INTRODUCTION
Inflammation is an essential defense mechanism of the body for the removal of noxious stimuli and invading pathogens (Abbas and Lichtman, 2003; Christgen and Kanneganti, 2020). The inflammatory process involves the migration of immune cells as well as the production and secretion of proinflammatory cytokines and mediators such as histamine, serotonin, prostaglandins (PGs), nitric oxide (NO), reactive oxygen species, tumor necrotic factor-(TNF-α), and interleukin (Christgen and Kanneganti, 2020; Christiansen et al., 2006; Fujiwara and Kobayashi, 2005; Kuebler, 2005). These inflammatory mediators are well known as essential biochemicals, which are involved in protecting body tissues and organs from pathogens or hazardous stimuli and initiating tissue repair (Bednash and Mallampalli, 2016; Christgen and Kanneganti, 2020; Oehmcke-Hecht and Köhler, 2018). However, excess and lasting production of inflammatory mediators and proinflammatory cytokines is associated with a number of inflammatory diseases (Bednash and Mallampalli, 2016; Christgen and Kanneganti, 2020; Tsuchiya and Hara, 2014).
Zingiberaceae, commonly known as the ginger family, include a large number of different plants comprising about 52 genera and 1,587 species (Srisook and Srisook, 2020). For centuries, various plants in this family have been consumed as food and used as herbs in many countries around the world, especially in the Asian zone (Larsen and Larsen, 2006). A number of natural compounds from plants in Zingiberaceae have been reported to show various pharmacological activities (Chongmelaxme et al., 2017; Jurenka, 2009; Martins et al., 2019; Mustafa and Srivastava, 1990), including anti-inflammatory effects. Zingiberaceae are recognized as a source of natural compounds for treating inflammation-related diseases, for instance, curcumin isolated from the rhizomes of Curcuma longa which has been used as an anti-inflammatory agent for rheumatic diseases and some inflammatory disorders (Yang et al., 2019).
Etlingera pavieana (Pierre ex Gagnep) R.M.Sm, known in Thailand as rew-hom, is one of the Zingiberaceae species, widely grown in Thailand, Vietnam, and other Southeast Asian countries (Poulsen and Phonsena, 2017). In Thailand, E. pavieana rhizomes are commonly consumed as a spice and herb which are sometimes mixed in salads. The rhizomes are also widely used as herbal medicine for diuresis (Poulsen and Phonsena, 2017) and to relieve fever, gastrointestinal disorders, and pharyngitis (Phonsena, 2007; Poulsen and Phonsena, 2017). Etlingera pavieana rhizome extracts have been shown to possess biological effects, for example, anti-inflammatory, antioxidant, and cytotoxic properties (Srisook et al., 2017, 2018; Iawsipo et al., 2018). Moreover, E. pavieana rhizomes have been demonstrated to consist of many phytochemical compounds, some of which have pharmacological properties (Wang et al., 2009; Srisook et al., 2017; Iawsipo et al., 2018; Naksang et al., 2020; Srisook et al., 2020). For example, the ethyl acetate fraction of E. pavieana and some phenolic compounds exhibits an anti-inflammatory effect in LPS-induced macrophages (Srisook et al., 2017). 4-Methoxycinnamyl p-coumarate (MCC), a phenylpropanoid derived from E. pavieana rhizomes, has been demonstrated to have anti-inflammatory, antitumor, and antibacterial effects (Tachai and Nuntawong, 2016; Srisook et al., 2017; Iawsipo et al., 2018; Mankhong et al., 2019; Srisook et al., 2020). Its anti-inflammatory effects are related to inhibition of Nuclear factor kappa B (NF-κB), AP-1, and Akt signaling cascades, resulting in the reduction of iNOS, COX-2, and proinflammatory cytokine expression and their subsequent protein biosynthesis (Mankhong et al., 2019). However, the anti-inflammatory effect and the safety profile of MCC in experimental animals have not been reported. This study aimed to examine the in vivo anti-inflammatory effect of MCC against acute inflammation in rats and to investigate the acute oral toxicity of MCC in mice.
MATERIALS AND METHODS
Preparation of MCC
Etlingera pavieana was collected from Chanthaburi Province, Thailand, and authenticated by Dr. B. Chewprecha, Botanist, at the Department of Biology, Faculty of Science, Burapha University. The voucher specimen (KS?SCBUU?0012?2) was kept at the Faculty of Science, Burapha University. MCC was separated from the ethyl acetate fraction of the ethanolic rhizome extract from E. pavieana as previously described by Srisook et al. (2017).
Experimental animals
Sprague Dawley rats and Institute of Cancer Research mice were purchased from Nomura Siam International Co., Ltd., Thailand. The female mice were used in the acute oral toxicity study, while the male rats were used in the anti-inflammatory activity study. The rats and mice were housed separately in open-mesh cages in a temperature-controlled room maintained at 21°C + 1°C with a 12:12 light-dark cycle. Standard diet and drinking water were available ad libitum. All animals were kept for at least 1 week to acclimatize prior to starting the experiments. Approval from the Animal Ethics Committee, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (no. 44/2561), and by the Institutional Animal Care and Use Committee, Burapha University (no. IACUC 005/2562), was obtained for all experimental procedures.
In vivo anti-inflammatory activity study of MCC
Ethyl phenylpropiolate(EPP) -induced rat ear edema
The EPP-induced ear edema was conducted as described by Brattsand et al. (1982). Nine male rats (40–60 g) were divided into three groups (three rats each): vehicle control, reference drug, and MCC-treated groups. Topical administration of acetone (20 μl/ear), phenylbutazone (1 mg/ear), or MCC (3 mg/ear) to the ears of each of the rats was performed prior to ear edema induction. Immediately after that treatment, inflammation was induced by topical application of EPP (1 mg/ear) to both ears of each rat. Ear thickness was measured using a digital Vernier caliper just prior to and at 15, 30, 60, and 120 minutes after EPP application. The level of ear edema formation was assessed by ear thickness.
Carrageenan-induced rat paw edema
The carrageenan-induced hind paw edema experiment was performed according to the method described by Winter et al. (1962). Thirty male rats (weight 100–120 g) were divided into five groups (n = 6/group) including control, aspirin, and three different doses of MCC-treated groups. The vehicle (5% Tween 80), aspirin (300 mg/kg), or MCC (25, 75, and 150 mg/kg) was administered orally to each rat 1 hour before injection of 1% carrageenan prepared in a normal saline solution into the subplantar side of the right hind paw of each rat.
Paw volume of the injected feet was assessed with a plethysmometer (Ugo Basile, Italy) after injection of carrageenan at 0, 1, 3, and 5 hours. After paw volume measurement, all rats were immediately sacrificed. The right hind paws of all rats were collected for histopathological examination.
Acute toxicity study in mice
The acute toxicity test was carried out according to the OECD guideline 420: acute oral toxicity-fixed dose procedure [OECD (The Organisation of Economic Co-operation and Development), 2001]. After acclimatization for 7 days, the female mice were divided into two groups of five each. The test group was treated orally with MCC (2,000 mg/kg). The control group was given an equal volume of 5% Tween 80. Mortality and signs of toxicity were monitored at 1, 2, 4, and 6 hours after oral administration and then once daily for 14 days after dosing. The body weights of the mice were recorded prior to and at 7 and 14 days after treatment. On day 15, all remaining mice were anesthetized and sacrificed. Necropsy and a gross pathological examination of internal organs, including thymus, bronchi, lungs, spleen, liver, heart, kidneys, stomach, intestine, sex organs, and adrenal glands, were performed.
Statistical analysis
Data are reported as mean ± standard error of the mean (SEM). Statistically significant differences between groups were analyzed by one-way analysis of variance followed by the post hoc least significant difference test. Differences were considered statistically significant when p values < 0.05.
RESULTS
Effects of MCC on EPP-induced rat ear edema
After topical administration of EPP, the vehicle group showed the greatest extent of ear edema. Figure 1 shows the effects of the test compound MCC and phenylbutazone, the reference anti-inflammatory drug, on EPP-induced rat ear swelling. Both MCC (3 mg/ear) and phenylbutazone (1 mg/ear) significantly reduced the ear edema when compared with the vehicle control group at all assessment times (p < 0.001).
Anti-inflammatory effects of MCC on carrageenan-induced rat paw edema
The results of the effects of MCC and aspirin (reference drug) on paw edema induced by carrageenan are shown in Figure 2. Various doses of MCC (25–150 mg/kg) produced dose-dependent inhibition of paw edema at every time point after carrageenan injection (p < 0.05–p < 0.001). After 5 hours of injection, MCC at the highest dose (150 mg/kg) and aspirin showed significant levels of edema inhibition, 44.89% and 67.82%, respectively.
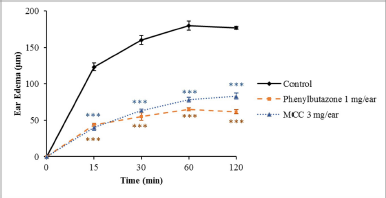 | Figure 1. Effects of MCC and phenylbutazone on rat edema induced by EPP. Results are shown as mean ± SEM (n = 6). ***Significantly different from control, p < 0.001. [Click here to view] |
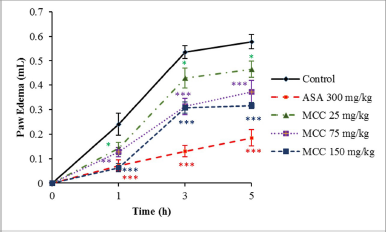 | Figure 2. Effects of MCC and aspirin (ASA) on rat paw edema induced by carrageenan injection. Results are shown as mean ± SEM (n = 6). p < 0.05, p < 0.01, and ***p < 0.001 significantly different from control. [Click here to view] |
Figure 3 shows the histopathological images of rat paw tissues at 5 hours after carrageenan injection. Marked edema with infiltration of inflammatory cells was observed in the tissues from carrageenan-injected paws of the control group. The MCC- (150 mg/kg) treated group as well as the reference drug group (aspirin 300 mg/kg) exhibited less interstitial edema than the control rats.
Acute toxicity study of MCC in mice
In the acute oral toxicity test, a single treatment of MCC (2,000 mg/kg) did not show mortality in any of the tested mice during the period of observation. No signs of toxicity or general behavioral changes were observed. Body weights on days 7 and 14 after treatment were not significantly different between the MCC-treated mice and the control mice. The organ weights of female mice in the MCC-treated group had no significant difference from the control group (Table 1).
DISCUSSION
From our previous study, MCC, a phenolic compound derived from E. pavieana rhizomes, has demonstrated its in vitro anti-inflammatory effect in LPS-stimulated macrophages (Mankhong et al., 2019) and in TNF-α-induced human endothelial cells (Srisook et al., 2020). This study is the first to demonstrate the anti-inflammatory effect of MCC in in vivo rat models. Although further toxicity studies are needed, MCC safety data from the acute toxicity study shows the potential of that compound for drug development.
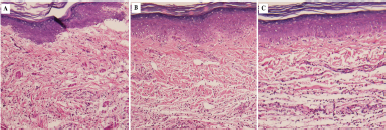 | Figure 3. Histopathological feature of rat paw tissues at 5 hours after carrageenan injection. Tissue sections from the rat hind paw were stained with H&E for histopathological examination at 5 hours after carrageenan injection (100× magnifications). (A) Control group. (B) Aspirin- (ASA-) treated group. (C) MCC- (150 mg/kg) treated group. [Click here to view] |
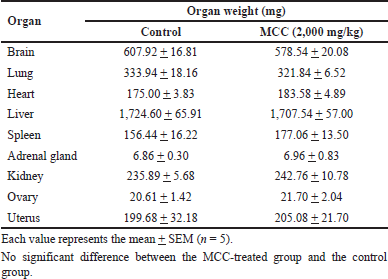 | Table 1. Organ weights of female mice in the acute toxicity study of MCC. [Click here to view] |
In this study, the in vivo anti-inflammatory effect of MCC using two experimental models of acute inflammation was examined. EPP-induced ear edema in rodents is generally used as a screening and evaluation model for testing the anti-inflammatory effect of compounds (Brattsand et al., 1982). EPP causes ear swelling by stimulating the production and secretion of several inflammatory mediators, for example, histamine, bradykinin, PGs, and serotonin (Carlson et al., 1985), leading to vasodilatation and an increase in vascular permeability. The data obtained from the present study demonstrate the anti-inflammatory effect of MCC as this phenolic compound significantly inhibited EPP-stimulated rat ear edema formation. The second model of the present study is hind paw edema induced by carrageenan, an acute inflammation model which is a conventional method for investigating the anti-inflammatory effect of test compounds. Carrageenan injection into a rat’s paw causes a biphasic inflammatory mediator release response. The first phase (0–2.5 h after carrageenan injection) results from the release of histamine, bradykinin, and serotonin. The late phase at 2.5–6 hours after injection is attributed to the formation and secretion of inflammatory mediators, especially PGs and NO (Liao et al., 2012; Mansouri et al., 2015). In the present study, MCC reduced paw edema caused by carrageenan injection, thereby demonstrating the anti-inflammatory effect of MCC in this model. In our previous in vitro study, we showed that MCC induces inactivation of the NF-κB and Akt signaling cascades, resulting in the inhibition of the expression of inflammatory mediators, NO and PGE2, in murine RAW 264.7 macrophage cells (Mankhong et al., 2019). Recently, MCC also exhibited the downregulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, endothelial adhesion molecules which mediate leukocyte emigration into inflamed sites (Srisook et al., 2020). These activities might be the mechanisms of the anti-inflammatory action of MCC observed in the EPP-induced ear edema and carrageenan-induced paw edema in rat models.
In the acute oral toxicity study, MCC (2,000 mg/kg) did not show mortality or produce any signs of acute toxicity in female mice during the 14 days of the study which would indicate that the LD50 value with oral administration of MCC is higher than 2,000 mg/kg for mice. However, further toxicity studies should be performed to confirm its safety in long-term use.
CONCLUSION
In conclusion, MCC has an anti-inflammatory effect in rats. MCC’s activity against acute inflammation might be due to suppression of the production and release of inflammatory mediators. MCC does not exhibit acute oral toxicity in mice, indicating its relative safety. Taken together, these findings strongly support the conclusion that there is significant potential for developing MCC as a new therapeutic agent for the treatment of inflammation-related disorders.
ACKNOWLEDGMENTS
This work was financially supported by a research grant from Burapha University through the National Research Council of Thailand (Grant no. 118/2560) and the Research Unit of Natural Bioactive Compounds for Healthcare Products Development. The authors thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Higher Education, Science, Research and Innovation, Thailand. The authors are also thankful to Dr. G. Lamar Robert for editing the manuscript and to Ms. Petchrat Sawai for valuable technical assistance.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
Authors declared that they do not have any conflicts of interest.
ETHICAL APPROVALS
Approval from the Animal Ethics Committee, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (no. 44/2561), and by the Institutional Animal Care and Use Committee, Burapha University (no. IACUC 005/2562), was obtained for all experimental procedures.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbas AK, Lichtman AH. Cellular and molecular immunology. 5th edition. Saunders, Philadelphia, PA, 2003.
Bednash JS, Mallampalli RK. Regulation of inflammasomes by ubiquitination. Cell Mol Immunol, 2016; 13:722–8. CrossRef
Brattsand R, Thalén A, Roempke K, Källström L, Gruvstad E. Influence of 16 alpha, 17 alpha-acetal substitution and steroid nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J Steroid Biochem, 1982; 16:779–86. CrossRef
Carlson RP, O’Neill-Davis L, Chang J, Lewis AJ. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions, 1985; 17:197–04. CrossRef
Chongmelaxme B, Sruamsiri R, Dilokthornsakul P. Dhippayom T, Kongkaew C, Saokaew S, Chuthaputti A, Chaiyakunapruk N. Clinical effects of Zingiber cassumunar (Plai): a systematic review. Complement Ther Med, 2017; 35:70–7. CrossRef
Christgen S, Kanneganti TD. Inflammasomes and the fine line between defense and disease. Curr Opin Immunol, 2020; 62:39–44. CrossRef
Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med, 2006; 11:302–8. CrossRef
Daily JW, Zhang X, Kim DS, Park S. Efficacy of ginger for alleviating the symptoms of primary dysmenorrhea: a systematic review and meta-analysis of randomized clinical trials. Pain Med, 2015; 16:2243–55. CrossRef
Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy, 2005; 4:281–6. CrossRef
Iawsipo P, Srisook E, Ponglikitmongkol M, Tatiyar S, Singaed O. Cytotoxic effects of Etlingera pavieana rhizome on various cancer cells and identification of a potential anti-tumor component. J Food Biochem, 2018; e12540:1–9. CrossRef
Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev, 2009; 14:141–53.
Kuebler WM. Inflammatory pathways and microvascular responses in the lung. Pharmacol Rep, 2005; 57:196–05.
Larsen K, Larsen SS. Gingers of Thailand. Queen Sirikit Botanic Garden, Chiang Mai, Thailand, 2006.
Liao JC, Deng JS, Chiu CS. Hou WC, Huang SS, Shie PH, Huang GJ. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med, 2012; 2012:429320; doi:10.1155/2012/429320 CrossRef
Mankhong S, Iawsipo P, Srisook E, Srisook K. 4-methoxycinnamyl p-coumarate isolated from Etlingera pavieana rhizomes inhibits inflammatory response via suppression of NF-κB, Akt and AP-1 signaling in LPS-stimulated RAW 264.7 macrophages. Phytomedicine, 2019; 54:89–97. CrossRef
Mansouri MT, Hemmati AA, Naghizadeh B, Mard SA, Rezaie A, Ghorbanzadeh B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J Pharmacol, 2015; 47:292–8. CrossRef
Martins LB, Rodrigues AMDS, Rodrigues DF, Dos Santos LC, Teixeira AL, Ferreira AVM. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) addition in migraine acute treatment. Cephalalgia, 2019; 39:68–76. CrossRef
Mustafa T, Srivastava KC. Ginger (Zingiber officinale) in migraine headache. J Ethnopharmacol, 1990; 29:267–73. CrossRef
Naksang P, Tongchitpakdee S, Thumanu K, Oruna-Concha MJ, Niranjan K, Rachtanapun C. Assessment of antimicrobial activity, mode of action and volatile compounds of Etlingera pavieana essential oil. Molecules, 2020; 25:E3245. CrossRef
OECD (The Organisation of Economic Co-operation and Development). The OECD guideline for testing of chemicals: 420 acute oral toxicity-fixed dose procedure. The Organisation of Economic Co-operation and Development, Paris, France, 2001.
Oehmcke-Hecht S, Köhler J. Interaction of the human contact system with pathogens-an update. Front Immunol, 2018; 9:312. CrossRef
Phonsena P. Medicinal plants in Khao Hin Son Herb Garden. Jettanaromphun Printing, Prachinburi, Thailand, 2007.
Poulsen DA, Phonsena P. Morphological variation and distribution of the useful ginger Etlingera pavieana (Zingiberaceae). Nord J Bot, 2017; 35:467–75. CrossRef
Srisook E, Palachot M, Mankhong S, Srisook K. Anti-inflammatory effect of Etlingera pavieana (Pierre ex Gagnep.) R.M.Sm. rhizomal extract and its phenolic compounds in lipopolysaccharide-stimulated macrophages. Pharmacogn Mag, 2017; 13:s230–5. CrossRef
Srisook K, Potiprasart K, Sarapusit S, Park CS, Srisook E. Etlingera pavieana extract attenuates TNF-α induced vascular adhesion molecule expression in human endothelial cells through NF-κB and Akt/JNK pathways. Inflammopharmacol, 2020; 28:1649–62. CrossRef
Srisook K, Srisook E. 2020. Pharmacological activities and phytochemicals of Etlingera pavieana (Pierre ex Gagnep) R.M.Sm. In: Hassan BAR (ed.). Medicinal plants – use in prevention and treatment of diseases. London, UK: IntechOpen, pp 1–13, 2020. CrossRef
Srisook K, Udompong S, Sawai P, Thongyen T. Etlingera pavieana rhizome extract decreases oxidative stress and activates eNOS activity via stimulation of Akt phosphorylation in human endothelial cells. Naresuan Phayao J, 2018; 11:23–8.
Tachai S, Nuntawong N. Uncommon secondary metabolites from Etlingera pavieana rhizomes. Nat Prod Res, 2016; 30:2215–9. CrossRef
Tsuchiya K, Hara H. The inflammasome and its regulation. Crit Rev Immunol, 2014; 34:41–80. CrossRef
Wang KC, Chang JS, Chiang LC, Lin CC. 4-Methoxycinnamaldehyde inhibited human respiratory syncytial virus in a human larynx carcinoma cell line. Phytomedicine, 2009; 16:882–6. CrossRef
Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med, 1962; 111:544–7. CrossRef
Yang M, Akbar U, Mohan C. Curcumin in autoimmune and rheumatic diseases. Nutrients, 2019; 11:1004. CrossRef