INTRODUCTION
Liver cancer is common in countries in Southeast Asia, including Indonesia. More than 800,000 people are diagnosed with this type of cancer each year throughout the world. Liver cancer is also a leading cause of cancer deaths worldwide, accounting for more than 700,000 deaths each year (American Cancer Society, 2019). In 2018, liver cancer contributed to 8.8% of 207,210 cancer deaths in Indonesia (Cancer Country Profile, 2020). The most common form of liver cancer in adults is hepatocellular carcinoma (HCC), which occurs more frequently in men, with the disease affecting men about three times more often than women. The incidence rate of the disease is also increasing exponentially (American Cancer Society, 2019). Hence, liver cancer is a vital public health problem, especially for developing countries like Indonesia, where the endemicity is often either intermediate or even high.
Hepatitis B virus (HBV) and hepatitis C virus infections are the primary cause of HCC. However, HBV infections are commonly found in Indonesia with a 4.7%–11.2% endemicity range (Janevska et al., 2015; Yano et al., 2015). The high endemicity of HBV infection in Indonesia which leads to HCC complications would pose a heavy burden not only for the Indonesian healthcare system but also for Indonesian people due to the various healthcare payments applied in Indonesia. Indonesian people could use the out-of-pocket payment system and also the main government health insurance program, namely Jaminan Kesehatan Nasional, which is the national insurance scheme for all Indonesian citizens. For the health coverage provided by this national insurance, Indonesia has implemented Indonesian Case Base Groups tariff rates according to the diagnosis grouping based on clinical and homogeneity of resource utilization approaches (Satibi et al., 2019). On the other hand, very little data exists about the financial burden caused by liver cancer in Indonesia. Given this, we carried out a cost-of-illness study of liver cancer by reviewing data from a large referral hospital in Indonesia. The results of this study should provide Indonesian health policymakers data to prioritize health interventions to allocate resources efficiently in devising health insurance schemes.
METHODS
Study design and study population
This study complies with the standards of the bottom-up approach in cost-of-illness analyses which demonstrate the quantification of the direct medical cost of liver cancer (Tan et al., 2009). The study proposal was reviewed and approved by the Medicine and Health Ethics Committee of Universitas Gadjah Mada with approval number KE/FK/0908/EC/2020. This study included patients 18 years of age and older who were diagnosed and categorized into three mutually exclusive groups, compensated cirrhosis (CC), decompensated cirrhosis (DC), and other chronic liver diseases indicating the disease severity at the initial stage of HCC diagnosis, and having complete data documented. The investigators identified potentially eligible patients from consecutive visits monitored in the Integrated Cancer Installation Unit visit register.
Data sources
Data were collected from 157 liver cancer patients’ medical and financial records in Sardjito Hospital, Yogyakarta, Indonesia, from January to December 2020. The records for both inpatients and outpatients treated at the Integrated Cancer Installation Unit were included. We identified and obtained information on liver cancer patients by using the codes established by the International Statistical Classification of Diseases, 10th Revision, such as K74 (CC and DC) and C22 (HCC). Patients coded with K74 were differentiated as either CC or DC through clinical diagnosis from patient case notes.
Calculation of direct medical costs
The direct medical cost consisted of the costs of hospitalization, outpatient visits, surgeries, costs of prescription drugs for liver diseases, costs of transarterial chemoembolization (TACE), and costs of interferons (IFNs) which were categorized per month for the length of each individual’s follow-up (per patient per month). The financial burden to the Indonesian society during this study period is estimated by the total direct medical cost multiplied by the number of liver cancer patients in Referral Hospital Dr. Sardjito, a large referral hospital in Yogyakarta, Indonesia. The study examined resource utilization based on hospital charges in 2020, which is expressed in US$ (US$1 = Indonesian Rupiah 14,241).
Statistical analysis
Descriptive analysis was carried out in the form of mean with standard deviation for numerical data and frequency with percentage for categorical data. A normality test of the cost data was carried out using a histogram, a Q-Q plot, and the Shapiro–Wilk test. Data were analyzed using the Statistical Package for the Social Sciences version 21 and Microsoft Excel 2016. All results were demonstrated in the form of tables.
RESULTS
General characteristic of the study participants
Table 1 presents the characteristics of 157 patients, consisting of 34 patients with CC, 76 patients with DC, and 47 patients with HCC. The mean age was 54.12 (±10.12) years with male predominance (74.5%) observed among the sample. Approximately 49.7% of the participants were primary school graduates, 35.7% were secondary school graduates, and 14.6% were university graduates. Around 35.7% of the participants had a monthly household income of not more than US$100 and only 7.6% of the participants had a monthly family income of more than US$500. More than half of the patients (63.1%) resided in the urban area and the majority of the patients were unemployed (49.7%). The majority of patients had a Charlson comorbidity index (CCI) score of more than 3 (37.6%) and only 13.4% of patients had a 0 score of CCI.
Types of direct cost for caring for liver cancer patients
The details of health service use and costs among liver cancer patients are demonstrated in Table 2. The total cost of hospitalization accounted for US$5,096 with HCC treatment predominance (US$2,032). The outpatient visits cost was found to be the highest for DC patients (US$341) with the cumulative cost accounting for US$827. The cost of prescription drugs for liver cancer patients accounted for US$1,851 for the cumulative three liver cancer stages. Surgery cost was the major cost driver in treating liver cancer patients with a cumulative US$55,697 for all three stages. The total payment for TACE was around US$2,485, and the total payment for IFNs accounted for US$5,705. Overall, the results show that the direct cost of the treatment increased with disease progression, with the total direct medical costs for treating liver cancer patients during the study period being approximately US$71,661.
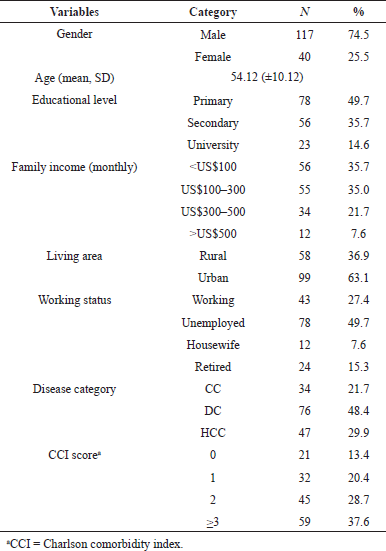 | Table 1. Characteristics of liver cancer patients in a tertiary hospital in Indonesia (N = 157). [Click here to view] |
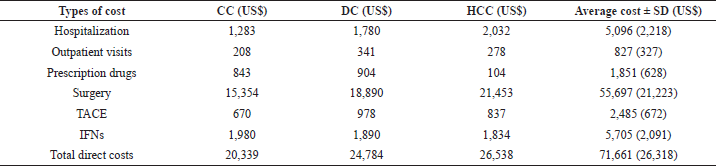 | Table 2. Types of direct cost and average cost per patient among liver cancer patients. [Click here to view] |
The case-based estimation of annual direct medical cost among liver cancer patients
The estimation of annual direct medical cost based on disease category in treating liver cancer patients was roughly US$3,822,375. The highest treatment cost was found in treating HCC patients with the total cost accounting for US$26,538, followed by DC patients with a cumulative US$24,784 and CC patients treated with an estimated total payment of around US$20,339. However, as the consequence of the majority of DC patients involved in this study (48.4%), the estimated annual direct medical cost for DC patients’ treatments was found to be the highest (US$1,883,554). The particular case-based estimation of the annual direct medical cost of the liver cancer treatment is summarized in Table 3.
Discussion
To our knowledge, this is the first study carried out in Indonesia to estimate the cost of illness of liver cancer in Indonesia. Our results demonstrated that liver cancer obtrudes a substantial financial burden on the Indonesian society and the healthcare system. Moreover, the burden of liver cancer described in this study would become a baseline study not only for the government to initiate the most effective regulation related but also for the Indonesian society to begin healthy lifestyle changes to prevent liver diseases. Therefore, the appropriate management of liver disease-related risk factors is very important to reduce the financial burden. Our key finding from this study was the cumulative annual direct medical cost of liver cancer patients that accounted for US$3,833,375. The increase of the direct medical costs with disease progression showed in our study was in line with the previously published study in Vietnam (Tu et al., 2012).
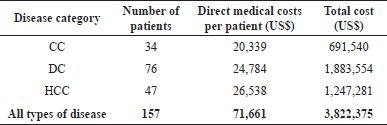 | Table 3. Annual direct medical cost based on disease category among liver cancer patients. [Click here to view] |
The direct medical cost was high among patients with HCC (US$26,538), resulting from a high cost of surgical interventions and a high hospitalization rate. These findings were similar to prior studies worldwide which demonstrated that the medical costs for treating HCC patients with advanced liver disease were higher than other etiologies of liver diseases (Nguang et al., 2018; Thein et al., 2013; Tu et al., 2012). Expenses on surgery were the largest cost driver for the direct medical costs for all CC, DC, and HCC categories, contributing more than 70% to the total direct medical costs. This might be explained as the consequence of the high surgical intervention prices in Indonesia. However, expenses on outpatient visits, prescription drugs, and TACE intervention for DC patients are identified to be the highest among the other disease categories. These findings might be connected with prior studies which identified that the overall survival rate for DC patients was the lowest compared to patients with mild-to-moderate chronic liver disease (Miquel et al., 2018; Nguang et al., 2018).
Expanses on IFNs were higher in treating CC patients than other disease categories (US$1,980). This result might be explained by the frequent use of IFNs in the management of CC in Indonesia based on the national consensus (Indonesian Association for the Study of the Liver, 2012). Cirrhotic patients with compensated stage disease were likely to be offered treatment with carefully persistent side effects monitoring. The majority of patients who participated in this study were DC patients. Therefore, this group was the major contributor to the estimated total direct medical cost (US$1,883,554). Overall, despite the difference in the type of treatment expenses in each stage, this study highlighted that the total cost for treating liver cancer patients was estimated to be more than US$20,000 per patient.
Liver cancer is highly fatal and is considered as the most common cancer that causes mortality in Indonesia. This study provides a comprehensive view of the cost burden of liver cancer care in Indonesia’s healthcare setting. However, the majority of patients aged more than 50 years were in late stages or with comorbidities. Further research is warranted to investigate the detailed costs of screening and other therapies in practice. Several prevention strategies have been proposed to reduce the ever-increasing cost of liver cancer treatment. Early detection and chemoprevention of HCC occurrence or recurrence are examples of secondary or tertiary prevention strategies, accordingly, in patients who already received aetiological agents (Hoshida et al., 2012). HCC screening was identified to be cost-effective and associated with improved early tumor detection, curative treatment rates, and survival based on the results of a series of prior cohort studies and model-based simulation (Fujiwara et al., 2018; Mittal et al., 2016; Singal et al., 2014).
The preventive strategies of liver diseases may help the reduction of the incidence which could decrease the cost. Unfortunately, in developing countries like Indonesia, people often begin seeking medical help only when the disease has already developed. Hence, this study would be helpful for the health policymakers in designing future practical interventions related to liver cancer and also providing adequate liver cancer screening as the most effective strategy to reduce the financial burden for both the government and patients. Additionally, this study would help the healthcare provider and the patient to not only identify and quantify the liver cancer attributable cost in Indonesia but also take the preventive actions appropriately.
As a prevalence-based cost-of-illness study from the patient’s perspective, the strength of this study was the calculation of the cost which covered all the possible direct cost components based on real patient data. Even though the cost estimations accounted solely for one large referral hospital in Yogyakarta, Indonesia, the results of the analysis are still considered important since no relevant study analyzing the cost of liver cancer has been conducted before in Indonesia. Nevertheless, this study has several limitations that should be acknowledged. Firstly, there were a limited number of participants in this study. Secondly, direct nonmedical costs and indirect and intangible costs related to liver disease were not addressed in this study. Moreover, this was a descriptive cost-of-illness study that did not provide information on the efficiency of resource uses. Thus, the higher cost could not be interpreted as better services or value for money.
CONCLUSION
The current study highlights that the medical costs of liver cancer to the healthcare system are significant. This study’s findings representing real-world cost estimates of liver cancer care provide insightful information for the future economic evaluation of early detection interventions, resource allocation, and health insurance reimbursement policies.
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Higher Education of Indonesia for supporting the research funding, the board of directors and hospital staff of the Sardjito Hospital, Yogyakarta, Indonesia, and also the participants of this study. They would also like to thank the pharmacists in Universitas Gadjah Mada, Yogyakarta.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest in this study.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
American Cancer Society. What is liver cancer? American Cancer Society, Atlanta, GA, vol. 2009, pp 1–6, 2019. Available via https://www.cancer.org/cancer/liver-cancer/about/what-is-liver-cancer.html
Cancer Country Profile. Indonesia: burden of cancer, 2020. Available via https://www.who.int/cancer/country-profiles/IDN_2020.pdf?ua=1
Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol, 2018; 68(3):526–49; doi:10.1016/j.jhep.2017.09.016 CrossRef
Hoshida Y, Fuchs BC, Tanabe KK. Prevention of hepatocellular carcinoma: potential targets, experimental models, and clinical challenges. Curr Cancer Drug Targets, 2012; 12(9):1129–59. Available via https://pubmed.ncbi.nlm.nih.gov/22873223
Indonesian Association for the Study of the Liver. Indonesia National Consensus of hepatitis B virus infection management. Ina ASL, Jakarta, Indonesia, 2012.
Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis, and treatment. Open Access Maced J Med Sci, 2015; 3(4):732–6; doi:10.3889/oamjms.2015.111 CrossRef
Miquel M, Cleries M, Vergara M, Vela E. Economic burden of cirrhosis in Catalonia: a population-based analysis. BMJ Open, 2018; 8(3):1–10; doi:10.1136/bmjopen-2017-018012 CrossRef
Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, Davila JA, El-Serag HB. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol, 2016; 65(6):1148–54; doi:10.1016/j.jhep.2016.07.025 CrossRef
Nguang SH, Wu CK, Liang CM, Tai WC, Yang SC, Ku MK, Yuan LT, Wang JW, Tseng KL, Hung TH, Hsu PI, Wu DC, Chuah SK, Hsu CN. Treatment and cost of hepatocellular carcinoma: a population-based cohort study in Taiwan. Int J Environ Res Pub Health, 2018; 15(12); doi:10.3390/ijerph15122655 CrossRef
Satibi S, Andayani TM, Endarti D, Suwantara IPT, Agustini NP D. Comparison of real cost versus the Indonesian case base groups (INA-CBGs) tariff rates among patients of high-incidence cancers under the National Health Insurance Scheme. Asian Pac J Cancer Prev, 2019; 20(1):117–22; doi:10.31557/APJCP.2019.20.1.117 CrossRef
Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med, 2014; 11(4):e1001624; doi:10.1371/journal.pmed.1001624 CrossRef
Tan SS, Rutten FFH, van Ineveld BM, Redekop WK, Hakkaart-van Roijen L. Comparing methodologies for the cost estimation of hospital services. Eur J Health Econ, 2009; 10(1):39–45; doi:10.1007/s10198-008-0101-x CrossRef
Thein HH, Isaranuwatchai W, Campitelli MA, Feld JJ, Yoshida E, Sherman M, Hoch JS, Peacock S, Krahn MD, Earle CC. Health care costs associated with hepatocellular carcinoma: a population-based study. Hepatol, 2013; 58(4):1375–84; doi:10.1002/hep.26231 CrossRef
Tu HAT, Woerdenbag HJ, Riewpaiboon A, Kane S, Le DM, Postma MJ, Li SC. Cost of illness of chronic hepatitis B infection in Vietnam. Value Health Reg Issues, 2012; 1(1):23–8; doi:10.1016/j.vhri.2012.03.008 CrossRef
Yano Y, Utsumi T, Lusida MI, Hayashi Y. Hepatitis B virus infection in Indonesia. World J Gastroenterol, 2015; 21(38):10714–20; doi:10.3748/wjg.v21.i38.10714 CrossRef