INTRODUCTION
Hyperglycemia is known as an obvious characteristic of diabetes mellitus, leading to type 1 diabetes with a relative or absolute decrease in insulin secretion or type 2 diabetes due to systemic resistance of insulin. Glycation, presenting in fluids and tissues of the human body, results from the spontaneous interaction of reducing sugars like glucose and fructose with amino residues of macromolecules, including nucleic acids, lipids, and proteins associated with the production of advanced glycation end products (AGEs). In addition, endogenous AGE accretion is the consequence of an increase in the rate of glycation responses induced by hyperglycemia (Bellier et al., 2019). Reactive carbonyl species, including glyoxal (GO) and methylglyoxal (MGO), play important roles in AGE formation as precursors or intermediates (Yamagishi and Matsui, 2010). Diabetic complications are caused by a rise in GO or MGO levels in plasma and tissues (Kilhovd et al., 2009). It is revealed that AGE accumulation caused impairment of nitric oxide signal pathway as well as oxidative stress, which may be responsible for the production of atherosclerotic plaques, myocardial and arterial inflexibility, and endothelial dysfunction (Peppa and Vlassara, 2005). Moreover, the structural and functional integrity of macromolecules is irreversibly damaged because of AGEs’ overproduction, leading to activate several aging-related pathologies such as neurodegenerative diseases together with diabetes and its complications. Also, MGO- and MGO-originated AGEs are believed to induce alterations in structures as well as functions of several tissues and organs in the human body. For instance, dysfunction of three microvascular tissues including the peripheral nervous system, eyes, and kidneys, considered as typical target tissues in diabetes, is induced by the presence of MGO. Additionally, the significant impact of MGO on endothelial function is attributed to the reduction of glyoxalase 1 activity, which increases MGO level, and modification of gene expression related to coronary artery disorder as well as upregulation of collagen expression, accompanied with apoptotic and endothelial inflammatory activation (Schalkwijk and Stehouwer, 2020).
Mimosa pudica Linn. has been used in traditional medicine in order to treat a variety of illnesses such as dysentery, urogenital disorders, piles, sinus, and healing wounds because of several potent effects like anti-infection, antioxidant, antimicrobe, antidepressant, antiproliferation, anticancer, and antidiabetes (Abramson et al., 2016). Inspired by the traditionally effective treatment of M. pudica, many studies have been conducted to identify phytochemicals in the different parts, containing phenolic compounds, alkaloids, flavonoids, glycoproteins, quinone, coumarins, tannins, and saponins. It is reported that M. pudica has a potent ability against the hyperglycemic state and hence may be considered as a promising alternative source of diabetes mellitus treatment (Tunna et al., 2014). Because of the substantial decrease in glucose, triglycerides, low-density lipoprotein, very-low-density lipoprotein, and total cholesterol concentrations in streptozotocin-induced diabetic rats, this plant has antidiabetic and antihyperlipidemic properties (Parasuraman et al., 2019). In addition, an in vitro experiment shows the antihyperglycemic effect of M. pudica ethanol extract through inhibition of diabetes-related enzymes as α-glucosidase and α-amylase in comparison with acarbose (Tasnuva et al., 2019). However, reports on the molecular mechanism of M. pudica antidiabetic effects related to glucotoxicity and AGE target are currently lacking. In the present study, to identify phytoconstituents in the ethanol extract of M. pudica, we used liquid chromatography-mass spectrometry (LC-MS). Furthermore, we have investigated the protective effect against MGO-induced glucotoxicity on human umbilical vein endothelial cells (HUVECs) as well as inhibition of MGO- and GO-caused AGE formation of M. pudica ethanol extract.
MATERIALS AND METHODS
Materials
2′,7′-Dichlorofluorescein diacetate, aminoguanidine (AG), bovine serum albumin (BSA), MGO, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). Lonza (Walkersville, MD) provided Endothelial Cell Growth Medium-2 (EGM-2) medium, and American Type Culture Collection provided fetal bovine serum (Rockville, MD).
Preparation of M. pudica extracts
The aerial parts of M. pudica were collected from Nam Dinh province, Vietnam. The plant samples were authenticated, and a voucher specimen has been deposited at the Department of Pharmacology and Clinical Pharmacy, University of Medicine and Pharmacy, Vietnam National University, Hanoi, Vietnam.
The dried plant material (20kg) was extracted with 80% ethanol at room temperature using the cold maceration method for 3 times/72 hours with the ratio of plant sample to ethanol being 1/10 (kg/l). Following that, the extracts were filtered, combined, and subsequently evaporated at reduced pressure to achieve ethanolic extract of M. pudica (1.785 kg).
LC-MS analysis
Phytochemical exploration of M. pudica extracts was conducted using the LC-MS method. The freeze samples were dissolved in equivalent amounts of LC-MS-grade acetonitrile to obtain filtrates for LC-MS analysis and then filtered through a polyvinylidene difluoride membrane (0.45 m). For phytochemical analysis, an Agilent 6520 (Agilent Technologies, Inc., Santa Clara, CA) accurate mass LC-MS was used in conjunction with an Agilent LC 1200, and sample separation was performed using an Extend-C18 column (1.8 m, 2.1, and 50 mm). The mobile gradient phase consisted of water containing 0.05% formic acid as a solvent A and acetonitrile as solvent B. The constant flow rate was 0.9 ml/minutes with column temperature kept at 30°C. Mass Spectrometer LTQ Orbitrap XL™ (Thermo Scientific Company, Waltham, MA) was used for MS analysis. Electrospray ionization was performed using the positive mode with 3,000 volts in the capillary, 125 V in the fragmented voltage, Oct RF Vpp of 750 V, drying gas (nitrogen) at 5 l/minutes, drying gas temperature at 300°C, and nebulizer pressure at 40 psi. To obtain the fragmentation patterns, collision-caused dissociation was used in tandemauto tandem MS (MS/MS) mode with varying collision energy of 3-4 V/100 DA with an offset of 8–10 V, and further mass fragmentation was done in targeted MS/MS mode with constant collision energy for accuracy. The detection of typical phytochemicals in the different M. pudica extracts was based on the obtained data of fragmentation patterns accompanied with the referred data reported in previous studies.
Cell culture
The HUVEC line was purchased from the American Type Culture Collection (Manassas, VA). The cells were cultured in EGM-2 containing 4% fetal bovine serum and maintained at 37°C in a humidified incubator with 5% CO2. All the cells used for subsequent experiments were of passage numbers between five and eight.
Measurement of cell viability
The viability of the cells was evaluated by using the MTT assay based on the method of Figarola et al. (2014), with some modifications. HUVECs were seeded into 96-well plates at a density of 1.0 × 104 cells/well, followed by incubating for 24 hours. In the next steps, these cells were pretreated with AG and different doses of M. pudica ethanol extract for 1 hour, subsequently incubated with MGO. After 24 hours, the addition of MTT solution was performed to possess a final concentration of 0.1 mg/ml. This mixture was then incubated for 2 hours, then removed medium and added dimethyl sulfoxide(100 μl/well). Measurement of the absorbance at a wavelength of 570 nm was performed by utilizing a microplate reader (Molecular Devices, San Jose, CA). AG at a concentration of 1 mM was referred to as a positive control.
Inhibition of AGE formation
Inhibitory effects of M. pudica ethanol extract on protein glycation were investigated through AGE formation assay followed the protocol of Kiho et al. (2005). The formation of AGEs was determined by fluorescence using a VICTORTM ×3 multilabel plate reader (Perkin Elmer, Waltham, MA), with excitation wavelengths of 355 and emission wavelengths of 460 nm.
AGE breaking activity of M. pudica ethanol extract
Breaking effects of M. pudica ethanol extract on the performed AGEs were evaluated by using trinitrobenzene sulfonate (TNBS) assay following the slightly modified protocol compared to the original method of Furlani et al. (2015). A microplate reader at a wavelength of 340 nm was used for measuring the breaking activity of M.pudica (MP) ethanol extract.
Statistical analysis
For statistical analysis of data obtained from the in vitro sample, GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA) was used, and values were expressed as mean, standard deviation. One-way analysis of variance and Bonferroni’s test were used to evaluate the findings better. Statistical significance was described as p values less than 0.05.
RESULTS
Phytochemical of M. pudica ethanol extract
The M. pudica ethanol extract was analyzed by using the LC-MS method to identify chemical compounds in comparison with molecular weight in the reported data. The identified compounds are listed in Table 1.
LC-MS profiling of M. pudica ethanol extract showed a maximum number of phytochemicals containing phenols like myoinositol, 4-phenylbutan-2-ol, 4-(2-phenylethyl)-phenol, 2-hydroxy-benzene ethanol, flavonoids such as luteolin, fisetin, apigenin, quercetin, and naringenin, and some acids, namely, gallic acid, p-hydroxybenzoic acid, caffeic acid, jasmonic acid, monoamidomalonic acid, and ferulic acid. Among them, 4-(2-phenylethyl)-phenol was detected by the highest mass ionization signal m/z 104.0 consistent with the parent ions of m/z 141.09 [M + H + NH4]. Also, the ion mass with m/z 104.0 could be the fragment of 2-tert-butyl-2-phenyl-1,3-dioxolane with m/z 206.99 [M + 2H]. The addition of kali to ferulic acid (m/z 194.14) provided the fragment with m/z 233.1 [M + K]. The ion with the mass number 180.24 [M + K] produced a fragment with the mass number 219.2, which was identified as caffeic acid. Apigenin [M + 2K + H] with m/z 270.28 could produce another fragment with m/z 347.2. The compound exhibited an [M + 2K + H] ion at m/z 247.3, which was identified as gallic acid (m/z 170.38). Fragmentation of the [M + NH4] ion at m/z 302.17 resulting in production with m/z 320.2 by the addition of ammonia was identified as quercetin.
Inhibitory effects of M. pudica ethanol extract on glucotoxicity in HUVECs
To investigate the protective effects of M. pudica on MGO-induced glucotoxicity, an MTT assay was performed through the results of cell viability. As shown in Figure 1, HUVECs exhibited a remarkable suppression in cell viability following MGO treatment (400 μM). Meanwhile, the inhibition of MGO-caused cytotoxic effects leading to an increase in the number of healthy cells within a population was observed in the cells pretreated with M. pudica ethanol extracts in a concentration-dependent manner. However, the M. pudica groups' inhibitory effects were lower compared to the positive control group treated with AG at a dose of 1 mM. Besides, M. pudica ethanol extracts only showed cytotoxicity at a high concentration (100 μg/ml).
Inhibitory effects of M. pudica ethanol extract on AGE formation
The evaluation of AGE formation was presented by measurement of fluorescence with AG as a positive control. As shown in Figure 2A and B, a significant reduction of the MGO-AGE formation was obtained due to the incorporation of M. pudica extracts in a concentration-dependent manner. Meanwhile, M. pudica was not influential on the GO-AGE formation. Additionally, the inhibitory effects on MGO-caused AGE formation of M. pudica extract at doses of 5 and 10 μg/ml were stronger than the positive control.
Breaking effects of M. pudica ethanol extracts on MGO- and GO-AGEs
TNBS assay was used for examining the braking ability of M. pudica ethanol extract on the performed AGEs via the amounts of remaining glycation. A significant reduction of free amines was seen after MGO- and GO-BSA incubations (Fig. 3A and B). Nevertheless, the treatment with M. pudica extracts at a dose of 0.1, 0.5, and 1 μg/ml led to an increase in the percentages of free amines and restored the amine levels of MGO- and GO-BSA after 24 hours, inducing the breakage of AGEs. Additionally, the M. pudica ethanol extracts at high concentrations induced stronger breaking effects on AGEs in comparison with positive control in both MGO and GO groups.
DISCUSSION
Mimosa pudica has been widely studied due to its potential pharmacological properties, including antidiabetic, antioxidant, antitoxin, wound healing, and antihepatotoxic effects (Joseph et al., 2013). The β-cell differentiated phenotype is maintained by steady physiological stimulation of glucose, and glucotoxicity is defined as toxic or deleterious impacts on the β-cell phenotype in extended or persistent contact with high glucose levels in vitro as well as in vivo studies. The hyperglycemic level in type 2 diabetes leads to glucotoxicity and glucolipotoxicity (Bensellam et al., 2012). Through MTT assay, M. pudica ethanol extract significantly inhibited glucotoxicity induced by MGO in a dose-dependent manner. The previous study has shown that M. pudica has antidiabetic activity. In in vivo experiments on diabetic rats induced by alloxan or streptozotocin, M. pudica extracts exhibited hypoglycemic effects via a decrease in blood glucose level, insulin, and plasma lipoprotein, which might be responsible for the antidiabetic property (Manosroi et al., 2011; Rajendiran et al., 2019). Rizwan Bashir et al. (2013) showed that the root powder of M. pudica has antidiabetic efficacy at a dose rate of 6 mg/kg body weight in albino rabbits. Rajendiran et al. (2017) also reported that the ethanol extract of Mp leaves at a dose of (300 mg/kg) daily for 30 days significantly increased the production of insulin and the decline of gluconeogenic enzymes in type 2 diabetes rats.
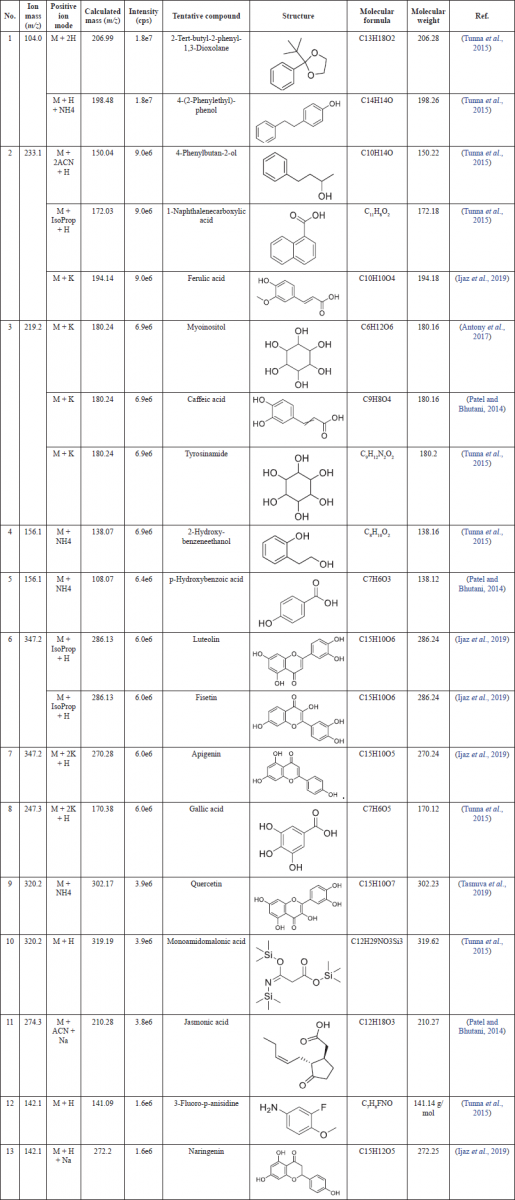 | Table 1. Chemical profiling of M. pudica ethanol extract. [Click here to view] |
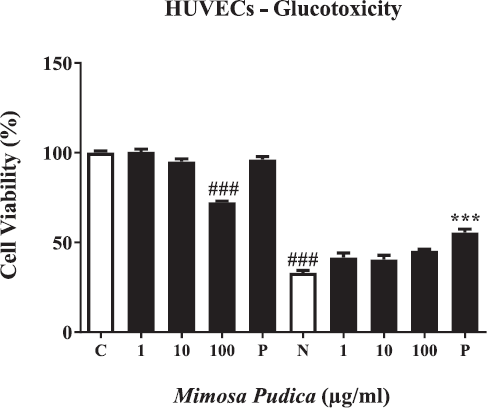 | Figure 1. Viability of HUVECs after being treated by M. pudica ethanol extract and aminoguanidine (AG) in both two groups without MGO and with MGO. From left to right, (C) control; (1) M. pudica (1 μg/ml); (10) M. pudica (10 μg/ml); (100) M. pudica (100 μg/ml); (P) AG (1 mM); (N) MGO (400 μM); (1) MGO + M. pudica (1 μg/ml); (10) MGO + M. pudica (10 μg/ml); (100) MGO + M. pudica (100 μg/ml); (P) MGO + AG (1 mM). The presentation of percent cell viability is performed as the mean ± SD of three independent experiments (###p < 0.001 vs. M. pudica treatment at 100 μg/ml without MGO and MGO treatment only and ***p < 0.001 vs. positive control with MGO treatment). [Click here to view] |
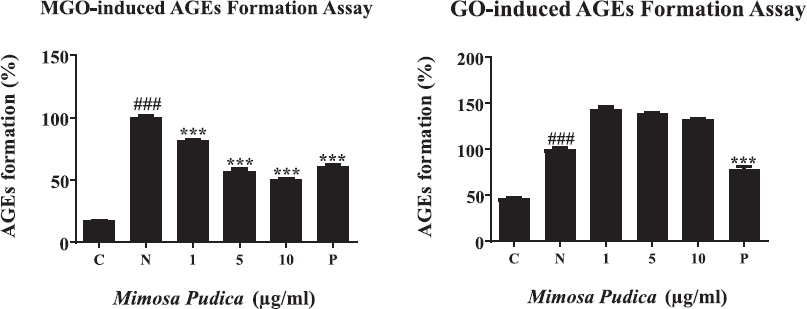 | Figure 2. (A) The inhibition of AGE formation induced by MGO after treatment with M. pudica ethanol extracts. From left to right, (C) control; (N) MGO (2 mM); (1) MGO + M. pudica (1 μg/ml); (5) MGO + M. pudica (5 μg/ml); (10) MGO + M. pudica (10 μg/ml); and (P) MGO + AG (1 mM). Bar values are performed as mean ± SD of three independent experiments (***p < 0.001 vs. MGO treatment only and ###p < 0.001 vs. MGO treatment). (B) The effects of M. pudica ethanol extracts on AGE formation caused by GO. From left to right, (C) control; (N) GO (2 mM); (1) GO + M. pudica (1 μg/ml); (5) GO + M. pudica (5 μg/ml); (10) GO + M. pudica (10 μg/ml); and (P) GO + AG (1 mM). The presentation of bar values is mean ± SD of three independent experiments (###p < 0.001 vs. MGO treatment only and ***p < 0.001 vs. positive control). [Click here to view] |
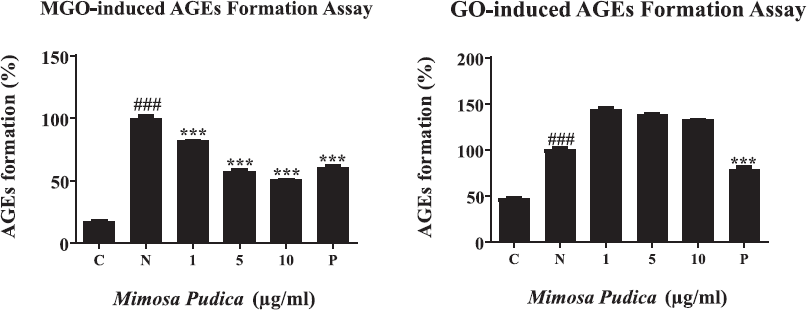 | Figure 3. (A) The AGE-breaking capability of M. pudica ethanol extracts was investigated by the number of free amines following the MGO-BSA reaction. From left to right, (N) MGO-BSA (1 μg/ml); (0.1) MGO-BSA M. pudica (0.1 μg/ml); (0.5) MGO-BSA + M. pudica (0.5 μg/ml); (1) MGO-BSA + M. pudica (1 μg/ml); (P) MGO-BSA + AG (1 mM). (B). The AGE-breaking capability of M. pudica ethanol extracts was investigated by the number of free amines following the GO-BSA reaction. From left to right, (N) GO-BSA (1 μg/ml); (0.1) GO-BSA + M. pudica (0.1 μg/ml); (0.5) GO-BSA + MP (0.5 μg/ml); (1) GO-BSA + M. pudica (1 μg/ml); (P) GO-BSA + AG (1 mM). [Click here to view] |
The progression of AGE production and accumulation is observed in the normal aging process as well as diabetes with an accelerated rate. It is confirmed that the interaction between AGEs and receptor for advanced glycation end products (RAGE) plays an important role in the development of diabetic vascular complications due to the generation of oxidative stress in various cell types associated with stimulation of vascular inflammation, thrombosis, and platelet activation. Also, AGEs may be responsible for fibrin stabilization and platelet aggregation, leading to a predisposition to thrombogenesis, and thus promoting retinopathy in diabetes. Furthermore, the accumulation of AGEs such as pentosidine, malondialdehyde, lysine, and carboxymethyl lysine (CML) in thickened glomerular basement membranes, extended mesangial matrix, and nodular lesions of the advanced disease leads to the remarkable effects of AGEs on diabetic nephropathy (Yamagishi, 2011). As expected, the treatment with M. pudica inhibited formation as well as exhibited breaking ability to AGE-MGO and AGE-GO. Glycation induced by MGO and GO leads to reduced free amines, which can break down AGEs, and hence, these results were found by estimating a significant increase in free amines (Do et al., 2017). The remarkable effects of M. pudica on AGE target contribute to explain the molecular mechanism of the antidiabetic property of this plant in this study.
Exploration of M. pudica major phytoconstituents was conducted by using Liquid chromatography (LC)/mass spectrometry (MS) method, and ethanol extract was tested for in vitro study. The existence of many bioactive compounds such as ferulic acid, apigenin, catechin, caffeic acid, naringenin, and quercetin may be responsible for M. pudica’s inhibitory effects on glucotoxicity and AGE formation and accumulation. Ferulic acid, a derivative of cinnamic acid, is well-known for its anti-inflammatory effects and ability to act as AGE inhibitor. Ferulic acid reduces AGEs and is linked to protein carbonyl content, CML levels, amyloid cross β-structure, and fructosamine. Hence, ferulic acid is considered an effective agent against oxidative stress and protein glycation related to preventing pathologies mediated by AGEs in diabetic complications (Dariya and Nagaraju, 2020; Sompong et al., 2013). It is found that apigenin has the ability to form AGEs by directly trapping MGO and then generating apigenin-MGO adducts. Apigenin often inhibits the formation of reactive oxygen species and suppresses the expression of adhesion molecules and proinflammatory cytokines, preventing inflammation and oxidative stress induced by AGEs in HUVECs. Apigenin’s defensive mechanism may be based on suppressing the extracellular-signal-regulated kinase 1/2 (ERK)/transcription factor kappa-light-chain-enhancer of activated B cells signal transduction pathway, which is activated by the AGE-RAGE interaction, as well as inducing the ERK/transcription factor (erythroid-derived 2)-like 2 pathway, which leads to upregulation of antioxidant protection molecules (Zhou et al., 2019). The treatment with catechin results in significant enhancement of renal dysfunction in type 2 diabetic mouse model via a decrease in AGE formation and proinflammatory cytokines because of MGO trapping.
Moreover, human endothelium-derived cells under high glucose levels treated by catechin exhibited cellular signaling suppression and MGO trapping (Zhu et al., 2014). Additionally, it has been demonstrated that the treatment by caffeic acid and naringenin combined with other components significantly inhibited the formation of AGEs (Do et al., 2017; Gugliucci et al., 2009). Also, quercetin has the inhibitory ability to AGE formation by simultaneous MGO and GO trapping. The six and eight regions of the polyphenol A-ring are shown to be responsible for MGO trapping and the splitting of AGEs (Do et al., 2017; Li et al., 2014). Furthermore, the BSA-MGO system treated by quercetin was presented in AGE inhibitory effect; meanwhile, BSA-GO system did not exhibit this effect. The findings may be because MGO, not GO, is the main dicarbonyl compound responsible for albumin glycation. The glycation was slowed by the conversion of hydrated monomer, dimer, and trimer to free GO (Li et al., 2014). Besides, the effectiveness of bioactive compounds identified from M. pudica extracts that may reduce glucotoxicity caused by MGO needs to be investigated. Mimosa pudica extract has shown the practical ability to prevent diabetic complications caused by MGO-associated endothelial cellular dysfunction.
CONCLUSION
Our study has identified some bioactive compounds in M. pudica such as 2-tert-butyl-2-phenyl-1,3-dioxolane, 4-(2-phenylethyl)-phenol, 4-phenylbutan-2-ol, 1-naphthalenecarboxylic acid, ferulic acid, myoinositol, caffeic acid, tyrosinamide, 2-hydroxy-benzene ethanol, p-hydroxybenzoic acid, luteolin, fisetin, apigenin, gallic acid, quercetin, monoamidomalonic acid, jasmonic acid, 3-fluoro-p-anisidine, and naringenin. We also showed that M. pudica ethanol extract may reduce glucotoxicity and GO- and MGO-induced metabolic dysfunction associated with AGE target in HUVECs. M. pudica ethanol extract can be considered a promising supplement for the treatment and prevention of endothelial dysfunction caused by GO and MGO. A detailed mechanism of action of M. pudica ethanol extract against the GO- and MGO-induced metabolic dysfunction should be conducted in future.
ACKNOWLEDGMENTS
This research has been done under the research project QG 21.52, “Study the ameliorates effects of Mimosa pudica Linn. leaves extract on streptozotocin-induced diabetic type 2 in mice” of Vietnam National University, Hanoi.
LIST OF ABBREVIATIONS
AGEs, advanced glycation end products; BSA, bovine serum albumin; CML, carboxymethyl lysine; ERK, extracellular-signal-regulated kinase; GO, glyoxal; HUVECs, human umbilical vein endothelial cells; LC-MS, liquid chromatography in tandem with mass spectrometry; MGO, methylglyoxal; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RAGE, receptor for advanced glycation end products; TNBS, trinitrobenzene sulfonate.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Not applicable.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abramson CI, Chicas-Mosier AM. Learning in plants: lessons from Mimosa pudica. Front Psychol, 2016; 7:417. CrossRef
Antony PJ, Gandhi GR, Stalin A, Balakrishna K, Toppo E, Sivasankaran K, Ignacimuthu S, Al-Dhabi NA. Myoinositol ameliorates high-fat diet and streptozotocin-induced diabetes in rats through promoting insulin receptor signaling. Biomed Pharmacother, 2017; 88:1098–113. CrossRef
Bashir R, Aslam B, Javed I, Muhammad F, Sarfraz M, Fayyaz A. Antidiabetic efficacy of Mimosa pudica (Lajwanti) root in albino rabbits. Int J Agric Biol, 2013; 15(4):782–6.
Bellier J, Nokin MJ, Lardé E, Karoyan P, Peulen O, Castronovo V, Bellahcène A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res Clin Pract, 2019; 148:200–11. CrossRef
Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol, 2012; 364(1–2):1–27. CrossRef
Dariya B, Nagaraju GP. Advanced glycation end products in diabetes, cancer and phytochemical therapy. Drug Discov Today, 2020; 25(9):1614–23. CrossRef
Do MH, Lee JH, Wahedi HM, Pak C, Lee CH, Yeo EJ, Lim Y, Ha SK, Choi I, Kim SY. Lespedeza bicolor ameliorates endothelial dysfunction induced by methylglyoxal glucotoxicity. Phytomedicine, 2017; 36:26–36. CrossRef
Figarola JL, Singhal J, Rahbar S, Awasthi S and Singhal SS. LR-90 prevents methylglyoxal-induced oxidative stress and apoptosis in human endothelial cells. Apoptosis, 2014; 19(5): 776-788. CrossRef
Furlani RE, Richardson MA, Podell BK, Ackart DF, Haugen JD, Melander RJ, Basaraba RJ and Melander C. Second generation 2-aminoimidazole based advanced glycation end product inhibitors and breakers. Bioorganic & medicinal chemistry letters, 2015; 25(21): 4820-4823. CrossRef
Gugliucci A, Bastos DHM, Schulze J, Souza MFF. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia, 2009; 80(6):339–44. CrossRef
Ijaz S, Khan HMS, Anwar Z, Talbot B, Walsh JJ. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed Pharmacother, 2019; 109:865–75. CrossRef
Joseph B, George J, Mohan J. Pharmacology and traditional uses of Mimosa pudica. Int J Pharm Sci Drug Res, 2013; 5(2):41–4.
Kiho T, Kato M, Usui S, Hirano K. Effect of buformin and metformin on formation of advanced glycation end products by methylglyoxal. Clin Chim Acta, 2005; 358(1–2):139–45. CrossRef
Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, Laakso M. Increased serum levels of methylglyoxal-derived hydroimidazolone-AGE are associated with increased cardiovascular disease mortality in nondiabetic women. Atherosclerosis, 2009; 205(2):590–4. CrossRef
Li X, Zheng T, Sang S, Lv L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J Agric Food Chem, 2014; 62(50):12152–8. CrossRef
Manosroi J, Moses ZZ, Manosroi W, Manosroi A. Hypoglycemic activity of Thai medicinal plants selected from the Thai/Lanna medicinal recipe database MANOSROI II. J Ethnopharmacol, 2011; 138(1):92–8. CrossRef
Parasuraman S, Ching TH, Leong CH, Banik U. Antidiabetic and antihyperlipidemic effects of a methanolic extract of Mimosa pudica (Fabaceae) in diabetic rats. Egypt J Basic Appl Sci, 2019; 6(1):137–48. CrossRef
Patel NK, Bhutani KK. Suppressive effects of Mimosa pudica (L.) constituents on the production of LPS-induced pro-inflammatory mediators. EXCLI J, 2014; 13:1011.
Peppa M, Vlassara H. Advanced glycation end products and diabetic complications: a general overview. Hormones (Athens), 2005; 4(1):28–37. CrossRef
Rajendiran D, Kandaswamy S, Sivanesan S, Radhakrishnan S, Gunasekaran K. Potential antidiabetic effect of Mimosa pudica leaves extract in high fat diet and low dose streptozotocin-induced type 2 diabetic rats. Int J Biol Res, 2017; 2(4):55–62.
Rajendiran D, Khan HBH, Packirisamy S, Gunasekaran K. Dose dependent antidiabetic effect of Mimosa pudica leaves extract in type 2 diabetic rat model. Pharma Innov J, 2019; 8:1–4.
Schalkwijk C, Stehouwer C. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev, 2020; 100(1):407–61. CrossRef
Sompong W, Meeprom A, Cheng H, Adisakwattana S. A comparative study of ferulic acid on different monosaccharide-mediated protein glycation and oxidative damage in bovine serum albumin. Molecules, 2013; 18(11):13886–903. CrossRef
Tasnuva S, Qamar U, Ghafoor K, Sahena F, Jahurul M, Rukshana A, Juliana M, Al-Juhaimi FY, Jalifah L, Jalal K. α-glucosidase inhibitors isolated from Mimosa pudica L. Nat Prod Res, 2019; 33(10):1495–9. CrossRef
Tunna T, Zaidul I, Ahmed Q, Ghafoor K, Al-Juhaimi F, Uddin M, Hasan M, Ferdous S. Analyses and profiling of extract and fractions of neglected weed Mimosa pudica Linn. traditionally used in Southeast Asia to treat diabetes. S Afr J Bot, 2015; 99:144–52. CrossRef
Tunna TS, Ahmed QU, Uddin A, Sarker M, Islam Z. (2014). Weeds as alternative useful medicinal source: Mimosa pudica Linn. on diabetes mellitus and its complications. Adv Mat Res.
Yamagishi SI. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol, 2011; 46(4):217–24. CrossRef
Yamagishi SI, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev, 2010; 3(2):101–8. CrossRef
Zhou Q, Cheng KW, Gong J, Li ET, Wang M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem Pharmacol, 2019; 166:231–41. CrossRef
Zhu D, Wang L, Zhou Q, Yan S, Li Z, Sheng J, Zhang W. (+)s methylglyoxal-adduct inhibit advancedthy by trapping methylglyoxal in type 2 diabetic mice. Mol Nutr Food Res, 2014; 58(12):2249–60. CrossRef