils in the food products. Molecules, 2020b; 25:1–28. CrossRef
Rohman A, Irnawati. Pumpkin (Cucurbita maxima) seed oil: chemical composition, antioxidant activities and its authentication analysis. Food Res, 2020c; 4(3):578–84. CrossRef
Rohman A, Riyanto S, Utari U. Antioxidant activity and total phenolic content of ethyl acetate extract of Mengkudu (Morinda citrifolia L.) fruit. Indones J Pharm, 2006; 17(2):136–42.
Rohman A, Widodo H, Lukitaningsih E, Windarsih A, Rafi M, Nurrulhidayah AF. Review on in vitro antioxidant activities of curcuma species commonly used as herbal components in Indonesia. Food Res, 2020a; 4(2):286–93. CrossRef
Saucedo-Hernández Y, Lerma-García MJ, Herrero-Martínez JM, Ramis-Ramos G, Jorge-Rodríguez E, Simó-Alfonso EF. Classification of pumpkin seed oils according to their species and genetic variety by attenuated total reflection fourier-transform infrared spectroscopy. J Agric Food Chem, 2011; 59(8):4125–9. CrossRef
Siano F, Straccia MC, Paolucci M, Fasulo G, Boscaino F, Volpe MG. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J Sci Food Agric, 2016; 96:1730–5. CrossRef
Valasi L, Arvanitaki D, Mitropoulou A, Georgiadou M, Pappas CS. Study of the quality parameters and the antioxidant capacity for the Ftir-chemometric differentiation of pistacia vera oils. Molecules, 2020; 25(7):1614. CrossRef
Vigli G, Philippidis A, Spyros A, Dais P. Classification of edible oils by employing 31P and 1H NMR spectroscopy in combination with multivariate statistical analysis. A proposal for the detection of seed oil adulteration in virgin olive oils. J Agric Food Chem, 2003; 51(19):5715–22. CrossRef
Widodo H, Sismindari S, Asmara W, Rohman A. Antioxidant activity, total phenolic and flavonoid contents of selected medicinal plants used for liver diseases and its classification with chemometrics. J Appl Pharm Sci, 2019; 9(6):99–105. CrossRef
Widyastuti I, Luthfah HZ, Hartono YI, Islamadina R, Can AT, Rohman A. Antioxidant Activity of Temulawak (Curcuma xanthorrhiza Roxb.) and its classification with chemometrics. Indones J Chemom Pharm Anal, 2021; 1(1):29–42. CrossRef
Windarsih A, Irnawati, Rohman A. Application of FTIR-ATR spectroscopy and chemometrics for the detection and quantification of lard oil in bovine milk fat. Food Res, 2020; 4(5):1732–8. CrossRef
Zhang Q, Liu C, Sun Z, Hu X, Shen Q, Wu J. Authentication of edible vegetable oils adulterated with used frying oil by fourier transform infrared spectroscopy. Food Chem, 2012; 132(3):1607–13. CrossRef