INTRODUCTION
Radiographic techniques are used to examine the body’s internal organs for the diagnosis of disease and tissue damage. Radiography, assisted by various agents, can differentiate tissue density differences, and trained experts in the field can pick up any damage and inconsistencies. Contrast media (CM) are chemicals that enhance the contrast between different body tissues by differential increases in the tissues’ radiopacity depending on their densities and interstitial spaces. Their use was integral to radiography and begun in the late 19th century when X-rays were discovered. Early on, it was realized that elements with a high atomic number would improve the X-ray images’ contrast. In fact, the first-ever image assisted by the use of CM was that of an amputated hand as reported by Haschek (1896). For their study, Haschek (1896) used Lead, barium, and bismuth salts. It was also realized that these metal salts were harmful to living tissues and thus could not be used in living individuals. Several decades later in Osborne et al (1923), used iodine as a contrast agent, which was already in use for syphilis (Osborne et al., 1923). The variety of CM agents and their use for different radiography has since seen exponential growth. Contrast-enhanced computer-assisted tomography has also relied on the use of CM for image enhancement, and this has become the imaging method of choice for a wide variety of reasons. Other early contrast agents included strontium bromide and sodium iodide (Berberich and Hirsch, 1923; Brooks, 1924).
Currently, routinely used CM include derivatives of 2, 4, 6-tri-iodinated benzene, carbon dioxide (CO2), and gadolinium. There are advantages and disadvantages for both of them. The addition of iodine to produce tri-iodinated benzene derivatives makes it more radiopaque, produces a lower incidence of toxicity, and is highly stable, making it a good choice in radio CM. The addition of side chains makes the iodinated CM more lipid-soluble, such as iothalamate and diatrizoate. Iodinated CM is classified based on chemical structure, iodine content, and iodination properties. Because of the carboxyl moiety addition, there is a net negative charge on the iodinated derivatives of benzene as CMs. Thus, they are used as sodium, calcium, or methylamine salts (Yoshikawa, 1992). Based on this, there are four major classes of iodinated CM: ionic monomer; consisting of a tri-iodinated benzene ring with a carboxylate-containing benzene substituent, ionic dimer; contains two benzene rings that are tri-iodinated in which at least one carboxylate-containing group is substituted on at least one benzene ring, non-ionic monomer; single tri-iodinated benzene ring without a carboxylate-containing benzene substituent, and non-ionic dimer: two linked tri-iodinated benzene rings that do not contain a carboxylate functional group within any benzene substituent (Solomon et al., 1994). In addition to the structural variation, iodinated CM is also classified into ionic and non-ionic types which differ in the presence of a carboxyl group (-COO-) moiety on the organic side chains of the CM. The four categories of CM have different properties and toxicity profiles, which makes them useful for different imaging examinations. One distinction, for instance, is the osmolarity of the CM. This can be an essential factor to be considered for choosing a CM for imaging. Non-ionic dimer iodinated CM is iso-osmolar to blood with an osmolarity of 290 mOsm/l while ionic monomers have high osmolarity and ionic dimers and non-ionic monomers have low osmolarity (Weininger et al., 2011). Iodixanol is a non-ionic dimeric iodinated derivative of benzene consisting of two benzene rings. It is commonly used as a CM in coronary angiography. It is the only CM that is iso-osmolar to blood with an osmolarity of 290 mOsm/kg H2O. It shows very low to negligible protein binding and is eliminated from the body with an elimination half-life of 2.1 hours, making it the radio CM of choice in a wide variety of imaging examinations. Although iodixanol has been in use for quite a while now and is considered a safe CM with a 71% reduced risk of neuropathy (McCullough, 2006), which is the most concerning side effect of CM, a small number of previous studies have shown some adverse reactions to its intravenous administration (Sutton et al., 2001). Iodixanol’s advantage is that it offers the best opacification of anatomical structures, which makes it suitable for radio and angiography, and angioplasty.
The use of CM for scans greatly aid image quality and the process of diagnosis. In a significant proportion of patients, CM may lead to adverse reactions such as thyroid dysfunction and contrast-induced nephropathy, as well as allergic reactions that might be as severe as anaphylactoid reactions (Leung and Braverman, 2012; Nadolski and Stavropoulos, 2013). As prophylaxis, patients are prescribed steroids and antihistamines before the scans (Small et al., 1982). However, factors attributable to these reactions vary, and the most significant risk factor is chronic kidney disease. History of previous adverse reactions to radio CMs also increases the risk of developing an allergic reaction to CM (Leung and Braverman, 2012). To ensure that proper care is provided, patients are monitored while the CM is injected and for few days following its use until it is entirely excreted from the body. If an allergic reaction is suspected, the infusion of the CM should be stopped immediately, and treatment with antihistamines should be started. If unnoticed and untreated, CM reactions can be fatal. Although the allergic response to CM can manifest in many forms and although the mortality due to adverse reactions to CM is less than 1 in 100,000, it can cause significant distress and discomfort to the patient along with additional complications, especially in patients with chronic kidney diseases (Andreucci et al., 2014). Thus, it is advised that patients’ medical history be assessed carefully to ensure the absence of any risk to CM use, a safe dosage (with appropriate dilution) of CM is used, and proper management strategies adopted if an adverse reaction occurs.
The aim of this study is to assess the incidence and type of allergic reactions induced by the administration of iodixanol CM in patients who underwent angiography.
METHODOLOGY
Study design
A cross-sectional retrospective study was conducted at Mohammed Bin Khalifa Cardiac Centre, Bahrain. Data from the period between January 2017 until October 2020 was included in this research. The start date of the data collection was chosen because the adverse drug reporting system was only initiated in 2017. The study sample was until October 2020, in order to prepare the required data analysis since the study is used as a part of an educational course. To construct the study methodology and objectives, a systematic search of the available literature was done using several search engines including the National library of medicine “PubMed” and Google scholar where related studies and similar researches were identified and reviewed during the study design phase.
Ethical approval
Ethical approval was obtained from BDF - Royal Medical Services – Military Hospital –Research & Research Ethics committee under Ref. No. BDF/R&REC/2020-458.
Study sample
A convenient sampling technique was used. Data was extracted from adverse drug reaction reporting system in the cardiac center. All patients who developed an allergic reaction (according to this system) within the specified study period were included in the study. The data collected were arranged from various categories of patients who suffered an allergic reaction due to intravenously administered iodixanol. The categories of patients were based on the patients’ demographic data, iodixanol CM dose, the severity of the allergic reaction, management, and finally, the Angioplasty date that relates to the season. The other system used to retrieve data is the “Apollo system.” This system is mainly used by the medical staff at the center and contains patients related information, which was used in order to obtain the iodixanol doses used if it was missing from the adverse drug reporting system. Additionally, the logbook from the catheterization laboratory was used to identify the missing Iodixanol doses along with the Apollo system which also involved relevant information about the patient’s history. Eventually, the patients’ previous medications list was collected from the AI-CARE system which is the main patient record system used in Bahrain Defense Force Hospital.
Statistical Analysis
SPSS version 23 was used for data entry and analysis. Frequencies and percentages were computed for all categorical variables. The chi-square test was used to determine a statistically significant relationship between any two categorical variables. In those statistical tests, a p-value of less than 0.05 was considered statistically significant.
RESULTS
The number of patients who reported adverse drug reactions in the mentioned period was found to be 230 patients out of 10,000 patients who underwent angiography or angioplasty. The adverse reaction was defined as a reaction that occurred within the first hour of administration of the iodixanol to several days. The patients included in this study were aged between 1 to 84 years with a median of 56 years and an average of 53 years. Both males and females were considered for this study. The number of male patients was 152 (66.1%) and the female patients was 78 (33.9%).
Demographical data of the 230 patients who developed an allergic reaction to iodixanol after undergoing angiography in the period between January 2017 to October 2020 and who were included in this study are shown in Table 1. As shown, the majority of the patients were males (66.1%) in the 45–59 (42.2%) years age group.
The clinical characteristics of the patients who suffered from an allergic reaction to the drug are shown in Table 2. Most (73.5%) of the patients included in this study received less than 200 mg of Iodixanol. In relation to the drugs that the patients were using, antiplatelets were the most common in over half of the patients (58.3%), while the least (10%) were diuretics. The incidence of allergic reactions was found to be highest during the winter season where it was reported by 28.3% of the patients, and this was lowest during spring (20%).
The relationship between the demographic data of the patients who suffered from an allergic reaction and their clinical characteristics is shown in Table 3. A statistically significant relationship existed between the iodixanol dose used and the age and gender of the patients and also in patients using beta-blockers, antiplatelets, statins, and ACEi (refer to Table 3 for details). In relation to the nature of the allergic reaction (clinical manifestation), a statistical significance (p-value 0.029) was seen with urticaria occurring most commonly among the age group 30–44 (33.3%). The relationship between the drugs used for management of suffered allergic reactions and the age groups was found to be statistically significant in both drugs classes used. In regard to the severity of the allergic reactions experienced by the patients, the severe cases were added to the moderate due to their limited number. The statistical significance (p-value 0.002) was only seen in the relationship between the severity and the gender, with males being more likely to develop “moderate to severe” allergic reactions compared to females.
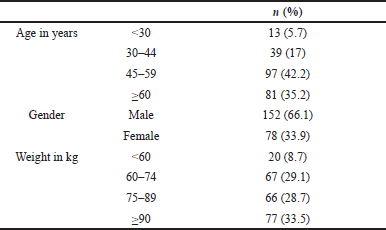 | Table 1. Demographical characteristics. [Click here to view] |
The relationship between the allergic reaction clinical manifestation, iodixanol dose, and the season during which the reaction occurred is shown in Table 4. Spring season in Bahrain starts in March, Summer season starts in June, Autumn season starts in September, while Winter season starts in December (Seasons of the Year, 2020). The statistical significance was only seen in the relationship between the clinical manifestation of rash, previously was noted as the most commonly reported symptoms, and the season. The highest incidence of rashes was found to be during the winter season.
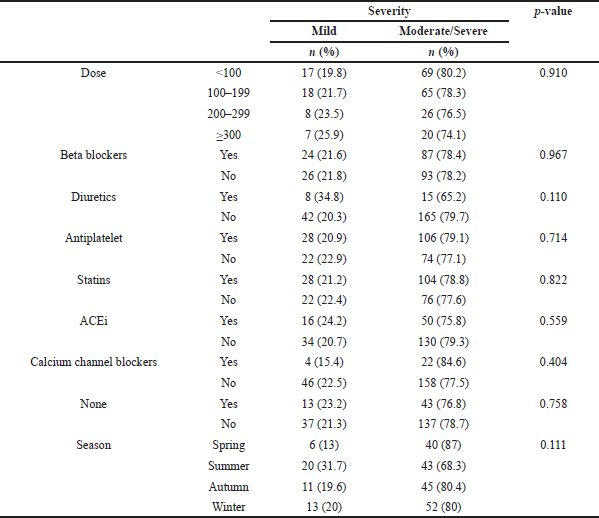
The relationship between the severity of the allergic reaction experienced and the iodixanol dose used, patients’ medication, and the season during which the reaction
occurred is shown in (Table 5), though no statistical significance was noted.
DISCUSSION
The approximate incidence of the anaphylactic reactions following the administration of iodinated CM was reported to be one in 75,000 population (Saljoughian, 2012). Although the specific mechanism of allergy of the iodinated CM is not clear, the iodine was regarded as the culprit (Carr and Walker, 1984). The Incidence of moderate CM allergic reactions (defined as a reaction that increases hospitalization by at least 1 day and requires modification in the drug regimen and/or administration of specific antidotes) was found to be approximately 1% (Saljoughian, 2012). Mild allergic reactions were described as late skin rash (Gharekhanloo and Torabian, 2012), transient (Muschick et al., 1995), or low-frequency discomfort (Jorgensen et al., 1992). In the current study, the overall reactions were found to be 2.3%, where most of the allergic reactions were reported to be moderate, while severe reactions were the least. This variance in the severity of the allergic reactions developed could be attributed to the differences between human body’s immune responses within the participants as well as the different genetic makeup of this study population when compared with similar studies done in other countries like Europe. Anaphylactic reactions were not reported by any patient within the study period and no life-saving treatment such as oxygen or atropine was needed. This is in concordance with the available literature which stated that CM-related severe immediate reactions are extremely rare when compared to mild reactions (Katayama et al.,1990; Kopp et al., 2008). Furthermore, a previous study stated that the mortality due to adverse reactions to CM is less than 1 in 100,000 (Andreucci et al., 2014), which also supports the finding of lower incidence rates of severe drug allergic reactions.
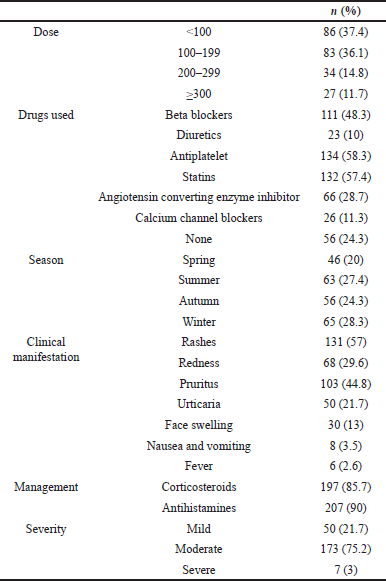 | Table 2. Clinical characteristics. [Click here to view] |
The overall incidence of allergic reactions in males was higher than females might be attributed to the higher incidence of cardiovascular disease in males (Center of disease control and prevention, 2017). The impact of gender on the incidence of allergic reactions is debatable in the literature. Some previous studies are reporting it to be higher in females (Lang et al., 1995) and other studies are reporting the opposite (National Institute for Health and Care Excellence, 2018). However, in the current study, this incidence was higher in female patients who received iodixanol at doses less than 100 mg which highlights the need of addressing allergic reactions with the help of practical advice to avoid it and further investigation.
No significant difference was seen in the relationship between the dose of iodixanol used and the severity or type of reported allergic reaction in this current study and this is in accordance with previously published studies which showed that most of the adverse effects of the CM are non-dose dependent (Maddox, 2002).
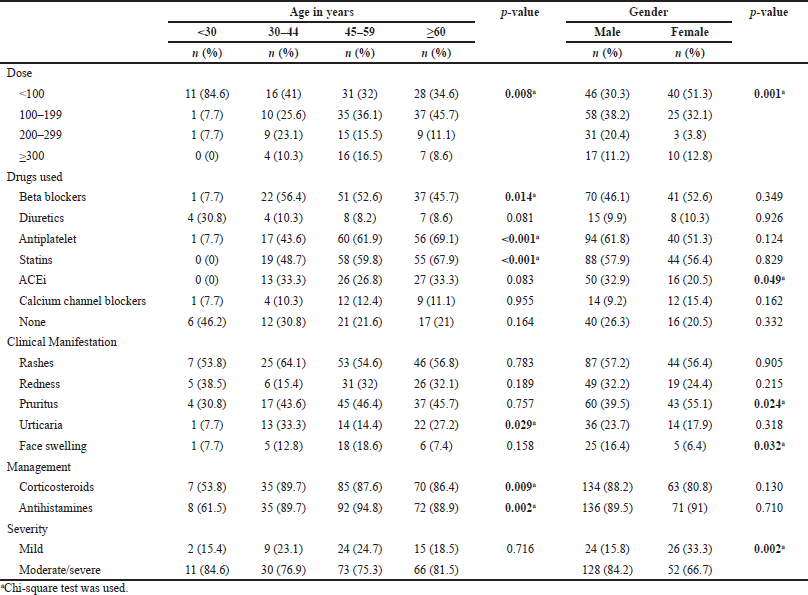 | Table 3. Relation between demographical characteristics and clinical characteristics. [Click here to view] |
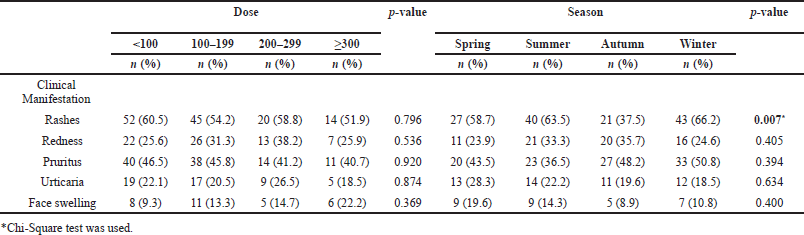 | Table 4. Relationship between diagnosis and each of dose and season. [Click here to view] |
 | Table 5. Relationship between severity and each of dose, drugs used, and season. [Click here to view] |
The findings of this research indicated that the allergic reactions to iodixanol were found to be significantly higher in patients who are taking anti-platelets medications, statins lipid lowering agents, and beta-blockers, in that order. This finding might be related to a possible drug-drug interaction between iodixanol with these medications. As a result, practitioners should be cautious and take into consideration the concomitant medications of the patients before the administration of iodixanol. Additionally, a few days pre-procedure discontinuation of antiplatelets medications could be considered. In agreement with the impact of concomitantly used drugs, previous studies were found to support the higher incidence rates of allergic reactions in elderly patients using beta-blockers (American College of Radiology, 2020), but none in regard to the use of antiplatelets or statins use. The association of a known history of a previous CM drug allergy with CM allergy is well known among published studies (Dahlstrom et al., 1985; Dawson, 2006; Torres et al., 2008; Vernassiere et al., 2004). This further emphasizes the need for a detailed history that includes all previous allergic reactions a patient experienced to known agents or drugs. When there is doubt, skin testing with CM has a high specificity, but its role in diagnosis is limited by a low sensitivity in mild to moderate reactions to CM (Saljoughian, 2012). The initiation of institutional policy for referring such patients to a dermatologist or specialized allergy care, to identify the real cause of allergic reactions can provide the basis for classifying the type of allergic or hyper-reactivity reactions.
The relationship between the development of an allergic reaction to iodixanol CM and the season was found to be statistically significant in this study population. The highest incidences were found to be during the winter months. Possible reasoning for this could be attributed to the people’s low intake of water and fluids during this season that might cause dehydration. This is particularly significant because iodixanol is excreted mainly renally, hence, resulting in delayed excretion of the agent and possibly a higher incidence of allergic reactions. Patients, especially those with previous allergic reactions, must be advised to rehydrate with drinkable fluids 2–3 hours before angiographic procedures (ACR Manual on Contrast Media, 2020; Andreucci et al., 2014). Intravenous hydration of the patient during and after the procedure could help in this aspect.
It is worth mentioning that most of the patients in the current study received antihistamines and corticosteroids for the management of allergic reaction. However, the use of antihistamines was slightly more than the corticosteroids which could be due to the higher safety profile of antihistamines in comparison to the corticosteroids when it comes to cardiovascular diseases (Sholter and Armstrong, 2000).
Additionally, a connection between iodine allergy and seafood allergy was proved in multiple published studies (Asero, 2001; Lalli, 1974). This marks an important point that must be addressed while taking the history of the patient prior to administering iodixanol. Other factors that have an impact on CM allergy were described in the literature. History of previous allergic reactions was found to increase the incidence of allergy to CM (Morcos, 2008). Other comorbidities might also have an impact on the CM allergy such as the history of thyroid gland disorders, asthma, cardiovascular diseases, and diabetes mellitus (National Institute for Health and Care Excellence, 2018).
Factors affecting the risk of developing an allergic reaction could be explored and identified by conducting bigger research studies, eventually helping in developing a clinical scoring system that physicians can use, after taking a detailed history of the patient, to predict the risk of developing an allergic reaction to iodixanol prior to its administration. Predicting the risk will help in decreasing the incidence of allergic reaction by possibly altering the administered dose or decreasing the severity by taking the necessary preventive measures. Although the effectiveness of premedication for the prevention of CM-induced allergic reactions is still under discussion, a clinical trial published in 1982 concluded that prophylactic use of steroids and antihistamines can reduce radiographic CM-induced allergic reactions (Small et al., 1982). The small sample size of this research study and the non-routine administration of prophylactic antihistamine makes drawing a conclusion on the preventive effect of premedication with steroids and antihistamines from this set of data not ideal and conducting larger structured studies is recommended.
There were limitations to this study. This is a retrospective study where data was collected from previously documented reports, which can result in bias from natural variation among different physicians’ documentation and assessment methods. An example: what one physician might describe as a moderate reaction could be labeled mild by another. Additionally, information about subsequent adverse events or previous contrast-related adverse events at other institutions was limited as hospitals do not share a united database system. It is advisable to initiate an electronic system that gives an alert whenever the patient is admitted with a previous allergic reaction, or any medication or food allergy in order to avoid such types of scenarios and the occurrence of a very serious allergic reaction.
Post-marketing studies reported a significant difference between intraarterial and intravenous administration of CM, with higher rates of reactions associated with the intraarterial application (Bettmann et al., 1997; Kopp et al., 2008; Mita et al., 1998). It is difficult to comment on that issue in this study because the majority of CM in Mohammed Bin Khalifa Cardiac Center were administered by the intravenous route. If there is a clinical need to investigate this area further, structured research would be applicable.
Multiple recommendations have been discussed. Despite the results found, future large-scale and long-term registries involving continuous data collection with standardized protocols may still be required to unravel all aspects contributing to the development of allergic reactions.
In conclusion, this study showed that male patients are more prone to get allergic reactions than female patients, which could be due to women having a lower risk of cardiovascular disease, and hence the lower need for angiography procedures. Moreover, among the allergic severity range, the moderate severity was found to be the most common and required brief hospitalization and management with corticosteroids and/or antihistamines. Additionally, it has been found that the incidence increased during the winter season which can be attributed to so many factors with the most emphasized factor is the issue of low fluid intake and higher rates of dehydration. Finally, rashes were found to be the most frequent diagnosed allergic reaction within the examined number of patients. The current study could confirm the high safety profile of iodixanol CM in regard to the allergic reactions patients who underwent angiography in Mohammed bin Khalifa Cardiac Centre, Bahrain. However, the findings further emphasized the necessity for standardized assessment strategies, reporting protocols, and the establishment of a nationwide integrated registry for iodinated CM-related allergic reaction as the basis for establishing a strategy to prevent recurrent allergic reaction.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to this project design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
CONFLICT OF INTERESTS
Authors declare that they do not have any conflicts of interest related to this work.
FUNDING
No financial support was received from any source.
REFERENCES
ACR Manual on Contrast Media. ACR committee on drugs and contrast media. 2020. Available via https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf (Accessed 17 December 2020).
Andreucci M, Solomon R, Tasanarong A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. BioMed Res Int, 2014; 131(2):19–39. CrossRef
Asero R. Multiple drug allergy syndrome: a distinct clinical entity. Curr Allergy, 2001; 1(1):18–22. CrossRef
Berberich J, Hirsch S. Die röntgenographische darstellung der arterien und venen am lebenden menschen. Klinische Wochenschrift, 1923; 2(49):2226–8. CrossRef
Bettmann M, Heeren T, Greenfield A, Goudey C. Adverse events with radiographic contrast agents: results of the SCVIR Contrast Agent Registry. Radiology, 1997; 20(3):611–20. CrossRef
Brooks B. Intra-arterial injection of sodium iodide: preliminary report. J Am Med Assoc, 1924; 82(13):1016–9. CrossRef
Carr DH, Walker AC. Contrast media reactions: experimental evidence against the allergy theory. Br J Radiol, 1984; 57(678):469–73.? CrossRef
Center of Disease Control and Prevention. Men an heart disease. 2017. Available via https://www.cdc.gov/heartdisease/men.htm (Accessed 15 December 2020).
Dahlstrom K, Shaw DD, Clauss W, Andrew E, Sveen K. Summary of U.S. and European intravascular experience with iohexol based on the clinical trial program. Invest Radiol, 1985; 20(1):117–21. CrossRef
Dawson P. Adverse reactions to intravascular contrast agents. BMJ, 2006; 33(3):663–4. CrossRef
Gharekhanloo F, Torabian S. Comparison of allergic adverse effects and contrast enhancement between iodixanol and iopromide. Iran J Radiol, 2012; 9(2):63–6. CrossRef
Haschek LO. A contribution to the practical use of photography according to Roentgen. Wien Chir Wochenschr, 1896; 9(63):63–4.
Jorgensen NP, Nossen JO, Borch KW, Kristiansen AB, Kristoffersen DT, Lundby B, Theo L. Safety and tolerability of iodixanol in healthy volunteers concerning two monometric X-ray contrast media. Euro J Radiol, 1992;15(3):252–7. CrossRef
Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese committee on the safety of contrast media. Radiology, 1990; 175(3):621–8.? CrossRef
Kopp A, Mortele K, Cho Y, Palkowitsch P, Bettmann M, Claussen C. Prevalence of acute reactions to iopromide: postmarketing surveillance study of 74,717 patients. Acta Radiol, 2008; 49(1):902–11. CrossRef
Lalli AF. Urographic contrast media reactions and anxiety. Radiology, 1974; 112(1):267–71. CrossRef
Lang DM, Alpern MB, Visintainer PF, Smith ST. Gender risk for anaphylactoid reaction to radiographic contrast media. J Allergy Clin Immunol, 1995; 95(4):813–7. CrossRef
Leung AM, Braverman LE. Iodine-induced thyroid dysfunction. Curr Opin Endocrinol Diabetes Obes, 2012; 19(5):414–9. CrossRef
Maddox T. Adverse reactions to contrast material: recognition, prevention and treatment. Am Fam Physician, 2002; 66(7):1229–35.
McCullough PA. Renal safety of iodixanol. Exp Rev Cardiovasc Ther, 2006; 4(5):655–61. CrossRef
Mita H, Tadokoro K, Akiyama K. Detection of IgE antibody to a radiocontrast medium. Allergy, 1998; 5(3):1133–40. CrossRef
Morcos S. Adverse reactions to iodinated contrast media. Cancer Imaging, 2008; 2:97–106. CrossRef
Muschick P, Wehrmann D, Schuhmann-Giampieri G, Krause W. Cardiac and hemodynamic tolerability of iodinated contrast media in the anesthetized rat. Invest Radiol, 1995; 45(4):745–53. CrossRef
Nadolski GJ, Stavropoulos SW. Contrast alternatives for iodinated contrast allergy and renal dysfunction: options and limitations. J Vascu Surg, 2013; 57(2):593–8. CrossRef
National Institute for Health and Care Excellence. Point-of-care creatinine tests to assess kidney function before administering intravenous contrast for computed tomography (CT) imaging final scope. 2018. Available via https://www.nice.org.uk/guidance/dg37/documents/final-scope (Accessed 20 December 2020).
Osborne ED, Sutherland CG, Scholl AJ, Rowntree LG. Roentgenography of urinary tract during excretion of sodium iodide. J Am Med Assoc, 1923; 80(7):920–2 CrossRef
Saljoughian M. Intravenous radiocontrast media: a review of allergic reactions. US Pharm, 2012; 37(5):14–6.
Seasons of the Year. Season in Bahrain. 2020. Available via https://seasonsyear.com/Bahrain (Accessed 25 December 2020).
Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol, 2000; 16(4):505–11.
Small P, Satin R, Palayew MJ, Hyams B. Prophylactic antihistamines in the management of radiographic contrast reactions. Clin Exp Allergy, 1982; 12(3):289–94. CrossRef
Solomon R, Werner C, Mann D, D’Elia J, Silva P. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med, 1994; 331(21):1416–20. CrossRef
Sutton AG, Finn P, Grech ED, Hall JA, Stewart MJ, Davies A, De Belder MA. Early and late reactions after the use of iopamidol 340, ioxaglate 320, and iodixanol 320 in cardiac catheterization. Am Heart J, 2001; 141(4):677–83. CrossRef
Torres MJ, Mayorga C, Cornejo-Garcia JA, Lopez S, Chaves P, Rondon C, Fernandez T, Blanca M. Monitoring non-immediate allergic reactions to iodine contrast media. Clin Exp Allergy, 2008; 15(2):233–8. CrossRef
Vernassiere C, Trechot P, Commun N, Schmutz JL, Barbaud A. Low negative predictive value of skin tests in investigating delayed reactions to radio-contrast media. Contact Derm, 2004; 50(1):359–66. CrossRef
Weininger M, Barraza JM, Kemper CA, Kalafut JF, Costello P, Schoepf UJ Cardiothoracic CT angiography: current contrast medium delivery strategies. Am J Roentgenol, 2011; 196(3):260–72. CrossRef
Yoshikawa H. Late adverse reactions to nonionic contrast media. Radiology, 1992; 183(3):737–40. CrossRef