INTRODUCTION
Obesity is a global problem that is largely regarded as one of the greatest hazards to global health in the modern period (NHS, 2019). Worldwide, around 2 billion individuals are overweight, with 650 million of them clinically obese (WHO, 2021). There are at least two types of drugs available to treat obesity at the moment. The first class of drugs (orlistat) suppresses pancreatic lipase, resulting in decreased intestinal fat absorption. The second is based on appetite suppressants such as sibutramine. Both medications, however, have a number of adverse effects, including constipation, hypertension, xerostomia, headaches, and insomnia (Scheen, 2010; Westerink and Visseren, 2011). As a result, the hunt for a more effective and safe approach to reducing obesity continues. While it is common to use numerous traditional herbal medications on a daily basis for obesity surveillance, their effectiveness and safety have been little explored and have not been convincingly demonstrated in this context.
It is well established that digestive enzymes such as pancreatic lipase and amylase are responsible for the hydrolysis of triacylglycerols and carbohydrates into simple molecules (Tucci, 2010). Additionally, naturally occurring polyphenols inhibit certain digestive enzymes, which can regulate the caloric content of food by reducing its absorption and lengthening the digestive process (Wang et al., 2020). This way, weight loss may become a feasible goal, resulting in significant health benefits.
Numerous studies have emphasized the health benefits of traditional herbal medicine. Tamarindus indica was singled out for its overall wellbeing effects. It is an evergreen tropical shrub that grows plentifully in Indonesia. According to an ethnomedicine study, this leaf was used by Indonesians as a component of a traditional herbal medicine known as jamu sinom, which translates as “herbal drink for body refreshment” (Handayani et al., 2001). Tamarind is a member of the Fabaceae family, more precisely the subfamily Caesalpiniaceae. Its leaves contain limonene, caryophyllene, beta-sitosterol, linalool, malic acid, palmitic acid, tartaric acid, and flavonoids (luteolin and apigenin) (Escalona-Arranz et al., 2010). Although the tamarind fruit extract was claimed to lower blood LDL (low density lipoprotein) cholesterol levels when given as tamarind fruit pulp, a clinical trial found that triglyceride levels increased unexpectedly (Iftekhar et al., 2006). The polysaccharide content of the fruit could have contributed to the increase in TG. The tamarind leaf extract is expected to have a greater antihyperlipidemic effect than the tamarind fruit extract due to its lower polysaccharide content. According to Nofianti et al. (2019), the tamarind leaf extract was effective in reducing hyperlipidemia in rats in vivo and also exerted glucose-lowering activity (Yerima et al., 2014).
Tamarindus indica was previously investigated in terms of metabolites extracted using a variety of solvents (Escalona-Arranz et al., 2011). In this context, and motivated by widespread public use of traditional herbal medicine derived from T. indica and its well-documented biological properties, this study focused on the phytochemical constituents of three distinct tropical zones. To aid in the dereplication of metabolites, a mass spectral analysis from ultrahigh-performance liquid chromatography-Electrospray ionization-mass spectrometry (UHPLC-ESI-MS) was performed. Simultaneously, the inhibiting effects of three T. indica leaf extracts from various zones on pancreatic lipase and amylase enzymes were examined for their potential use in the treatment of obesity.
MATERIALS AND METHODS
Plant material
As it is known that the water content in the soil affects the production of secondary metabolites, several T. indica-producing areas were selected on the Island of Java, which has different rainfall and evaporation rates. Tamarindus indica leaves were collected from farmers in three distinct tropical zones on Java Island based on the Köppen–Geiger climate classification map for Indonesia (1980–2016) (Fig. 1), namely rainforest denoted by R [Cianjur district; minimum temperature (Tmin) ≥ +18°C, precipitation on driest season (Pdriest) ≥ 60 mm); monsoon denoted by M (Sleman district; Tmin ≥ +18°C, Pannual ≥ 25 mm); and savannah denoted by S (Gresik district; Tmin ≥ +18°C, Pdriest < 60 mm). The plants obtained were identified at the Department of Pharmaceutical Biology, Gadjah Mada University.
Chemicals
Pancreatin (P3292-25G), vitexin standard, gallic acid, quercetin, phosphate buffer saline (PBS) tablet, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, nitrophenol glucose, dinitrosalicylic acid, and p-nitrophenylbutyrate (N9867-1G) were obtained from Sigma-Aldrich (St. Louis, MO). Orlistat (Xenical) was obtained from a local distributor. The solvent for LC-HRMS (liquid chromatography - high resolution mass spectroscopy) was MS grade purchased from Thermo ScientificTM.
Preparation of the extracts
Fresh leaves were dried for 24 hours at 50°C in a tray oven before being powdered with a grinder. Maceration of the leaf powder in 70% ethanol at room temperature (25 ± 2°C) was performed for 24 hours. After filtration, a rotary evaporator was used to evaporate the filtrate into dryness.
Phytochemical contents: UHPLC-ESI-MS
The phytochemical content of the tamarind leaf extract was analyzed using the untargeted mode of the Q Exactive Orbitrap UHPLC-ESI-MS System (Thermo ScientificTM). The extract was dissolved in 100 μg/ml MS grade methanol and filtered through a 0.22 μm filter. The device was set up as follows: the mobile phases were (A) aqueous formic acid 0.1% and (B) acetonitrile-formic acid (0.1%); injection volume was 5 μl and flow rate was 0.3 ml/minute. The gradient system was 0–16 minutes (B 5%–90%); 16–20 minutes (B 90%); 20.1–25 minutes (B 5%). Ion source ESI 3.62 kV-positive mode was used. Furthermore, the raw chromatograms were analyzed using the Compound Discoverer 3.2 (Thermo ScientificTM) software with reference to local and online databases, including mzCloud (www.mzcloud.org) and ChemSpider (www.chemspider.com).
Determination of total phenolic content
Measurement of the total phenolic content was carried out by mixing 14 μl of extract (500 μg/ml in methanol), 208 μl of distilled water, and 14 μl of the Folin–Ciocalteu reagent. The mixture was incubated for 8 minutes. After incubation, the mixture was added with 14 μl of Na2CO3 (20%) and then incubated for 1.5 hours, followed by absorbance measurement at 765 nm. Gallic acid was used as a standard curve (series of 20–150 μg/ml). Total phenolic content was expressed as milligrams gallic acid equivalent per gram of extract (Wiyono et al., 2020).
Determination of total flavonoid content
The total flavonoid content was measured using a spectrophotometric method with an aluminum chloride reagent (Chandra et al., 2014). A total of 0.8 ml of extract (500 μg/ml in methanol) was mixed with 0.8 ml of 2% AlCl3. After incubation for 60 minutes, the absorbance of the solution was measured at 420 nm. Quercetin (20–150 μg/ml) was used as the standard. Flavonoid content was expressed as milligrams of quercetin equivalent per gram of extract.
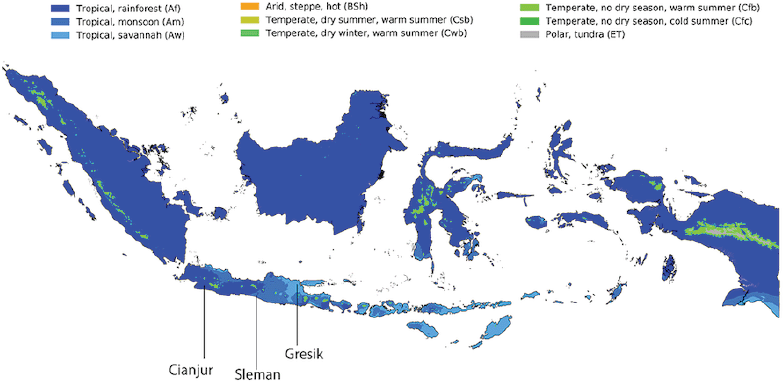 | Figure 1. Koppen–Geiger climate classification map for Indonesia (1980–2016) and sources of experimental plant. Cianjur represented Rainforest-zone, Sleman as Monsoon-zone, and Gresik as Savanah-zone. (Beck et al., 2018). [Click here to view] |
Determination of vitexin content
Vitexin levels in the tamarind leaf extract were determined using the Q Exactive Orbitrap UHPLC-ESI-MS System in the targeted mode (Thermo ScientificTM). The extract was dissolved in 100 μg/ml MS grade methanol and filtered through a 0.22 μm filter. The device was initially configured as follows: the mobile phase was (A) aqueous formic acid 0.1% and (B) acetonitrile-formic acid (0.1%); injection volume was 8 μl; flow rate was 0.3 ml/minute. Gradient system was 0–8 minutes (B 5%–90%); 8–13 minutes (B 90%); 13.01–18 minutes (B 5%). The ion source used was electrospray ionization in positive mode (3.32 kV). The vitexin standard was prepared by dissolving vitexin in methanol of MS grade in a series of concentrations of 0.5–20 μg/ml.
DPPH radical scavenging assay
Because the measurement of IC50 DPPH scavenging is often biased, depending on the concentration of DPPH used as mentioned in a previous critical review (Menezes et al., 2021), the measurement of antioxidant capacity was used in this study instead of IC50. A volume of 0.5 ml of the tamarind leaf extract at 500 μg/ml (in methanol) or ascorbic acid (in methanol) at concentrations ranging from 20 to 100 μg/ml was mixed with 0.5 ml of DPPH (1,1-diphenyl-2-picrylhydrazyl) at 0.04 mg/ml (in absolute ethanol) and stored at room temperature in a dark place. After 30 minutes of incubation, the absorbance at 520 nm was measured. The extract’s antioxidant capacity was expressed as gram ascorbic acid equivalent per 100 g of extract.
Pancreatic lipase inhibition
The lipase inhibitory activity was determined spectrophotometrically by monitoring the rate of nitrophenol formation as a product of pancreatin’s hydrolysis of p-nitrophenylbutyrate (Terra et al., 2016). The extract was prepared by dissolving the extract in DMSO (dimethyl sulfoxide) at concentrations of 10 and 100 μg/ml. About 10 μl of each extract solution (for final mixture concentrations of 0.38 and 3.8 μg/ml, respectively) were mixed with 10 μl of pancreatin (1 mg/ml in phosphate buffer saline, pH 6.8). After 5 minutes of incubation at 37°C, the substrate 240 ml (p-nitrophenylbutyrate 0.165 mM in PBS) was added. The absorbance was measured at 415 nm immediately after substrate addition and 35 minutes later. The lipase activity was expressed as μM nitrophenol released per minute. Orlistat 120 μg/ml (4.6 μg/ml final concentration) was used as a positive control. Nitrophenol standard curves were created by diluting nitrophenol to concentrations of 0, 1, 2, 5, 10, and 20 μg/ml. About 260 μl of each dilution was measured in a 96-well plate at 415 nm.
To ascertain the mode of inhibition, the enzyme kinetics values (Km and Vmax) were determined using the extract and orlistat (final concentrations of 3.8 and 4.6 μg/ml, respectively) at a range of substrate concentrations of 0.1, 0.2, 0.3, and 1.0 mM.
Pancreatic amylase inhibition
Tests on the inhibitory activity of the extract against the amylase enzyme were carried out by the sugar reducing method using pancreatin (1 mg/ml) in phosphate buffer saline of pH 6.8 referring to the method of Keharom et al. (2016) with modifications. The starch as amylase substrate was dissolved in distilled water by heating until it was clear. In summary, 50 μl of the extract in DMSO was mixed with 100 μl of the enzyme pancreatin, followed by 2,000 μl of the starch substrate (5,000 μg/ml). The mixture was incubated for 10 minutes at 37°C. Then the reaction was stopped by adding 500 μl of 1 M NaOH, followed by the addition of 200 μl dinitrosalicylic acid 2.75 mg/ml. The mixture was then heated in boiling water for 5 minutes. Absorbance reading was carried out by taking 100 μl of the solution that was diluted with 1,000 μl of distilled water in a cuvette and then measured at 410 nm. As the standard curve, standard glucose (serial concentration 2.5–500 μg/ml) was used.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 7.0 with the one-way analysis of variance followed by Dunnett’s multiple comparison test. Pearson’s correlation test was performed to determine the correlation between antioxidant activity and phytochemical level. Differences among comparisons were considered statistically significant for p values less than 0.05 (95% confidence level). Nonlinear regression analysis was also carried out to calculate the enzyme kinetics value (Km and Vmax). The data are presented as mean ± standard deviation.
RESULTS
Effect of various climate zones on total phenolic, flavonoid, and vitexin content
The gallic acid standard curve y = 0.0014x + 0.0501 (r2 = 0.9912) was obtained to determine total phenolic content. Meanwhile, the quercetin standard curve was y = 0.0192x + 0.0786 (r2 = 0.9992). The results indicated that tamarind leaves from the monsoon zone (M) had the highest total phenolic content (55.9 ± 1.9% GAE, gallic acid equivalent), which was proportional to their antioxidant activity (r = 0.998). Meanwhile, the highest total flavonoid content (2.1 ± 0.0% QE, quercetin equivalent) was owned by tamarind leaves grown in the savannah climate zone (S). The flavonoid content was poorly correlated (r = −0.379) to antioxidant activity based on Pearson’s correlation test. The total phenolic, flavonoid, and vitexin content are presented in Table 1.
Effect of various climates on vitexin content
The content of vitexin was quantified using targeted UHPLC-ESI-MS. Standard vitexin was injected into HRMS in series, and the peak area was determined, yielding the standard curve y = 11774802.32x + 1980946.77 (r2 = 0.9985). As shown in Supplementary Figure 1, both vitexin standard and vitexin in the extract had a retention time of 6.1–6.2 minutes. The results indicated that tamarind leaves from the savannah zone (S) had the highest vitexin content (9.63% of dry extract).
Effect of various climates on antioxidants
The standard curve of ascorbic acid for DPPH scavenging was calculated to be y = 0.43x – 2.75 (r2 = 0.9543). The antioxidant capacity of the dry extract was determined by extrapolating the extract’s absorbance reading to the standard curve and was expressed as percent ascorbic acid equivalent per dry extract, as shown in Table 1. Antioxidant capacities of the rainforest (R), monsoon (M), and savannah (S) extracts were 31.3%, 42.4%, and 41.1% AAE (ascorbic acid equivalent), respectively.
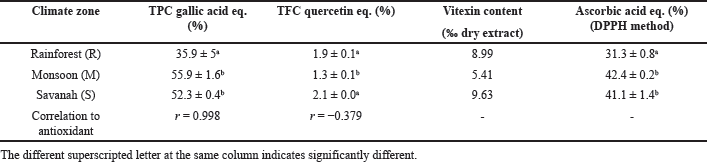 | Table 1. Total phenolic, flavonoid, vitexin and antioxidant capacity of different source of Tamarindus indica leaf extract. Except for vitexin content, values were presented as Mean ± SD, n = 3. [Click here to view] |
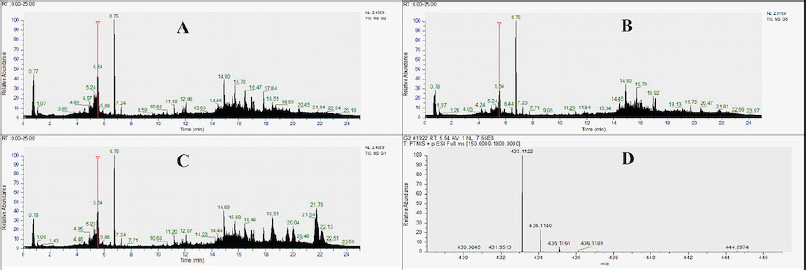 | Figure 2. Chromatogram of untargeted LC-HRMS from T. indica leaf exract. Label: R (Rainforest), M (Monsoon), S (Savanah), Vtx (MS1 spectra of vitexin). [Click here to view] |
UHPLC-ESI-MS metabolites annotation
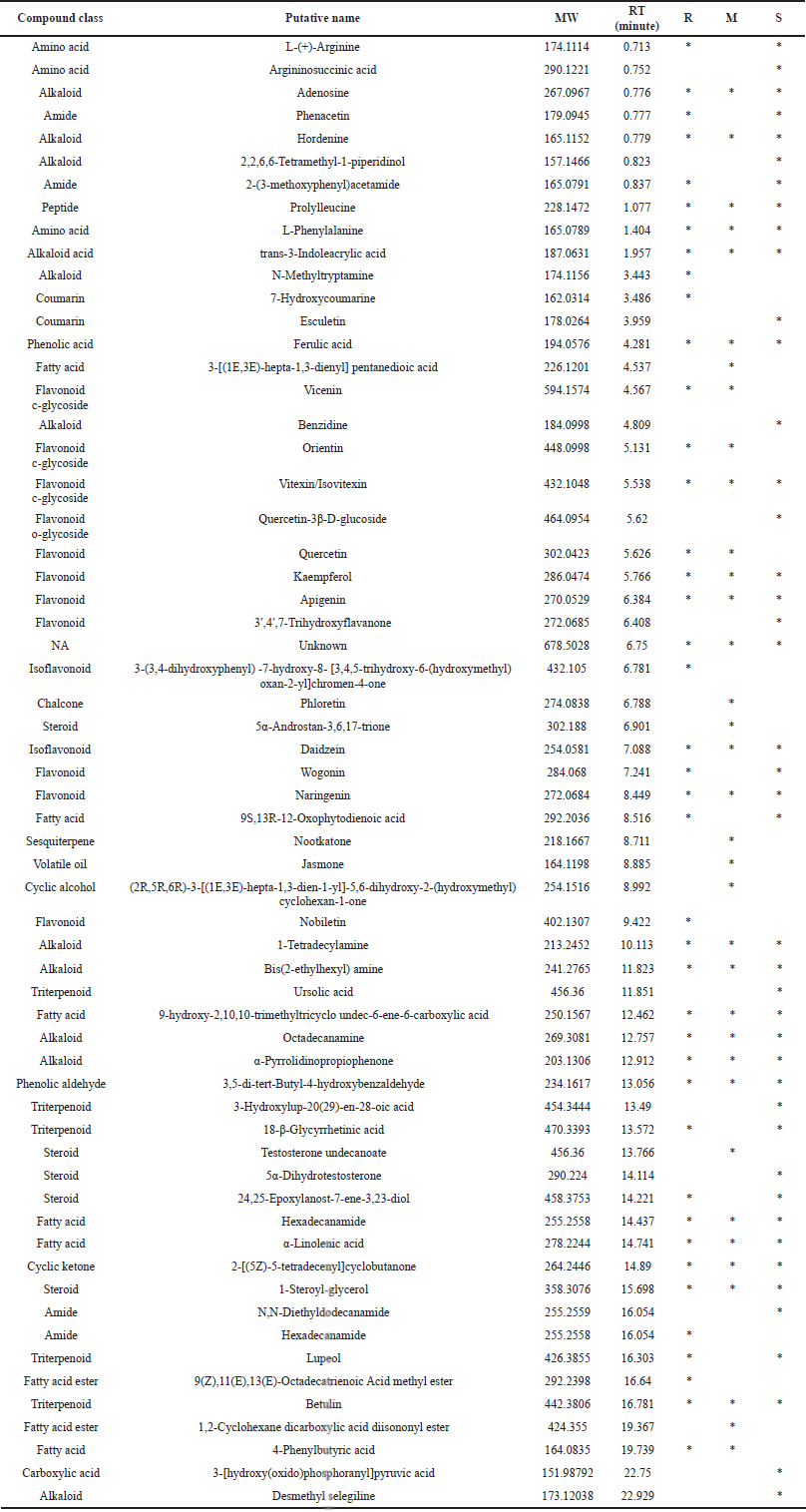
As shown in Figure 3, an analysis of the untargeted LC–MS chromatogram was performed. The analytical parameters for metabolite profile analysis were as follows: candidate molecules were chosen based on their conformation to the fragmentation pattern in mzCloud, and their molecular mass deviation was no greater than 2 ppm. In Table 2, predicted molecules are tabulated and classified according to their compound class.
In general, alkaloids, amides, amino acids, chalcones, coumarins, fatty acids and esters, flavonoids, peptides, phenolic acids and aldehydes, sesquiterpenes, steroids, triterpenoids, and volatile oils were detected in T. indica leaf extract. Based on the chromatogram shown in Figure 2, it can be seen that compounds with high abundance appeared at a retention time of 14–22 minutes. The flavonoid content was seen dominantly at the retention time of 4.56–8.45 minutes. The presence of steroid and triterpenoid compounds was more detected in T. indica from the savannah (S) zone.
Meanwhile, the distinctive compounds that were only detected in each zone are as follows: the rainforest zone: N-metiltriptamin, 7-methoxycoumarin, 3-(3,4-dihydroxyphenyl)-7-hydroxy-8-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] chromen-4-one, nobiletin, and octadecatrienoic acid methyl ester; the monsoon zone: 3-[(1E,3E)-hepta-1,3-dienyl] pentanedioic acid, phloretin, 5α-androstan-3,6,17-trione, nootkatone, jasmone, (2R,5R,6R)-3-[(1E,3E)-hepta-1,3-dien-1-yl]-5,6-dihydroxy-2-(hydroxymethyl) cyclohexan-1-one, and 1,2-cyclohexane dicarboxylic acid diisononyl ester; the savannah zone: argininosuccinic acid, 2,2,6,6-tetramethyl-1-piperidinol, esculetin, benzidine, quercetin-3β-D-glucoside, 3’,4’,7-trihydroxy flavanone, ursolic acid, 3-hydroxylupenoic acid, and N,N-diethyldodecanamide.
Pancreatic lipase inhibition
The lipase inhibitory activity measurements revealed that the compound with the highest inhibitory activity was M (monsoon zone) at 3.8 μg/ml giving 58.3% inhibition (p = 0.0026), followed by R (rainforest) 40.9% (p = 0.0454) and S (savannah) 38.6% (p = 0.0611), as compared to the solvent control (Fig. 3A) at the α = 0.05 significance level. The inhibitory activity of the T. indica leaf extract from the monsoon zone was even stronger than orlistat (4.6 μg/ml).
Additionally, the inhibition mode was determined by varying the substrate concentration so that the Michaelis–Menten curve was formed, as shown in Figure 3B. The mode of inhibition was determined by comparing the maximum reaction rate (Vmax), Michaelis constant (Km), and Lineweaver–Burk (LB) slope values in the presence and absence of an inhibitor. According to the data obtained, the results showed that all extracts decreased Vmax and Km and altered the value of the LB slope, as shown in Table 3.
Pancreatic amylase inhibition
The results of the amylase inhibitory activity test showed that the T. indica leaf extract did not significantly reduce the rate of starch hydrolysis by amylase. At 0.38 and 3.8 μg/ml of extract concentration, there was no decrease in the rate of starch hydrolysis by pancreatic amylase enzymes (p > 0.05), as shown in Figure 4.
DISCUSSION
Phytochemicals and antioxidants
Obesity develops as a result of an imbalance in calorie intake and activity. Calories enter the body in the form of carbohydrates (4 calories per gram), fat (9 calories per gram), and protein (4 calories per gram). The number of calories consumed is determined by the rate of absorption in the intestinal lumen and the metabolism of food prior to absorption. Carbohydrates are absorbed in the form of monosaccharide molecules, which were previously hydrolyzed by the amylase and alpha-glucosidase enzyme. Protein is absorbed in the form of amino acids, a process that is initiated by proteases. Meanwhile, fat is absorbed in the form of free fatty acids following the lipase enzyme’s hydrolysis of triglycerides (Brodkorb et al., 2019). Inhibition of these three enzymes, particularly lipase, has recently become a target for obesity prevention.
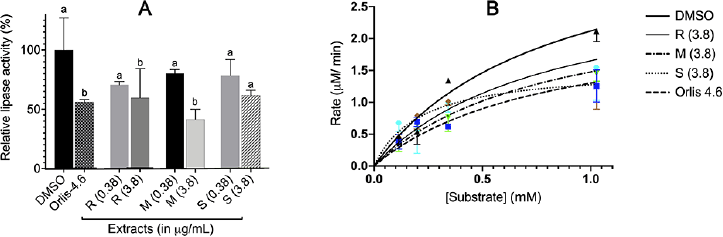 | Figure 3. (A) Pancreatic lipase inhibitory activity of T. indica leaf extract. The activity was measured using low (0.38 µg/ml) and high (3.8 µg/ml) concentration of extract with 0.165 mM p-nitrophenylbutyrate as substrate. (B) Kinetics of pancreatic lipase in the absent and present of extract. [Click here to view] |
 | Table 2. Predicted phytochemical content of Tamarindus indica leaf extract using untargeted HRMS method. Sample code: R (rainforest), M (monsoon), S (savanah). [Click here to view] |
Herbal medicine is growing in popularity year after year. As much as 60% of the world’s population is said to have begun to use herbal medicine. This proportion is even higher in developing countries, reaching nearly 80% (Ahmad Khan and Ahmad, 2019). Numerous studies have indicated that T. indica has lipid-lowering activity and may be used to treat obesity (Aprilia et al., 2017; Joyeux et al., 1995; Lahamado et al., 2017). It was estimated that this activity is mediated by both antioxidant activity and inhibitory activity against proteins/enzymes involved in obesity.
However, the production of plant metabolites as well as their pharmacological activity is strongly influenced by the conditions in which the plant grows. According to the current study, the total phenolic content of the T. indica leaf extract grown in the rainforest zone (R) was lower (35.95% GAE) compared to the monsoon zone (M) (55.96% GAE) and the savannah zone (S) (52.30% GAE). Similarly, the total flavonoid content is highest in the S extract (2.10% QE), which is derived from tropical savannah areas that are typically dry. These results were in line with previous reports where plants that grow in areas of high ecological stress produce more metabolites than those that grow in nutrient-rich areas (Mundim and Pringle, 2018; Oni et al., 2013).
The antioxidant capacity of the three extracts was found to be proportional to the total phenol content (r = 0.998) rather than the total flavonoid content (r = −0.379) based on Pearson’s correlation analysis. According to this information, it was revealed that the molecule responsible for the T. indica leaf extract’s antioxidant activity was a nonflavonoid phenolic compound. This study differed slightly from the previous one, which demonstrated antioxidant activity was closely correlated to both total phenolic and flavonoid content (Razali et al., 2012). This difference was estimated to be caused by ecological factors affecting the flavonoid production in tamarind leaves during the monsoon season (M) as well as the glycosylation and methylation of flavonoids.
To unravel this ecological influence, the aqueous ethanolic extracts of three plants were analyzed using an LC-HRMS system to predict the metabolite variation, as shown in Table 2. In general, classes of molecules detected in the T. indica extract included alkaloids, amides, amino acids, chalcones, coumarins, fatty acids and esters, flavonoids, peptides, phenolic acids and aldehydes, sesquiterpenes, steroids, triterpenoids, and volatile oils.
Tamarind leaves are considered to be flavonoid-dense plants. The three plants contained flavonoids such as kaempferol, apigenin, naringenin, vitexin/isovitexin, and daidzein. Additionally, wogonin, 3?-4?-7-trihydroxyflavanone, and quercetin-3b-D-glycoside were found in the savannah zone (S). Meanwhile, nobiletin and 3-(3,4-dihydroxyphenyl)-7-hydroxy-8-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] chromen-4-one were flavonoids found exclusively in the tamarind leaves from the rainforest zone (R).
 | Table 3. The value of Km and Vmax of Michaelis-Menten plot from lipase activity in the absent and present of extract. The values are presented as Mean ± SE. [Click here to view] |
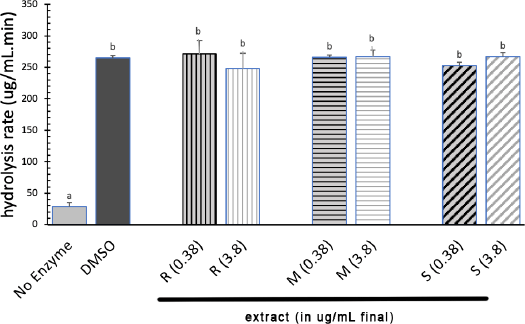 | Figure 4. Rate of starch hydrolysis by pancreatic amylase in the absent and present of extract. Extract were prepared at 0.38 and 3.8 ug/ml final. (R: Rainforest, M: Monsoon, S: Savanah). [Click here to view] |
On the other hand, phytochemicals only existing in the monsoon zone (M) were 3-[(1E,3E)-hepta-1,3-dienyl] pentanedioic acid, phloretin, 5α-androstan-3,6,17-trione, nootkatone, jasmone, (2R,5R,6R)-3-[(1E,3E)-hepta-1,3-dien-1-yl]-5,6-dihydroxy-2-(hydroxymethyl) cyclohexan-1-one, and 1,2-cyclohexane dicarboxylic acid diisononyl ester. Nootkatone and jasmone were sesquiterpenes of chemical class.
Phloretin, 3?,4?,7-trihydroxyflavanone, and quercetin-3-D-glucoside were the hydroxylated compounds that were not present in the rainforest climate tamarind (R). It was possible that the absence of these components, which have a hydroxyl group at the 4’ position, resulted in decreased antioxidant activity via DPPH radical scavenging. Materska (2008) reported that the hydroxyl group at position 4’ on flavones is critical for their antioxidant activity. This group’s antioxidant activity was said to be significantly reduced by glycosylation.
Alkaloids such as adenosine, hordenine, 1-tetradecylamine, bis(2-ethylhexyl) amine, octadecanamine, α-pyrrolidinopropiophenone, 2,2,6,6-tetramethyl-1-piperidinol, benzidine, N-methyltryptamine, and trans-3-indoleacrylic acid were almost evenly distributed across all climates. Alkaloids were slightly more abundant in the savannah climate (S) than in other climates. Additionally, sesquiterpenes and steroid components were found in greater abundance in tamarind monsoon climates (M), whereas terpenoids were found in greater abundance in savannah climates (S). This result corroborated previous research in which Li et al. (2020b) stated that ecological factors, particularly a scarcity of water, stimulated the production of sterol compounds in Artemisia annua and other plants. This increase was mediated by an increase in the expression of the sterol C-4 methyl oxidase (SMO1) gene, which was expressed in response to dehydration tolerance.
Pancreatic lipase and amylase inhibitory activity
Despite the fact that flavonoids were poorly absorbed in the intestine (Fakhrudin et al., 2019), several flavonoids have been shown to inhibit pancreatic lipase enzymes in addition to their antioxidant properties. Nonesterified flavanols like catechin, epicatechin, gallocatechin, and epigallocatechin have no effect on pancreatic lipase inhibition (Mohapatra et al., 2015; Rahim et al., 2015). When catechins, such as epigallocatechin gallate and epigallocatechin digallate, are esterified with gallic acid, they become active in inhibiting pancreatic lipase.
The inhibitory activity of pancreatic lipase was determined using pancreatin, a digestive enzyme mixture containing lipase (6 U/mg), amylase (75 U/mg), and protease (75 U/mg) (Terra et al., 2016). Despite its lower activity than fresh-prepared pancreatic juice, pancreatin was acceptable to be used for food digestibility assay (Salhi et al., 2020).
Test results on amylase activity of all T. indica leaf extracts failed to inhibit pancreatic amylase at both measured concentrations (p > 0.05). However, as shown in Figure 3, T. indica leaf extract was able to significantly inhibit the pancreatic lipase enzyme (p ≤ 0.05) at a concentration of 3.8 μg/ml (R and M extracts) compared to DMSO as solvent control using substrate concentration of 0.165 mM. This finding differed slightly from a previous report in which a 500 μg/ml final concentration of the T. indica leaf extract inhibited lipase by 28% less than orlistat (Abd Rahman, 2017). However, 3.8 μg/ml of the M extract was found to be more effective than orlistat in recent research. This activity was possibly influenced by the difference in its metabolites as well as growth location.
C-glycosidic flavonoids, such as vitexin, have been identified as potentially effective lipase inhibitors (Abdulai et al., 2021). A previous study found that kaempferol inhibited lipase via a competitive mode (Li et al., 2020a). Meanwhile, another study found that quercetin inhibits lipase competitively. Betulinic acid inhibited the lipase enzyme as well, but the mode of inhibition was unknown (Kim et al., 2012). Trendafilova et al. (2018) also discovered that sesquiterpene had potent lipase inhibitory activity. The mode of inhibition was poorly informed. The synergistic effect of these compounds was presumably responsible for the high lipase inhibition of the monsoon extract (M) at 3.8 μg/ml in this study. Sesquiterpenes from Alisma orientale were also reported to have high lipase inhibitory activity (Cang et al., 2017).
According to the enzyme inhibition criterion (Palmer and Bonner, 2011), the T. indica leaf extract inhibited pancreatic lipase on a mix-mode of inhibition. As shown in Table 3, all extracts reduced both the maximum reaction rate (Vmax) and the Michaelis constant (Km), as well as the slope of the Lineweaver–Burk curve. Competitive inhibition was defined as Vmax remaining constant while Km increased. Noncompetitive inhibition was defined as Vmax decreasing but Km remaining constant, while uncompetitive inhibition was defined as Km and Vmax changing but the Lineweaver–Burk slope remaining constant.
CONCLUSION
The lipase inhibitory activity of the T. indica leaf was found to be affected by its ecological environment. Among the three tropical climates, e.g., rainforest, monsoon, and savannah, T. indica growing in the monsoon climate inhibited lipase the most. It was thought that differences in antilipase activity were caused by differences in the types of flavonoids, alkaloids, fatty acids, and steroids. More research is needed, particularly to assess the extract’s activity against other enzymes or proteins involved in the obesity mechanism, such as lipoprotein lipase, leptin, and phosphatidic acid phosphatase.
ACKNOWLEDGMENT
This research was funded by collaborative work of Research Grant of Program Prioritas Nasional Kedeputian Ilmu Pengetahuan Teknik LIPI No. 26/A/DT/2021 and Saintek Kemenristek 2019 scholarship program.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to this research, including concept and research design (TW, ENS, WRP, and AF), data acquisition (TW and AF), data analysis (AF and TW), funding (AF), manuscript drafting (TW), and critical revision of manuscript and supervision (AF, ENS, and WRP), until approval of the final version of the manuscript (ENS).
CONFLICTS OF INTEREST
The authors declare there are no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abd Rahman RNZR. Anti-obesity potential of selected tropical plants via pancreatic lipase inhibition. Adv Obes Weight Manag Control, 2017; 6; doi:10.15406/aowmc.2017.06.00163. CrossRef
Abdulai IL, Kwofie SK, Gbewonyo WS, Boison D, Puplampu JB, Adinortey MB. Multitargeted effects of vitexin and isovitexin on diabetes mellitus and its complications. Sci World J, 2021; 2021:1–20; doi:10.1155/2021/6641128 CrossRef
Ahmad Khan MS, Ahmad I. Herbal Medicine: Current Trends and Future Prospects. New Look to Phytomedicine Adv. Herb. Prod. as Nov. Drug Leads, Massachusetts, United States: Academic Press; 2019, p. 3–13. https://doi.org/10.1016/B978-0-12-814619-4.00001-X. CrossRef
Aprilia CA, Ninditasari G, Walujo D. Hypolipidemic Effect and antioxidant activity of tamarind leaves extract in hypercholesterol-fed rats. Indones J Cardiol, 2017; 38:72–80. CrossRef
Brodkorb A, Egger L, Alminger M, Alvito P, Assunção R, Ballance S, Bohn T, Bourlieu-Lacanal C, Boutrou R, Carrière F, Clemente A, Corredig M, Dupont D, Dufour C, Edwards C, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie AR, Martins C, Marze S, McClements DJ, Ménard O, Minekus M, Portmann R, Santos CN, Souchon I, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W, Recio I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc, 2019; 14:991–1014; doi:10.1038/s41596-018-0119-1. CrossRef
Cang J, Wang C, Huo X-K, Tian X-G, Sun C-P, Deng S, Zhang B-J, Zhang H-L, Liu K-X, Ma X-C. Sesquiterpenes and triterpenoids from the rhizomes of Alisma orientalis and their pancreatic lipase inhibitory activities. Phytochem Lett, 2017; 19:83–8; doi:10.1016/j.phytol.2016.12.017 CrossRef
Chandra S, Khan S, Avula B, Lata H, Yang MH, ElSohly MA, Khan IA. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid Based Complement Altern Med, 2014; 2014:1–9; doi:10.1155/2014/253875 CrossRef
Escalona-Arranz JC, Pérez-Rosés R, Licea Jiménez I, Rodríguez-Amado JR, Argota-Coello H, Cañizares-Lay J, Morris HJ, Sierra G. Chemical constituents of Tamarindus indica L. leaves. Rev Cuba Química, 2010; XXII.
Escalona-Arranz JC, Rodríguez-Amado J, Pérez-Rosés R, Cañizares-Lay J, Sierra G, Morris HJ, Licea Jiménez I. Metabolites extraction optimization in Tamarindus indica L. leaves. Bol Latinoam y Del Caribe Plantas Med y Aromat, 2011; 10(4):369–7.
Fakhrudin N, Wiyono T, Putra AR, Widyarini S, Nurrochmad A. The evaluation on anti-platelet and antithrombosis activities of cinnamomum sintoc bark extract. Thai J Pharm Sci, 2019; 43(4):219–26. CrossRef
Handayani L, Suparto H, Suprapto A. Traditional system of medicine in Indonesia. In: Chaudhury RR, Rafei UM (eds.). Traditional Medicines in Asia, WHO, New Delhi, India, p 47, 2001.
Iftekhar ASMM, Rayhan I, Quadir MA, Akhteruzzaman S, Hasnat A. Effect of Tamarindus indica fruits on blood pressure and lipid-profile in human model: an in vivo approach. Pak J Pharm Sci, 2006; 19:125–9.
Joyeux M, Mortier F, Fleurentin J. Screening of antiradical, antilipoperoxidant and hepatoprotective effects of nine plant extracts used in Caribbean folk medicine. Phyther Res, 1995; 9:228–30; doi:10.1002/ptr.2650090316. CrossRef
Keharom S, Mahachai R, Chanthai S. The optimization study of α-amylase activity based on central composite design-response surface methodology by dinitrosalicylic acid method. Int Food Res J, 2016; 23:10–7.
Kim J, Lee YS, Kim C-S, Kim JS. Betulinic acid has an inhibitory effect on pancreatic lipase and induces adipocyte lipolysis. Phyther Res, 2012; 26:1103–6; doi:10.1002/ptr.3672. CrossRef
Lahamado OT, Sabang SM, Mustapa K. Ekstrak Daun Asam Jawa (Tamarindus Indica L.) Sebagai Antidiabetes. J Akad Kim, 2017; 6:1–6. CrossRef
Li S, Pan J, Hu X, Zhang Y, Gong D, Zhang G. Kaempferol inhibits the activity of pancreatic lipase and its synergistic effect with orlistat. J Funct Foods, 2020a;72:104041; doi:10.1016/j.jff.2020.104041 CrossRef
Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem, 2020b;148:80–9; doi:10.1016/j.plaphy.2020.01.006 CrossRef
Materska M. Quercetin and its derivatives: chemical structure and bioactivity—a review. Polish J Food Nutr Sci, 2008; 58:407–13.
Menezes BB de, Frescura LM, Duarte R, Villetti MA, da Rosa MB. A critical examination of the DPPH method: mistakes and inconsistencies in stoichiometry and IC50 determination by UV–Vis spectroscopy. Anal Chim Acta, 2021; 1157:338398; doi:10.1016/j.aca.2021.338398 CrossRef
Mohapatra S, Prasad A, Haque F, Ray S, De B, Ray SS. In silico investigation of black tea components on α-amylase, α-glucosidase and lipase. J Appl Pharm Sci, 2015; 5:042–7. CrossRef
Mundim FM, Pringle EG. Whole-plant metabolic allocation under water stress. Front Plant Sci, 2018; 9; doi:10.3389/fpls.2018.00852 CrossRef
NHS. Obesity: Causes, Diagnosis, and Treatment. Obes - NHS United Kingdom 2019. https://www.nhs.uk/conditions/obesity/ (accessed October 10, 2021).
Nofianti T, Nurmayasari S, Priatna M, Ruswanto R, Nurfatwa M. The effect of the ethanolic extract of Asam Jawa leaf (Tamarindus Indica L.) in total cholesterol, triglyceride, LDL and HDL concentration on male sprague dawley rats. J Phys Conf Ser, 2019; 1179:012175; doi:10.1088/1742-6596/1179/1/012175 CrossRef
Oni PI, Jimoh S., Adebisi L. Population pattern and phenological behaviours for selected medicinal plants in Nigeria; implications for ex-situ conservation. J Appl Pharm Sci, 2013; 3:052–60.
Palmer T, Bonner PL. Enzyme Inhibition. Enzymes, Sawston, United Kingdom: Woodhead Publishing; 2011, p. 126–52. https://doi.org/10.1533/9780857099921.2.126. CrossRef
Rahim ATMA, Takahashi Y, Yamaki K. Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Res Int, 2015; 75:289–94; doi:10.1016/j.foodres.2015.05.017. CrossRef
Razali N, Mat-Junit S, Abdul-Muthalib AF, Subramaniam S, Abdul-Aziz A. Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chem, 2012; 131:441–8; doi:10.1016/j.foodchem.2011.09.001 CrossRef
Salhi A, Amara S, Mansuelle P, Puppo R, Lebrun R, Gontero B, Aloulou A, Carrière F. Characterization of all the lipolytic activities in pancreatin and comparison with porcine and human pancreatic juices. Biochimie, 2020; 169:106–20; doi:10.1016/j.biochi.2019.07.004 CrossRef
Scheen AJ. Cardiovascular risk-benefit profile of sibutramine. Am J Cardiovasc Drugs, 2010; 10:321–34; doi:10.2165/11584800-000000000-00000 CrossRef
Terra GDP, Vinícius De Farias M, Trevisan MG, Garcia JS. Evaluation of pancreatin stability through enzyme activity determination. Acta Pharm, 2016; 66:423–31; doi:10.1515/acph-2016-0037 CrossRef
Trendafilova A, Todorova M, Kutova N, Guncheva M. Phytochemical profile and anti-lipase activity of balkan endemic jurinea tzar-ferdinandii. Nat Prod Commun, 2018; 13:1017–20; doi:10.1177/1934578x1801300823 CrossRef
Tucci S. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab Syndr Obes Targets Ther, 2010; 3:125–43; doi:10.2147/DMSOTT.S7005 CrossRef
Wang H-N, Xiang J-Z, Qi Z, Du M. Plant extracts in prevention of obesity. Crit Rev Food Sci Nutr, 2022; 62(8):2221–34; doi:10.1080/10408398.2020.1852171 CrossRef
Westerink J, Visseren FL. Pharmacological and non-pharmacological interventions to influence adipose tissue function. Cardiovasc Diabetol, 2011; 10:13; doi:10.1186/1475-2840-10-13 CrossRef
WHO. Obesity and overweight. WHO Factsheet, WHO, Geneva, Switzerland, 2021.
Wiyono T, Nurhayati R, Herawati ERN, Laila U. The effect of time, pH and solvent composition on cocoa shell polyphenol extraction and its antioxidant activity: response surface method approach. IOP Conf Ser Earth Environ Sci, 2020; 462:012029; doi:10.1088/1755-1315/462/1/012029 CrossRef
Yerima M, Anuka JA, Salawu OA, Abdu-Aguye I, Tanko Y. Antihyperglycaemic activity of the flavonoid-rich fraction of the extract of Tamarindus indica L. on experimentally induced hyperglycaemic wistar rats. J Appl Pharm Sci, 2014; 4:64–8; doi:10.7324/JAPS.2014.40813 CrossRef
SUPLEMENTARY MATERIAL
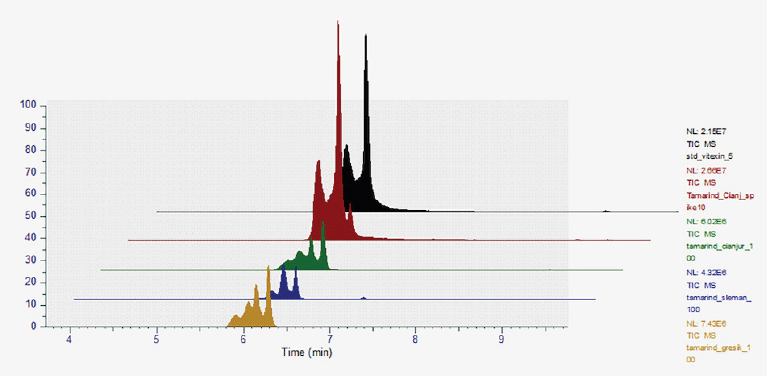 | Suplementary Figure 1. Vitexin retention time (6 minutes) of the standard, extracts, as well as in spiked extract. [Click here to view] |