INTRODUCTION
The family Moraceae, often called the mulberry family or the fig family, grows in a wide range of climatic conditions. Thus, it is widely distributed in various types of regions (Tomczyk et al., 2019). The genera that have been widely studied for their health benefits are Morus, Ficus, and Artocarpus (Afzan et al., 2019). These plants are widely utilized traditionally in cosmetics, agriculture, food, and additives in the pharmaceutical industry (Ghavami et al., 2020). These benefits are due to the secondary metabolites contained in them (Afzan et al., 2019).
Recently, a group of compounds that have increased attention because of their bioactive properties, is flavonoids (Li et al., 2020a). Flavonoids are widely present in every part of the Moraceae plants (Zhu et al., 2019). Flavonoids in Morus, Ficus, and Artocarpus have shown antidiabetic (Junior et al., 2017), anti-inflammatory (Ribeiro et al., 2019), anticancer (Boonyaketgoson et al., 2020), antiplasmodial (Boonyaketgoson et al., 2020), immunomodulator (Septama et al., 2018), and antispasmodic (Zoofishan et al., 2019) activity. Some studies also proved their ability to improve several diseases including neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease (Paudel et al., 2019; Suttisansanee et al., 2020) and osteoporosis (Yuan et al., 2017) and to help lower blood pressure (Alamgeer et al., 2017).
The hydroxyl group in flavonoids plays a role in providing antioxidant properties that can fight oxidative stress (Zhao et al., 2018). However, the flavonoids in plants vary and are influenced by environmental conditions such as climate, solar radiation, temperature, and precipitation rate (Dalmagro et al., 2018; Krishna et al., 2018). The total flavonoid is an important parameter in determining the quality of a plant (Afzan et al., 2019).
Therefore, the objectives of this review were to provide the flavonoids of Morus, Ficus, and Artocarpus (family Moraceae) and their antioxidant activity and to study the influence of the climate on flavonoid biosynthesis. To the best of authors’ knowledge, this review is the first one that compiles all the pieces of information mentioned.
METHODS
This review included studies published in the PubMed database obtained using the keywords (“Morus” OR “Ficus” OR “Artocarpus”) AND “flavonoids” NOT “opuntia” with “full-text” and “10 year” publication date filtered from February 2021 to June 2021. The inclusion criteria were articles about Morus, Ficus, and Artocarpus genera which contain flavonoids and biosynthesis mechanism, contain the list of flavonoids present in the plant, contain the antioxidant activity of the plant, and contain the environmental influence on the flavonoid biosynthesis. The articles obtained from the initial search were 548 studies. Articles published before 2011, reviews, non-English studies, and unrelated studies such as studies on processed foods and studies that do not contain information about flavonoid content were excluded. The information obtained from articles was then supplemented with information about climate obtained through the network sites https://climatecharts.net/ (Zepner et al., 2020) and https://gml.noaa.gov/grad/solcalc/ (Global Monitoring Laboratory). The flowchart of literature searching is shown in Figure 1.
BIOSYNTHESIS PATHWAY OF FLAVONOIDS
Flavonoids are a substantial secondary metabolites group present in plants which can be classified according to their basic skeleton into certain groups such as flavonols, flavones, flavanols, isoflavones, flavanones, anthocyanins, and proanthocyanidin(Li et al., 2020a). Other sources stated that flavanolol, aurone, furan chromone, isoflavanone, biflavones, xanthones, chalcones, and dihydrochalcone are also included in the flavonoid classification (Wang et al., 2018). Generally, a schematic presentation of the biosynthesis pathway of flavonoids is shown in Figure 2.
 | Figure 1. Flow chart of literature searching. [Click here to view] |
Biosynthesis of flavonoids begins with phenylalanine which is catalyzed by phenylalanine ammonium lyase (PAL) to form cinnamic acid. The cinnamic acid is further oxidized and then catalyzed with the help of cinnamic acid 4-hydroxylase (C4H) and 4-coumaroyl CoA ligase (4CL) to form p-coumaric acid and 4-coumaroyl CoA. These stages are included in the phenylpropanoid pathway. Then, the resulting product will interact with three malonyl-CoA molecules from the shikimic pathway and produce naringenin. The stages from naringenin to various other types of flavonoids are the entry stages of the flavonoid biosynthesis pathway (Li et al., 2020a).
The formation of flavonols from dihydroflavonol is catalyzed by flavonol synthase (FLS), which converts dihydrokaempferol, dihydroquercetin, and dihydromyricetin into kaempferol, quercetin, and myricetin, respectively (Huang et al., 2020). In mulberry fruit, flavonoid biosynthesis is influenced by the level of maturity, where the ripe fruit has higher levels of flavonoids (Huang et al., 2020). The same thing also happened to fig fruit (Ficus carica) which showed that the anthocyanin levels in fruits that had changed color to red could contain 28 times more anthocyanins compared to fruits that were still yellow(Li et al., 2020c)
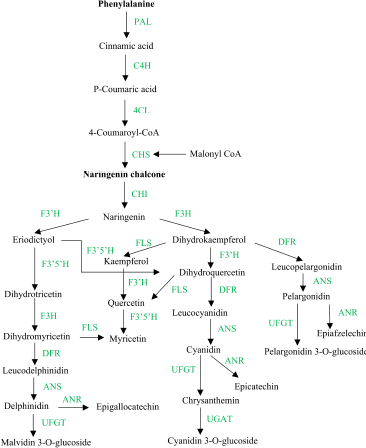 | Figure 2. A schematic presentation of the flavonoid biosynthesis pathway (adapted from Huang et al., 2020 ). PAL: phenylalanine ammonia-lyase, C4H: cinnamic acid 4-hydroxylase, 4CL: 4-coumarate-CoA ligase, CHS: chalcone synthase, CHI: chalcone isomerase, F3H: flavonoid 3-hydroxylase, F3’H: flavonoid 3’-hydroxylase, F3’5’H: flavonoid 3’5’-hydroxylase, FLS: flavonol synthase, DFR: dihydroflavonol 4-reductase, ANR: anthocyanin reductase, ANS: anthocyanidin synthase, UFGT: flavonoid 3-O-glucosyl transferase, UGAT: cyanidin-3-O-glucoside 2-O-glucuronosyltransferase. [Click here to view] |
Compounds contained in each part of the plant are different. This happens because there are differences in proteins expressed in each plant organ. These proteins or enzymes affect the synthesis process in the flavonoid biosynthetic pathway. Organ-specific metabolic analysis in M. alba showed that more flavonoids were accumulated in roots than leaves and twigs. Notably, the two root-specific proteins named flavonoid 3,5-hydroxylase and chalcone flavanone isomerase were accumulated in the flavonoid pathway (Zhu et al., 2019). The difference in the concentration of flavonoids in Morus atropurpurea showed the highest flavonoid content in root bark, followed by stem bark, twigs, and old leaves (Wang et al., 2017)
FLAVONOID COMPOUNDS IN MORUS, FICUS, AND ARTOCARPUS
Moraceae plants, especially genus Morus, can be widely cultivated in tropical, subtropical, and temperate climates in Asia, Europe, and South and West America (Paudel et al., 2019). In China, Morus alba and Morus nigra have been used as traditional medicines since ancient times (Hao et al., 2018; Zhao et al., 2018)Guangxi and Chongqing are emerging sericulture areas in China where the production of mulberry leaves is huge. In order to identity high quality mulberry leaves that are suitable for healthy products to expand planting, 24 samples from three regions (Guangdong, Guangxi, Chongqing. They are also important in the economic sector, especially in sericulture (Zou et al., 2012). Morus plants are known to be abundant with flavonoids. Hence, various studies of metabolic profiles to transcriptome analysis have been carried out on several Morus species, both to understand the biosynthesis of flavonoids in Morus and to determine the response of flavonoids as a defense against environmental conditions (Li et al., 2020a; Li et al., 2020b).
The biggest population of the family Moraceae is from Ficus (Farag et al., 2014). This genus consists of around 800 species and is widely spread from Asia to the Mediterranean region (Alamgeer et al., 2017; Farag et al., 2014). Ficus deltoidea is an indigenous plant in Indonesia, Thailand, and Malaysia and can be found easily in other Southeast Asian countries. The plant wildly grows near beaches, hilly forests, and peat soil (Afzan et al., 2019). It is complicated to find the distinction of the varieties based on the plant morphology, especially the leaves, because they tend to have a diverse leaf shape on both the same stem or a different stem of the same plant (Afzan et al., 2019; Shahinuzzaman et al., 2020). Therefore, the identification of secondary metabolite and chemical markers is needed to distinguish and choose the right plant to be used as a medicinal herb (Afzan et al., 2019). A previous study on F. deltoidea Jack leaves from Kalimantan, Indonesia, harvested more than 6 months after being planted, revealed the highest flavonoids and total phenolic content (TPC) compared to the younger leaves, unripe fruits, and stems. This plant was seeded in a conditioned soil with pH = 6.12, N = 0.688%, using NPK Mutiara (16:16:16) as a basic fertilizer (Manurung et al., 2017). Another study on F. carica collected in Lakhdaria, Algeria, also reported that the leaves of this plant contained a high flavonoid and TPC and antioxidant activity (Mahmoudi et al., 2016).
The genus Artocarpus consists of a tropical plant that is mainly cultivated in Asia, especially in South and Southeast Asia (Boonyaketgoson et al., 2020). This genus is a rich source of prenylated flavonoid (PF) and more than 300 PFs have been isolated (Ye et al., 2019).
From Table 1, we could see that the Morus species are mostly grown in a subtropical climate and humid climate. Despite their diverse growing place, every part of the Morus plants shows a similar type of flavonoid. The leaves mostly consist of flavonols derivatives, when anthocyanins are mostly found in the fruits, and the root and stem barks contain various flavone derivatives, 2-arylbenzofuran flavonoid, and PFs. Many types of kuwanon (flavone derivatives), mulberrofuran (2-arylbenzofuran flavonoid), and morusin (prenylated flavone) exist in the root and stem bark of any species in the genus Morus (Abdel Bar et al., 2019; Guo et al., 2019; Zheng et al., 2012). Surprisingly, morusin is also found in Artocarpus heterophyllus and Artocarpus xanthocarpus roots (Jin et al., 2015; Ye et al., 2019). This shows that morusin might be a typical compound of the family Moraceae.
Metabolic profiling of mulberry leaves shows a variety of flavonols and flavones (Li et al., 2020a). Kaempferol 3-O-glucoside (astragalin), quercetin 3-O-glucoside (isoquercitrin), and kaempferol/quercetin di-O-hexoside were found to be abundant in all Morus leaves samples (Li et al., 2020a). This is consistent with the data collected in Table 1, where astragalin and isoquercitrin were detected in all samples of M. alba leaves from various countries under different climatic conditions. This result is also in line with Kim et al. (2014) study where rutin, isoquercitrin, and astragalin were found to be the main flavonoid compounds in M. alba leaves with concentrations of 3.10, 5.68, and 2.41 mg/g, respectively (Kim et al., 2014) Based on the flavonoid compound information collected in this review, the flavonoid compounds of M. nigra leaves were identified by targeted screening so that only a few flavonoid compounds were detected. However, the flavonoids in M. nigra leaves showed the same characteristics as M. alba which was dominated by flavonols. Unlike flavonols which are abundant in O-glycosyl modification, flavones such as luteolin, apigenin, and chrysoeriol and their derivatives were also detected in Morus leaves with O-hexosylated and O-pentosylated modifications (Li et al., 2020a).
Luteolin and apigenin and their glycosides were identified in almost all Ficus plants. Luteolin was detected in two species of Ficus from Egypt and one species each from Cameroon, China, and the Ivory Coast. Besides that, based on Table 1, other flavones also can be found in most Ficus species.
Various PFs were identified in the roots of A. heterophyllus. PFs are chromone class derivatives that are structurally different and characterized by multiple prenyl units linked to the flavone core by C–C and/or C–O. Various prenyl substitution patterns in the flavone skeleton give PF a high structural diversity (Ye et al., 2019). Many PFs were also identified in Artocarpus nigrifolius twigs such as artocarmin which can also be found in other Artocarpus twigs and root barks and gemichalcone which also can be found in some Morus laevigata twigs and A. heterophyllus twigs (Di et al., 2013; Liu et al., 2018; Wang et al., 2015).
TOTAL FLAVONOID CONTENT (TFC) AND TPC
Various studies have estimated the flavonoid composition in some Moraceae plants. TFC and TPC have been determined from each part of Moraceae plants and summarized in Table 2. Most of the data originate from South Korea (Ju et al., 2018; Kim et al., 2014; 2020; Yu et al., 2021), Bangladesh (Khan et al., 2013; Sumi et al., 2016), China (Chen et al., 2020; Krishna et al., 2018), Brazil (Souza et al., 2018; Zeni et al., 2017), Malaysia (Abrahim et al., 2018; Jalal et al., 2015), Portugal (Palmeira et al., 2019), Pakistan (Alamgeer et al., 2017), Italy (Loizzo et al., 2014), India (Gupta et al., 2020), Serbia (Natic et al., 2015), Poland (Tomczyk et al., 2019), and Thailand (Suttisansanee et al., 2020).
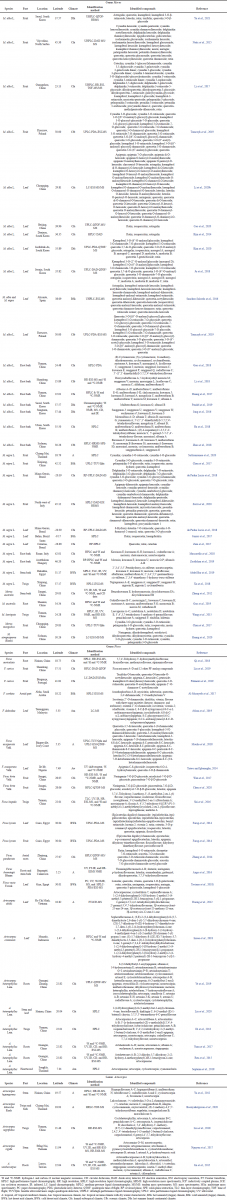 | Table 1. Flavonoid compounds in Morus, Ficus, and Artocarpus. [Click here to view] |
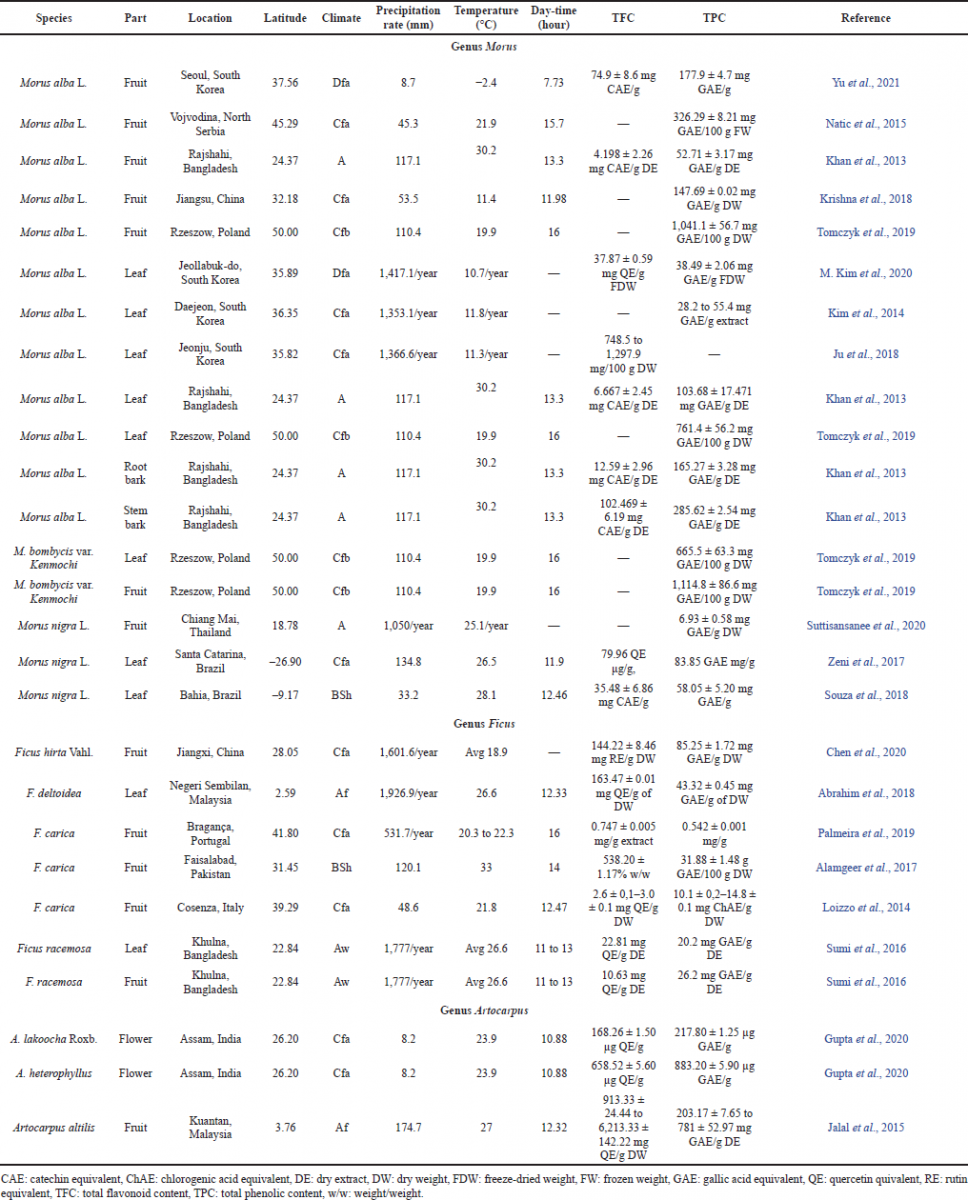 | Table 2. Total flavonoid and phenolic content in several Moraceae plants. [Click here to view] |
Studies have shown that the polyphenol content of mulberry leaves is influenced by the variety and the growing location (Krishna et al., 2018). Morus leaf TPC values ranged from 665.5 ± 63.3 mg gallic acid equivalent (GAE)/100 g dried matter (DW) or almost equivalent to 6.65 mg GAE/g DW to 103.68 ± 17.471 mg GAE/g DW. The lowest TPC is from the Morus bombycis species from Poland and the highest one is from M. alba from Bangladesh. The highest TFC of Morus leaves was achieved by M. nigra from Bahia, Brazil, with a 35.48 ± 6.86 mg catechin equivalent (CAE)/g extract. Both Brazil and Bangladesh are considered as low-latitude countries that are located between the equator (0°) and 30°N/S (Khan et al., 2013; Zeni et al., 2017). The low-latitude area receives more sunlight than the higher-latitude area which can be the reason for these TFC and TPC values.
The highest TFC value in the genus Morus was achieved by the stem bark of M. alba from Bangladesh with a value of 102.469 ± 6.19 mg CAE/g DE (Khan et al., 2013). Bangladesh is a tropical country with the highest temperature which might be related to the light intensity in that place. Solar ultraviolet-B (UVB) radiation can induce oxidative stress in the plant cells because of the overproduced reactive oxygen species (ROS) (Guan et al., 2018). Thus, the formation of flavonoids and phenolic compounds is induced to neutralize these free radicals (Li et al., 2020b; Mouho et al., 2018).
It is found that Morus fruits contain higher TFC and TPC in regions with lower temperature conditions. Compared to the M. alba fruit from Bangladesh which was collected when the average temperature was 30.2°C (Khan et al., 2013), the fruit from South Korea when the temperature was −2.4°C has almost 18 times higher TFC (Yu et al., 2021). The expression of the PAL enzyme could be induced in the lower temperature condition, which led to the enhancement of the flavonoid content (Hao et al., 2018).
Ficus hirta fruits, from Jiangxi, China, show the highest TFC among fruits and leaves in the same genus. Among F. carica fruits from Pakistan, Italy, and Portugal, figs from Pakistan had the highest flavonoid and phenolic contents with values of 538.20% ± 1.17% w/w or 5.832 g quercetin equivalent/g dry matter and 31.88 ± 1.48 g GAE/100 g dry matter, respectively (Alamgeer et al., 2017). The hot semiarid (steppe) climate of Pakistan is more favorable for fig cultivation than the wet and warm temperate (Cf) climate (Datiles, 2015). Pakistan’s higher average temperature at the time of fruit collection than the temperatures of the two countries may also contribute to the higher TFC values as F. carica fruits require higher heat and temperature to reach ripeness and good quality (Isa et al., 2020).
The highest levels of anthocyanins are at the fruit’s perfect maturity level, so if they were not ripe, the content would likely be less than in the fruit that was harvested at that time (Gupta et al., 2020). The Artocarpus altilis fruit shows a very high total flavonoid and phenolic content compared to the other species in Moraceae. The TFC of A. altilis varied from 913.33 ± 24.44 to 6,213.33 ± 142.22 mg quercetin equivalent (QE)/g DW. This species is known as breadfruit and grows best in a hot and humid climate. The fruits of A. altilis are commonly used as food, medicine, and also animal feed (Jalal et al., 2015).
As these plants are rich in flavonoids, they are relevant to their various activities such as antioxidants and anti-inflammatory properties. However, it cannot be avoided that the composition of flavonoids in plants in various studies is not constant because of several factors such as origin, fertilization, harvesting season, plant age, the process of drying, and storage conditions. In addition, the identification of these compounds is also influenced by the method of analysis (Ribeiro et al., 2019).
ANTIOXIDANT ACTIVITY OF MORACEAE PLANTS
Oxidative stress generally causes an increase in intracellular ROS levels which can cause fatal effects to oxygen toxicity and cellular function (Kim et al., 2020). Under normal circumstances, ROS participate against pathogens, which is considered the most efficient microbicidal mechanism. In addition to its defense purpose during infection, excessive ROS production can increase the inflammatory process (Septama et al., 2018).
The mechanism of action of antioxidants is based on the test method. Therefore, the antioxidant activity assay is carried out by various methods. The antioxidant activity of plants is notably affected by the concentration of phenolic compounds contained in them. Generally, flowers or fruits that have a darker color produce a stronger antioxidant potential (Gupta et al., 2020).
Among the various methods to determine antioxidant activity, the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay has been a preferred and widely used method to evaluate the free radical scavenging ability of various natural products (Krishna et al., 2018). This method is more rapid, simple, and inexpensive compared to other antioxidant activity assays, while the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) or Trolox equivalent antioxidant capacity assay is suitable for lipophilic and hydrophilic samples (Shahinuzzaman et al., 2020). A strong positive correlation between TPC and DPPH radical scavenging ability was shown in the study of Tomczyk et al. (2019) with a correlation coefficient above 0.9 (Tomczyk et al., 2019).
Flavonoids also show a capability to act against hydroxyl and superoxide radicals, the two most powerful radicals generated during metabolism, through hydroxyl radical scavenging activity (HRSA) and superoxide radical anion scavenging activity (SAS) assay (Zeni et al., 2017). A good correlation of TPC with SAS assay was demonstrated at over 0.933 (Natic et al., 2015). Both the ferric-reducing ability power (FRAP) and reducing power (RP) methods measure the reduction of Fe3+ to Fe2+ in the presence of antioxidants (Loizzo et al., 2014). Another antioxidant activity against ROS products such as malondialdehyde (MDA) can be measured by the lipid peroxidation assay. MDA is formed by the reaction of ROS with the side chain of phospholipids containing polyunsaturated fatty acid on the cell membrane (Li et al., 2020b).
Various antioxidant activity tests that have been carried out in the Moraceae plants showed strong antioxidant activities, and the values are provided in Table 3. The DPPH test, which was expressed as IC50, showed very strong to moderate activity in Morus, Ficus, and Artocarpus. The lowest IC50 of Morus was achieved by the stem bark of M. alba from Rajshahi, Bangladesh, with 36.5 μg/ml (Khan et al., 2013). This is in line with the total flavonoid and phenolic contents contained in the plant which are higher compared to other plants. Other methods also showed a good value of antioxidant ability. This shows that Moraceae plants are promising antioxidant agents with various mechanisms.
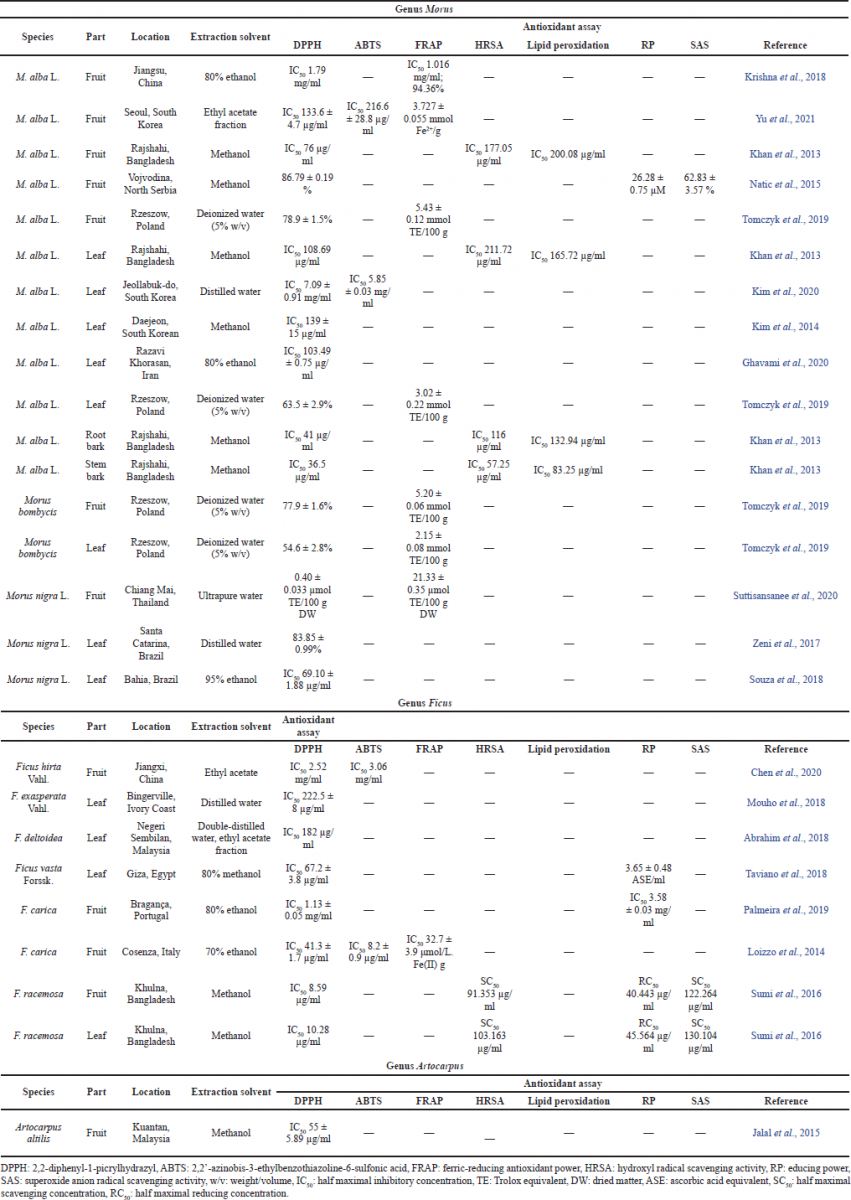 | Table 3. Antioxidant activity of Morus, Ficus, and Artocarpus plants using various methods. [Click here to view] |
PFs and Diels–Alder-type adduct flavonoids show remarkable ability to scavenge free radicals. This is also related to the abundance of free hydroxyl groups in these phenolic compounds which may contribute to the activity (Zhao et al., 2018). Rutin and quercetin which are present in the M. nigra leaves ethanolic extract have been reported to play a big role in the anti-inflammatory effect, feasibly by modulating bradykinin and serotonin pathways (Ribeiro et al., 2019). The anti-inflammatory effect was also shown by prenylated isoflavones which showed an inhibitory effect on nitric oxide (NO) production (Liu et al., 2019).
CLIMATE INFLUENCES ON FLAVONOIDS
In general, functional components in plants are influenced by differences in varieties and cultivation environments, including sunlight, amount of fertilizer, and temperature (Sugiyama et al., 2016). In observing the flavonoid content in plants, this is also influenced by season, temperature, and accumulation of rainfall. Observations made on M. nigra growing in Brazil by measuring quercetin levels regularly every season throughout the year showed that quercetin and flavonoids are routinely affected by climate (Dalmagro et al., 2018).
The continued depletion of the ozone layer in the last few years has led to increased damage to crops through ultraviolet (UV) radiation from the sun (Li et al., 2020b). The effects of UVB stress induction and dark treatment have been carried out to understand the genes that contribute to metabolic mechanisms in a plant under abiotic stress conditions. Transcriptomics of M. alba leaves which were treated with UVB and dark incubation showed an increase in flavonoid biosynthesis due to upregulation of gene expression involved in flavonoid biosynthesis pathways (Guan et al., 2018).
The effect of light deprivation was also observed on anthocyanin synthesis in F. carica cultivar Zibo, China. Lack of light greatly affects pigment synthesis in fruit. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment showed significant changes in phenylpropanoid biosynthesis and flavonoid biosynthesis pathways in fruits where significant repression occurs in the transcripts of chalcone synthase, chalcone isomerase, flavonoid 3’-hydroxylases, dihydroflavonol 4-reductase, and flavonoid 3-O-glucosyl transferase (Wang et al., 2019).
One of the most abundant secondary metabolites in plants and the largest subclass of flavonoids is flavones. Flavonoids, including flavones, have various functions that are useful for plants to adapt to complex and constantly changing environments. Flavone plays a role in protecting plants from solar UV radiation, giving color to flowers, interactions between species, and plant self-defense. Several studies have shown higher flavone content in the leaves of plants grown at higher altitudes. This indicates a correlation between flavones and plant tolerance to UV stress (Li et al., 2020b).
Based on climate, the flavonoid content is more abundant in plants that grow around the equator. Nevertheless, other factors such as cultivation, soil conditions, and the processing of samples could not be ignored (Kim et al., 2014). Interestingly, a report on the effect of the season on the flavonoid content had confirmed that both young and mature leaves collected in the dry season gave higher flavonoid production compared to that of the rainy season (Luengas-Caicedo et al., 2007).
CONCLUSION
Various species of Morus, Ficus, and Artocarpus show many variations of the flavonoid content. Each plant part has a characteristic in the flavonoid content; for example, the fruit contains a lot of anthocyanins, especially cyanidin glycosides; the leaves are rich in flavonols and their glycosides such as quercetin and kaempferol, while the roots and stems contain lots of flavones and their glycosides such as apigenin and luteolin. Several PFs and Diels–Alder adduct flavonoids were also found in this family, especially in the genus Morus. The largest flavonoid content in Morus plants is in the stems and roots, while the leaves of the Ficus genus are rich in flavonoids and TPC. More interestingly, climatic conditions, particularly the altitude and UV radiation, as well as the dry and rainy seasons, play a significant role in the flavonoid biosynthesis pathways. Furthermore, as these plants are plentiful in flavonoids, they have been proven to exhibit a strong antioxidant activity through various mechanisms. This review provides more insight into the potential of Moraceae plants as herbs to help improve various disease conditions induced by free radicals. Further research on the use of Moraceae plant extract as a functional food as well as in vivo and clinical trials is needed to ascertain the beneficial effects of these plant extracts on human health.
ACKNOWLEDGMENTS
The authors thank the Rector of Universitas Padjadjaran for funding the publication via the Unpad Academic-Leadership Grant of Prof. Dr. Jutti Levita in Batch 2021, managed by the Directorate of Research and Community Engagement of Universitas Padjadjaran.
AUTHORS’ CONTRIBUTIONS
Jutti Levita (JL) was principally responsible for the conception and design of the study. Dina Hawari (DH) searched and collected the articles. DH, Mutakin (M), and Gofarana Wilar (GW) participated in the processing, selecting, and analyzing of the data. DH, GW, and M contributed to the writing of the manuscript. JL checked, finalized, and revised the manuscript. All authors read and approved the final manuscript to be published.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel Bar FM, Abbas GM, Gohar AA, Lahloub MFI. Antiproliferative activity of stilbene derivatives and other constituents from the stem bark of Morus nigra L. Nat Prod Res, 2020; 34(24):3506–13. CrossRef
Abrahim NN, Abdul-Rahman PS, Aminudin N. The antioxidant activities, cytotoxic properties, and identification of water-soluble compounds of Ficus deltoidea leaves. PeerJ, 2018; 6(10):e5694. CrossRef
Afzan A, Kasim N, Ismail NH, Azmi N, Ali AM, Mat N, Wolfender JL. Differentiation of Ficus deltoidea varieties and chemical marker determination by UHPLC-TOFMS metabolomics for establishing quality control criteria of this popular Malaysian medicinal herb. Metabolomics, 2019; 15(3):35. CrossRef
Alamgeer, Iman S, Asif H, Saleem M. Evaluation of antihypertensive potential of Ficus carica fruit. Pharm Biol, 2017; 55(1):1047–53. CrossRef
Al-Musayeib N, Ebada S, Gad H, Youssef F, Ashour M. Chemotaxonomic diversity of three Ficus species: their discrimination using chemometric analysis and their role in combating oxidative stress. Pharmacogn Mag, 2017; 13(51):613. CrossRef
Ango PY, Kapche DWFG, Fotso GW, Fozing CD, Yeboah EMO, Mapitse R, Dermitas I, Ngadjui BT, Yeboah SO. Thonningiiflavanonol A and thonningiiflavanonol B, two novel flavonoids, and other constituents of Ficus thonningii Blume (Moraceae). Z Naturforsch C J Biosci, 2016; 71(3–4):65–71. CrossRef
Boonyaketgoson S, Du Y, Valenciano Murillo AL, Cassera MB, Kingston DGI, Trisuwan K. Flavanones from the twigs and barks of Artocarpus lakoocha having antiplasmodial and anti-TB activities. Chem Pharm Bull, 2020; 68(7):671–4. CrossRef
Cao X, Yang L, Xue Q, Yao F, Sun J, Yang F, Liu Y. Antioxidant evaluation-guided chemical profiling and structure-activity analysis of leaf extracts from five trees in Broussonetia and Morus (Moraceae). Sci Rep, 2020; 10(1):4808. CrossRef
Chen C, Peng X, Chen J, Wan C. Antioxidant, antifungal activities of ethnobotanical Ficus hirta Vahl. and analysis of main constituents by HPLC-MS. Biomedicines, 2020; 8(1):15. CrossRef
Chen H, Yu W, Chen G, Meng S, Xiang Z, He N. Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries. Molecules, 2017; 23(1):4. CrossRef
Dalmagro AP, Camargo A, da Silva Filho HH, Valcanaia MM, de Jesus PC, Zeni ALB. Seasonal variation in the antioxidant phytocompounds production from the Morus nigra leaves. Ind Crops Prod, 2018; 123(2018):323–30. CrossRef
de Padua Lucio K, Rabelo ACS, Araujo CM, Brandao GC, de Souza GHB, da Silva RG, de Souza DMS, Talvani A, Bezerra FS, Cruz CAJ, Costa DC. Anti-inflammatory and antioxidant properties of black mulberry (Morus nigra L.) in a model of LPS-induced sepsis. Oxid Med Cell Longev, 2018; 2018:1–13. CrossRef
Di X, Wang S, Wang B, Liu Y, Yuan H, Lou H, Wang X. New phenolic compounds from the twigs of Artocarpus heterophyllus. Drug Discov Ther, 2013; 7(1):24–8. CrossRef
Farag MA, Abdelfattah MS, Badr SEA, Wessjohann LA. Profiling the chemical content of Ficus lyrata extracts via UPLC-PDA-qTOF-MS and chemometrics. Nat Prod Res, 2014; 28(19):1549–56. CrossRef
Ghavami G, Muhammadnejad S, Amanpour S, Sardari S. Bioactivity screening of mulberry leaf extracts and two related flavonoids in combination with cisplatin on human gastric adenocarcinoma cells. Iran J Pharm Res, 2020; 19(2):371–82.
Global Monitoring Laboratory. NOAA solar calculator [Online] Available via https://gml.noaa.gov/grad/solcalc/ (Accessed 24 May 2021).
Guan Q, Yu J, Zhu W, Yang B, Li Y, Zhang L, Tian J. RNA-Seq transcriptomic analysis of the Morus alba L. leaves exposed to high-level UVB with or without dark treatment. Gene, 2018; 645:60–8. CrossRef
Guo S, Liu L, Zhang S, Yang C, Yue W, Zhao H, Ho CT, Du J, Zhang H, Bai N. Chemical characterization of the main bioactive polyphenols from the roots of Morus australis (mulberry). Food Funct, 2019; 10(10):6915–26. CrossRef
Guo YQ, Tang GH, Lou LL, Li W, Zhang B, Liu B, Yin S. Prenylated flavonoids as potent phosphodiesterase-4 inhibitors from Morus alba: isolation, modification, and structure-activity relationship study. Eur J Med Chem, 2018; 144:758–66. CrossRef
Gupta AK, Rather MA, Kumar Jha A, Shashank A, Singhal S, Sharma M, Pathak U, Sharma D, Mastinu A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. flowers: new sources of bioactive compounds. Plants, 2020; 9(10):1329. CrossRef
Ha MT, Seong SH, Nguyen TD, Cho WK, Ah KJ, Ma JY, Woo MH, Choi JS, Min BS. Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and α-glucosidase. Phytochemistry, 2018; 155:114–25. CrossRef
Hao JY, Wan Y, Yao XH, Zhao WG, Hu RZ, Chen C, Li L, Zhang DY, Wu GH. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. Lightfoot DA, editor. PLoS One, 2018; 13(6):e0198072. CrossRef
Huang G, Zeng Y, Wei L, Yao Y, Dai J, Liu G, Gui Z. Comparative transcriptome analysis of mulberry reveals anthocyanin biosynthesis mechanisms in black (Morus atropurpurea Roxb.) and white (Morus alba L.) fruit genotypes. BMC Plant Biol, 2020; 20(1):279. CrossRef
Huang QH, Lei C, Wang PP, Li JY, Li J, Hou AJ. Isoprenylated phenolic compounds with PTP1B inhibition from Morus alba. Fitoterapia, 2017; 122:138–43. CrossRef
Huong TT, Cuong NX, Tram LH, Quang TT, Duong L Van, Nam NH, Dat NT, Huong PTT, Diep CN, Kiem PV, Minh CV. A new prenylated aurone from Artocarpus altilis. J Asian Nat Prod Res, 2012; 14(9):923–8. CrossRef
Inoue M, Hitora Y, Kato H, Losung F, Mangindaan REP, Tsukamoto S. New geranyl flavonoids from the leaves of Artocarpus communis. J Nat Med, 2018; 72(3):632–40. CrossRef
Isa MM, Jaafar MN, Kasim KF, Mutalib MFA. Cultivation of fig (Ficus carica L.) as an alternative high value crop in malaysia: a brief review. IOP Conf Ser Mater Sci Eng, 2020; 864(1):0–6. CrossRef
Jalal TK, Ahmed IA, Mikail M, Momand L, Draman S, Isa MLM, Abdull RMSB, Nor OM, Ibrahim M, Abdul WR. Evaluation of antioxidant, total phenol and flavonoid content and antimicrobial activities of Artocarpus altilis (Breadfruit) of underutilized tropical fruit extracts. Appl Biochem Biotechnol, 2015; 175(7):3231–43. CrossRef
Jin YJ, Lin CC, Lu TM, Li JH, Chen IS, Kuo YH, Ko HH. Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis. Phytochemistry, 2015; 117:424–35. CrossRef
Ju WT, Kwon OC, Kim HB, Sung GB, Kim HW, Kim YS. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J Food Sci Technol, 2018; 55(5):1789–96. CrossRef
Jung JW, Park JH, Lee YG, Seo KH, Oh EJ, Lee DY, Lim DW, Han D, Baek NI. Three new isoprenylated flavonoids from the root bark of Morus alba. Molecules, 2016; 21(9):1112. CrossRef
Junior II da S, Barbosa HDM, Carvalho DCR, Barros RDA, Albuquerque FP, da Silva DHA, Souza GR, Souza NA, Rolim LA, Silva FMM, Duarte GIBP, Almeida JRG da S, Oliveira JFM de GDA, Lira EC. Brazilian Morus nigra attenuated hyperglycemia, dyslipidemia, and prooxidant status in alloxan-induced diabetic rats. Sci World J, 2017; 2017:1–10. CrossRef
Khan MA, Rahman AA, Islam S, Khandokhar P, Parvin S, Islam MB, Hossain M, Rashid M, Sadik G, Nasrin S, Mollah MNH, Alam AHMK. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae). BMC Res Notes, 2013; 6:24. CrossRef
Kim DS, Kang YM, Jin WY, Sung YY, Choi G, Kim HK. Antioxidant activities and polyphenol content of Morus alba leaf extracts collected from varying regions. Biomed Rep, 2014; 2(5):675–80. CrossRef
Kim M, Nam DG, Ju WT, Choe JS, Choi AJ. Response surface methodology for optimization of process parameters and antioxidant properties of mulberry (Morus alba L.) leaves by extrusion. Molecules, 2020; 25(22):5231. CrossRef
Krishna PGA, Sivakumar TR, Jin C, Li SH, Weng YJ, Yin J, Jia JQ, Wang CY, Gui ZZ. Antioxidant and hemolysis protective effects of polyphenol-rich extract from mulberry fruits. Pharmacogn Mag, 2018; 14(53):103. CrossRef
Li D, Chen G, Ma B, Zhong C, He N. Metabolic profiling and transcriptome analysis of mulberry leaves provide insights into flavonoid biosynthesis. J Agric Food Chem, 2020a; 68(5):1494–504. CrossRef
Li F, Zhang B, Chen G, Fu X. The novel contributors of anti-diabetic potential in mulberry polyphenols revealed by UHPLC-HR-ESI-TOF-MS/MS. Food Res Int, 2017; 100:873–84. CrossRef
Li H, Li D, Yang Z, Zeng Q, Luo Y, He N. Flavones produced by mulberry flavone synthase type I constitute a defense line against the ultraviolet-B stress. Plants, 2020b; 9(2):215.
Li J, An Y, Wang L. Transcriptomic analysis of Ficus carica peels with a focus on the key genes for anthocyanin biosynthesis. Int J Mol Sci, 2020c; 21(4):1245.
Li M, Wu X, Wang X, Shen T, Ren D. Two novel compounds from the root bark of Morus alba L. Nat Prod Res, 2018; 32(1):36–42.
Liu X, Kuang XD, He XR, Ren G, Wang Y, Xu LY, Feng LH, Wang B, Zhou ZW. Prenylflavonoids from the twigs of Artocarpus nigrifolius. Chem Pharm Bull, 2018; 66(4):434–8.
Liu YP, Guo JM, Yan G, Zhang MM, Zhang WH, Qiang L, Fu YH. Anti-inflammatory and antiproliferative prenylated isoflavone derivatives from the fruits of Ficus carica. J Agric Food Chem, 2019; 67(17):4817–23.
Liu YP, Yu XM, Zhang W, Wang T, Jiang B, Tang HX, Su QT, Fu YH. Prenylated chromones and flavonoids from Artocarpus heterophyllus with their potential antiproliferative and anti-inflammatory activities. Bioorg Chem, 2020; 101:104030.
Loizzo MR, Bonesi M, Pugliese A, Menichini F, Tundis R. Chemical composition and bioactivity of dried fruits and honey of Ficus carica cultivars Dottato, San Francesco and Citrullara. J Sci Food Agric, 2014; 94(11):2179–86.
Luengas-Caicedo PE, Braga FC, Brandao GC, de Oliveira AB. Seasonal and intraspecific variation of flavonoids and proanthocyanidins in Cecropia glaziovi Sneth. leaves from native and cultivated specimens. Z Naturforsch C J Biosci, 2007; 62c:701–9.
Mahmoudi S, Khali M, Benkhaled A, Benamirouche K, Baiti I. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac J Trop Biomed, 2016; 6(3):239–45.
Manurung H, Kustiawan W, Irawan WKIW, Marjenah. Total flavonoid content and antioxidant activity of tabat barito (Ficus deltoidea Jack) on different plant organs and ages. J Med Plants Stud, 2017; 5(6):120–5.
Datiles M. Ficus carica (common fig). Invasive species compendium [ONLINE]. 2015. Available via https://www.cabi.org/isc/datasheet/24078 (Accessed 19 July 2021).
Mascarello A, Orbem Menegatti AC, Calcaterra A, Martins PGA, Chiaradia-Delatorre LD, D’Acquarica I, Ferrari F, Pau V, Sanna A, De Logu A, Botta M, Bota B, Terenzi H, Mori M. Naturally occurring Diels-Alder-type adducts from Morus nigra as potent inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase B. Eur J Med Chem, 2018; 144:277–88.
Mouho DG, Oliveira AP, Kodjo CG, Valentao P, Gil-Izquierdo A, Andrade PB, Ouatta ZA, Bekro YA, Ferreres F. Chemical findings and in vitro biological studies to uphold the use of Ficus exasperata Vahl leaf and stem bark. Food Chem Toxicol, 2018; 112:134–44.
Natic MM, Dabic DC, Papetti A, Fotiric Aksic MM, Ognjanov V, Ljubojevic M, Tesic ZL. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem, 2015; 171:128–36.
Nguyen MTT, Le TH, Nguyen HX, Dang PH, Do TNV, Abe M, Takagi R, Nguyen NT. Artocarmins G-M, prenylated 4-chromenones from the stems of Artocarpus rigida and their tyrosinase inhibitory activities. J Nat Prod, 2017; 80(12):3172–8.
Palmeira L, Pereira C, Dias MI, Abreu RMV, Correa RCG, Pires TCSP, Alves MJ, Barros L, Ferreira ICFR. Nutritional, chemical and bioactive profiles of different parts of a Portuguese common fig (Ficus carica L.) variety. Food Res Int, 2019; 126(2019):108572.
Paudel P, Park SE, Seong SH, Jung HA, Choi JS. Novel Diels–Alder type adducts from Morus alba root bark targeting human monoamine oxidase and dopaminergic receptors for the management of neurodegenerative diseases. Int J Mol Sci, 2019; 20(24):6232.
Qi CC, Fu YH, Chen WH, Chen GY, Dai CY, Song XP, Han CR. A new isoflavone from the roots of Ficus auriculata. Nat Prod Res, 2018; 32(1):43–7.
Ren G, Peng J, Liu A, Liang J, Yuan W, Wang H, He J. Structure elucidation and NMR assignments of two new flavanones from the roots of Artocarpus heterophyllus. Magn Reson Chem, 2015; 53(10):872–4.
Ribeiro AEAS, Soares JMD, Silva HAL, Wanderley CW de S, Moura CA, de Oliveira-Junior RG, de Oliveira AP, Rolim LA, Costa EV, Almeida JRG da S, de Oliveira HP, Palheta-Junior RC. Inhibitory effects of Morus nigra L. (Moraceae) against local paw edema and mechanical hypernociception induced by Bothrops jararacussu snake venom in mice. Biomed Pharmacother. 2019; 111(2019):1046–56.
Sanchez-Salcedo EM, Tassotti M, Del Rio D, Hernández F, Martínez JJ, Mena P. (Poly)phenolic fingerprint and chemometric analysis of white (Morus alba L.) and black (Morus nigra L.) mulberry leaves by using a non-targeted UHPLC–MS approach. Food Chem, 2016; 212:250–5.
Septama AW, Jantan I, Panichayupakaranant P. Flavonoids of Artocarpus heterophyllus Lam. heartwood inhibit the innate immune responses of human phagocytes. J Pharm Pharmacol, 2018; 70(9):1242–52.
Shahinuzzaman M, Yaakob Z, Anuar FH, Akhtar P, Kadir NHA, Hasan AKM, Sobayel K, Nour M, Sindi H, Amin N, Sopian K, Akhtaruzzaman M. In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Sci Rep, 2020; 10(1):1–14.
Shi ZF, Lei C, Yu BW, Wang HY, Hou AJ. New alkaloids and α-glucosidase inhibitory flavonoids from Ficus hispida. Chem Biodivers, 2016; 13(4):445–50.
Souza GR, Oliveira-Junior RG, Diniz TC, Branco A, Lima-Saraiva SRG, Guimaraes AL, Oliveira AP, Pacheco AGM, Silva MG, Moraes-Filho MO, Costa MP, Pessoa C, Almeid JRGS. Assessment of the antibacterial, cytotoxic and antioxidant activities of Morus nigra L. (Moraceae). Brazilian J Biol, 2018; 78(2):248–54.
Sugiyama M, Katsube T, Koyama A, Itamura H. Effect of solar radiation on the functional components of mulberry (Morus alba L.) leaves. J Sci Food Agric, 2016; 96(11):3915–21.
Sumi SA, Siraj MA, Hossain A, Mia MS, Afrin S, Rahman MM. Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evid Based Complement Altern Med, 2016; 2016:1–9.
Suttisansanee U, Charoenkiatkul S, Jongruaysup B, Tabtimsri S, Siriwan D, Temviriyanukul P. Mulberry fruit cultivar ‘Chiang Mai’ prevents beta-amyloid toxicity in PC12 neuronal cells and in a Drosophila model of Alzheimer’s disease. Molecules, 2020; 25(8):1837.
Taiwo BJ, Igbeneghu OA. Antioxidant and antibacterial activities of flavonoid glycosides from Ficus exasperata Vahl-Holl (Moraceae) leaves. Afr J Tradit Complement Altern Med, 2014; 11(3):97–101.
Taviano MF, Rashed K, Filocamo A, Cacciola F, Dugo P, Mondello L, Bisignano C, Acquaviva R, D’Arrigo M, Miceli N. Phenolic profile and biological properties of the leaves of Ficus vasta Forssk. (Moraceae) growing in Egypt. BMC Complement Altern Med, 2018; 18(1):161.
Tomczyk M, Milek M, Sidor E, Kapusta I, Litwinczuk W, Puchalski C, Dzugan M. The effect of adding the leaves and fruits of Morus alba to rape honey on its antioxidant properties, polyphenolic profile, and amylase activity. Molecules, 2019; 25(1):84.
Wan C, Chen C, Li M, Yang Y, Chen M, Chen J. Chemical constituents and antifungal activity of Ficus hirta vahl. fruits. Plants, 2017; 6(4):1–9.
Wang C, Zhi S, Liu C, Xu F, Zhao A, Wang X, Tang X, Li Z, Huang P, Yu M. Isolation and characterization of a novel chalcone synthase gene family from mulberry. Plant Physiol Biochem, 2017; 115:107–18.
Wang M, Yu BW, Yu MH, Gao LX, Li JY, Wang HY, Li J, Hou AJ. New isoprenylated phenolic compounds from Morus laevigata. Chem Biodivers, 2015; 12(6):937–45.
Wang T, Li Q, Bi K. Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci, 2018; 13(1):12–23.
Wang Z, Song M, Li Y, Chen S, Ma H. Differential color development and response to light deprivation of fig (Ficus carica L.) syconia peel and female flower tissues: transcriptome elucidation. BMC Plant Biol, 2019; 19(1):217.
Xu LJ, Yu MH, Huang CY, Niu LX, Wang YF, Wu CZ, Yang PM, Hu X. Isoprenylated flavonoids from Morus nigra and their PPAR γ agonistic activities. Fitoterapia, 2018; 127(2018):109–14. CrossRef
Ye J, Ren G, Li W, Zhong G, Zhang M, Yuan J, Lu T. Characterization and identification of prenylated flavonoids from Artocarpus heterophyllus Lam. roots by quadrupole time-of-flight and linear trap quadrupole orbitrap mass spectrometry. Molecules, 2019; 24(24):4591. CrossRef
Yu JS, Lim SH, Lee SR, Choi CI, Kim KH. Antioxidant and anti-inflammatory effects of white mulberry (Morus alba L.) fruits on lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules, 2021; 26(4):920. CrossRef
Yu MH, Zhao T, Yan GR, Yang HX, Wang HY, Hou AJ. New isoprenylated flavones and stilbene derivative from Artocarpus hypargyreus. Chem Biodivers, 2012; 9(2):394–402. CrossRef
Yuan WJ, Yuan JB, Peng JB, Ding YQ, Zhu JX, Ren G. Flavonoids from the roots of Artocarpus heterophyllus. Fitoterapia, 2017; 117:133–7. CrossRef
Zeni ALB, Moreira TD, Dalmagro AP, Camargo A, Bini LA, Simionatto EL, Scharf DR. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An Acad Bras Cienc, 2017; 89(4):2805–15. CrossRef
Zepner L, Karrasch P, Wiemann F, Bernard L. ClimateCharts.net—an interactive climate analysis web platform. Int J Digit Earth, 2020; 14:338–56. [Online] Available via https://climatecharts.net/#home. (Accessed 24 May 2021).
Zhang X, Lv H, Li Z, Jiang K, Lee MR. HPLC/QTOF-MS/MS application to investigate phenolic constituents from Ficus pandurata H. aerial roots. Biomed Chromatogr, 2014; 29(6):860–8. CrossRef
Zhao Y, Kongstad KT, Jäger AK, Nielsen J, Staerk D. Quadruple high-resolution α-glucosidase/α-amylase/PTP1B/radical scavenging profiling combined with high-performance liquid chromatography–high-resolution mass spectrometry–solid-phase extraction–nuclear magnetic resonance spectroscopy for identification of antidiabetic constituents in crude root bark of Morus alba L. J Chromatogr A, 2018; 1556:55–63. CrossRef
Zheng ZF, Zhang QJ, Chen RY, Yu DQ. Four new flavonoids from Morus australis. J Asian Nat Prod Res, 2012; 14(3):263–9. CrossRef
Zhu W, Zhong Z, Liu S, Yang B, Komatsu S, Ge Z, Tian J. Organ-specific analysis of Morus alba using a gel-free/label-free proteomic technique. Int J Mol Sci, 2019; 20(2):365.
Zoofishan Z, Kúsz N, Csorba A, Toth G, Hajagos-Toth J, Kothencz A, Gaspar R, Hunyadi A. Antispasmodic activity of prenylated phenolic compounds from the root bark of Morus nigra. Molecules, 2019; 24(13):2497.
Zorzi M, Gai F, Medana C, Aigotti R, Peiretti PG. Identification of polyphenolic compounds in edible wild fruits grown in the north-west of Italy by means of HPLC-DAD-ESI HRMS. Plant Foods Hum Nutr, 2020; 75(3):420–6.
Zou Y, Liao S, Shen W, Liu F, Tang C, Chen CYO, Sun Y. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in southern China. Int J Mol Sci, 2012; 13(12):16544–53.