INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is an advancing pulmonary disorder distinguished by diminished airflow and enhanced chronic inflammatory reaction in the airways. The goal of COPD drug therapy is to diminish symptoms, boost exercise resistance and health condition, and reduce exacerbation recurrence. Long acting beta2 agonists (LABA), long-acting muscarinic antagonists (LAMA), and inhaled corticosteroids (ICS) remain the extensively used therapeutic options for COPD (Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2020). Inhaled triple therapy containing (ICS/LAMA/LABA) can improve pulmonary function, symptoms, and health status, and can reduce exacerbations compared with dual therapy (LAMA/LABA or ICS/LABA) (Ferguson et al., 2018; Lipson et al., 2017, 2018).
COPD costs colossal expenditures through drugs, clinician visits, hospitalizations, and emergency room visits and adversely affects the patient’s quality of life (Ehteshami-Afshar et al., 2016; Lopez-Campos et al., 2016). Various factors may affect the healthcare costs in COPD including but not limited to symptom severity and frequency and nature of exacerbations (Blasi et al., 2014; Iheanacho et al., 2020; Rutten-van Mölken and Lee, 2006; Toy et al., 2010). Apart from its economic strain, COPD decreases patient’s overall health status and negatively impacts the quality of life owing to high levels of anxiety and depression (Soler-Cataluna et al., 2016).
Cost-effectiveness analysis (CEA) is a kind of monetary evaluation that differentiates relative costs and outcomes (effects) of different therapeutic approaches. CEA is presented as a ratio where the numerator is the cost associated with the therapeutic approach and the denominator is health gain associated with the therapeutic approach. The most commonly used outcome measure of CEA is quality-adjusted life year (QALY). Frequently, CEA guides treatment decisions on the choice of therapeutic approaches for COPD patients. CEA aids stakeholders discover the way how clinical benefit observed in research translates into the quality of life and whether the additional costs justify the additional benefits as opposed to emphasizing costs alone (Drummond and Jefferson, 1996).
Numerous studies have been performed across the globe to evaluate the cost-effectiveness of triple therapy (ICS/LAMA/LABA) versus dual therapy (LAMA/LABA or ICS/LABA) (Driessen et al., 2018; Ismaila et al., 2019; Schroeder et al., 2019, 2020). To the authors’ knowledge, no previous meta-analysis has been performed evaluating the cost-effectiveness of triple therapy (ICS/LAMA/LABA) versus dual therapy (LAMA/LABA or ICS/LABA). Thus, the aim of the present study was to meta-analyze the cost-effectiveness of triple therapy (ICS/LAMA/LABA) versus dual therapy (LAMA/LABA or ICS/LABA) from the United States (US) National Healthcare System (NHS) perspective.
MATERIALS AND METHODS
Search strategy
Medline and Scopus databases were hand checked to locate relevant economic studies evaluating triple therapy (ICS/LAMA/LABA) versus dual therapy (LAMA/LABA or ICS/LABA). The search was conducted to include all relevant papers from 2011 (January) to 2021 (January). Search strategy used to find the relevant articles in the PubMed database is as follows:
#1 (COPD[Title]) OR (“chronic obstructive pulmonary disease”[Title]))
#2 (“cost-effectiveness”[Title]) OR (“cost-utility”[Title]) OR (“economic evaluation”[Title])
#3 #1 AND #2
The data were extracted individually by two reviewers based on the following inclusion criteria:
Inclusion criteria
- Economic studies evaluating triple therapy (ICS/LAMA/LABA) versus dual therapy (LAMA/LABA or ICS/LABA).
- Economic studies which are designed as cost-effectiveness or cost-utility analysis.
- Patients with moderate to severe COPD.
- English Language publications.
- Only original research papers.
- Full text available.
The discrepancies between the two reviewers were resolved by the third reviewer. Titles and abstracts were first reviewed followed by a full-text review of papers comparing triple therapy versus dual therapy. All the studies included in this meta-analysis were model-analysis of already published randomized clinical trials. Costs, QALYs, Life years (LY’s), net monetary benefit (NMB), and net health benefit (NHB) were used as evaluation parameters. The present study was done in agreement with the preferred reporting items for systematic reviews and meta-analyses guidelines (Moher et al., 2015).
Data extraction
Data collection was done taking into consideration Consolidated Health Economic Evaluation Reporting Standard checklist (Husereau et al., 2013). Extracted data included baseline characteristics of the study population, intervention and comparator drug, target population, country, modeling approach, time horizon, discounting, perspective of study, sensitivity analysis, most sensitive parameter, funding, costs, effectiveness (QALY/LY), incremental cost-effectiveness ratio (ICER), and Exacerbations.
Data analysis
To standardize costing data, all costs were converted and presented as annual costs in the year 2020 US dollars ($). As none of the included studies reported standard deviation (SD) of the costs and utility values, we made use of sample size, 95% confidence interval (CI), and standard error to derive the SD. Alternatively, Revman software calculators were also used for calculating the SD (Higgins et al., 2021).
Comparative efficiency research provides a way for meta-analyzing cost-effectiveness studies by pooling incremental net benefits (INB) (Crespo et al., 2014). Net Benefit Calculations combine cost, effectiveness, and willingness to pay (WTP) into a single value. INMB = (E2−E1) * WTP – (C2−C1)
INHB = (E2−E1) – (C2−C1)/WTP
Where E2 and E1 are the effectiveness of the two strategies
and C2 and C1 are the costs of the two strategies. The optimal strategy will have the highest Net Benefit value. The new treatment is said to be cost-effective if the INB is positive (Stinnett, 1998; Willan, 2004).
Costs per QALY, costs per LY, and ICER were used to evaluate cost-effectiveness. If the new treatment is more costly and less effective than the standard treatment, it is said to be dominated. If it is less costly and more effective, it is said to be dominant. However, if the new treatment is more costly and more effective, it is said to be cost-effective if ICER is less than the WTP for that particular country. Error Propagation methods were applied to costs and utility values to obtain the SD of cost-effectiveness (Taylor, 1997). The ICER was calculated by the formula given below:
ICER = (C2−C1)/(E2−E1)
Where E2 and E1 are the effectiveness of the two strategies and C2 and C1 are the costs of the two strategies. ICER represents the average incremental cost associated with one additional unit of the measure of effect. Costs are estimated in monetary units whereas effectiveness is measured in health status values (McFarlane and Bayoumi, 2004) in this study the QALY and LY gained.
Statistical analysis
As all the outcomes in this study were continuous, standardized mean differences (SMD) with its 95% CI were used to present the comparison between triple therapy and dual therapy. The random-effects model was used because the incorporated studies had variations in geographical location and population characteristics. Statistical heterogeneity between trials was assessed using the I2 statistic, with I2 values greater than 50% indicating significant heterogeneity between studies. Variation in the studies was evaluated using Meta-regression analysis. Data were analyzed using RevMan v5.3 software and Comprehensive Meta-analysis v3 software.
RESULTS AND DISCUSSION
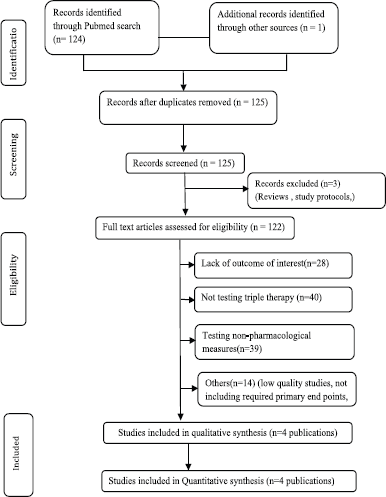
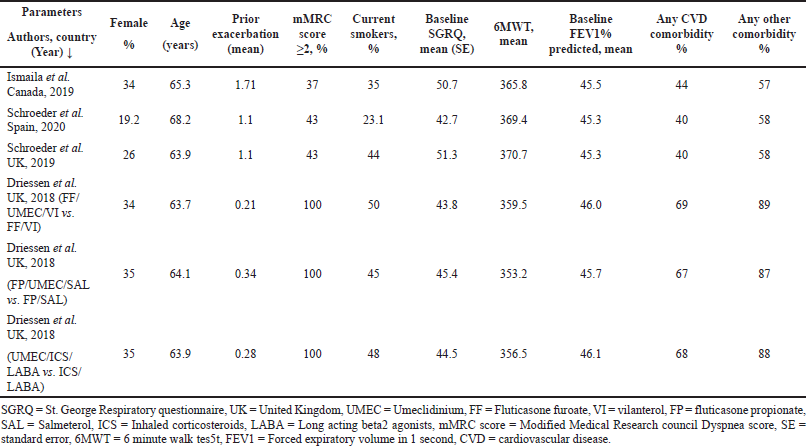
A comprehensive literature search resulted in 125 research articles considering cost-effectiveness in COPD patients. Following meticulous assessment by the reviewers, four model analyses published between 2011 and 2021 were included in this meta-analysis based on the inclusion criteria. A summary of the study selection procedure is presented in Figure 1. Study baseline characteristics used as model inputs in the model analysis are given in Table 1.
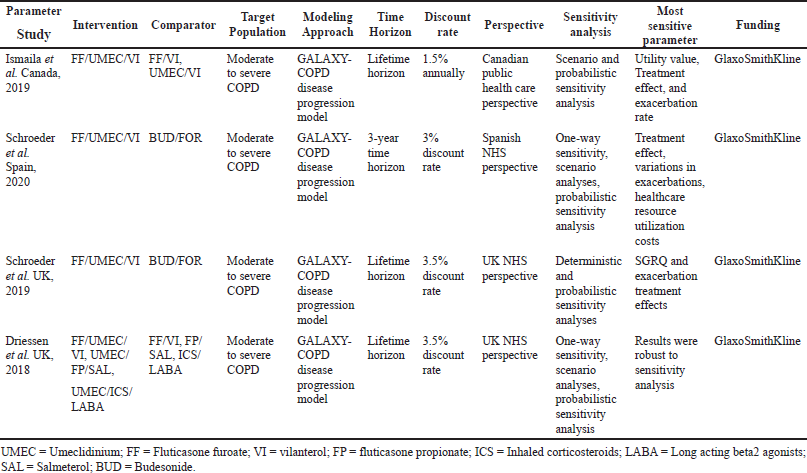
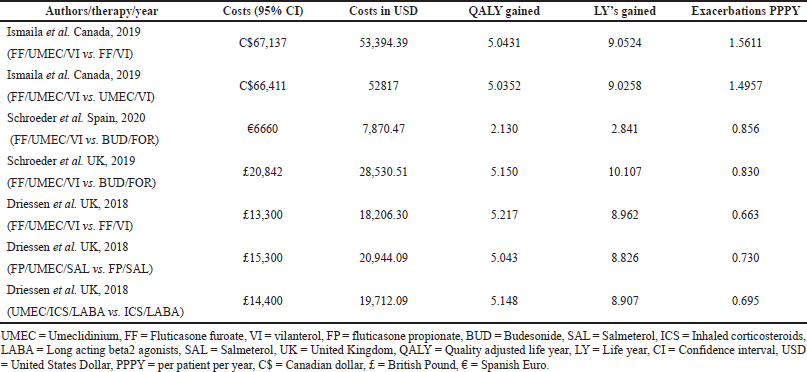
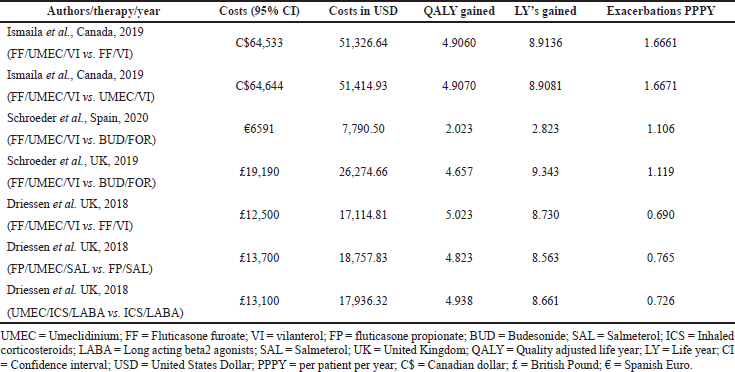
In the current meta-analysis, four new articles presented the cost-effectiveness of triple therapy (LABA/LAMA/ICS) versus dual therapy (LABA/LAMA or ICS/LABA) (Tables 2–5). Two studies were conducted from the UK National Health service perspective while the remaining two were conducted from the Spain National Healthcare system and Canadian public healthcare perspective. In general, all the studies were performed from the National healthcare system perspective.
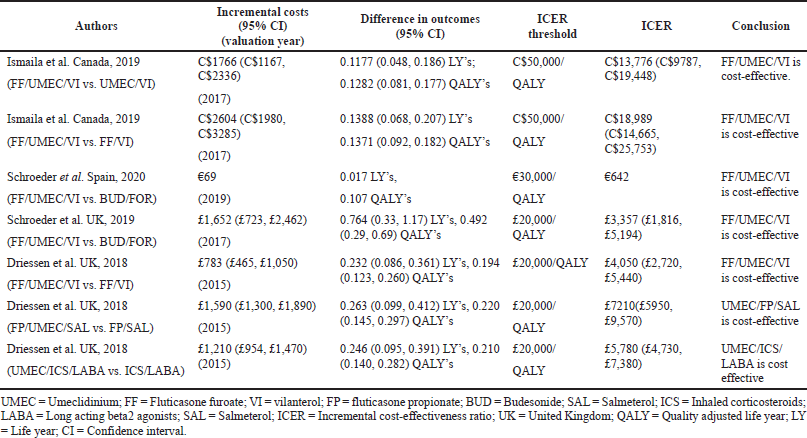
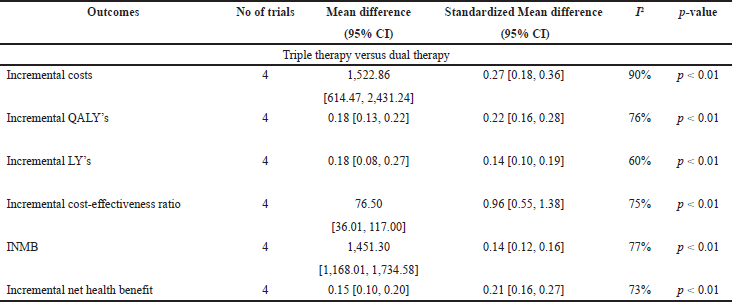
The ICS evaluated were Fluticasone furoate, Budesonide and Fluticasone propionate, LAMA was Umeclidinium, and LABA were Vilanterol, Salmeterol, and formoterol. All the studies were model analyses of previously published clinical trial data, were sponsored by the pharmaceutical company, and used QALY/LY as an outcome measure for effectiveness analysis. One study (Ismaila et al., 2019) used IMPACT trial data (Lipson et al., 2018), the other two (Schroeder et al., 2019, 2020) used FULFILL trial data (Lipson et al., 2017), and the fourth study (Driessen et al., 2018) used data from three different trials (Siler et al., 2015, 2016; Sousa et al., 2016). In all the four studies, triple therapy (ICS/LABA/LAMA) was found to be cost-effective than dual therapy (LABA/LAMA and ICS/LABA) as the ICER remained below the WTP threshold of that particular country.
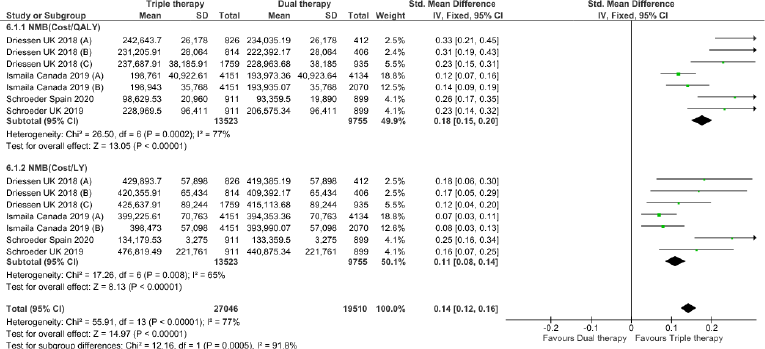
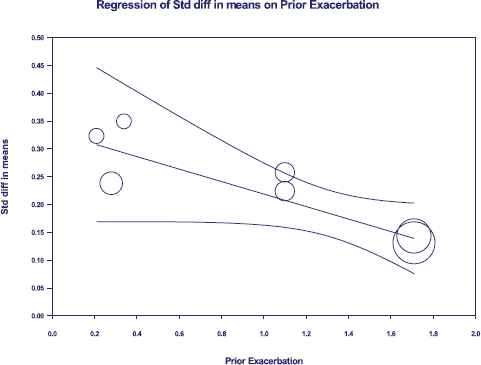
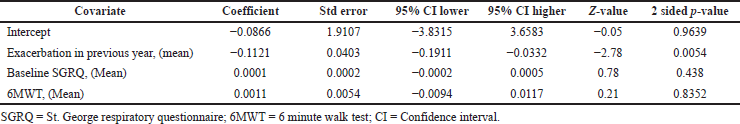
Triple therapy demonstrated higher costs compared with dual therapy (SMD: 0.27; 95% CI: 0.18, 0.36) which was statistically significant (Fig. 2). Meta-analysis of QALY revealed greater utility associated with triple therapy compared to dual therapy (SMD: 0.22; 95% CI: 0.16, 0.28) (Fig. 3). Triple therapy resulted in greater LY gained compared to dual therapy (SMD: 0.14; 95% CI: 0.10, 0.19) (Fig. 4). The cost-effectiveness of triple therapy was better than dual therapy as the ICER was well below the WTP (SMD: 0.96; 95% CI: 0.55, 1.38) (Fig. 5). INMB revealed NMB associated with triple therapy was higher than dual therapy (SMD: 0.14; 95% CI: 0.12, 0.16) (Fig. 6). Similarly, triple therapy resulted in more NHB than dual therapy as evidenced from positive INHB (SMD: 0.17; 95% CI: 0.13, 0.21) (Fig. 7).Meta-regression analysis was performed to evaluate the effect of baseline variables that may act as potential effect modifiers for additional QALY gain from triple therapy versus dual therapy. These variables included previous exacerbations in 1 year, baseline St. George Respiratory Questionnaire (SGRQ), and 6-minute walk test (6MWT). Among all the included variables, only exacerbations in the previous year significantly negatively impacted QALY (p < 0.01) (Fig. 8 and Table 6). The summary of findings is presented in Table 7.
This study is the first to meta-analyze the cost-effectiveness of triple therapy versus dual therapy from the US National healthcare system perspective. This meta-analysis revealed higher gains in QALY, LY, NMB, and NHB with triple therapy compared to dual therapy. Although cost was more with triple therapy, this was offset by the benefits in QALY and LY than dual therapy. This was evidenced by the fact that ICER was cost-effective well below the WTP in favor of triple therapy when both QALY and LY were taken into account. However, the subtleness of QALY and LY gain from triple therapy should make stakeholders think about the higher costs associated with triple therapy especially when adverse events decrease the QALY and increase the costs of triple therapy (Lopez-Campos et al., 2018; Suissa and Drazen, 2018).
Costs of triple therapy were higher compared to both ICS/LABA and LABA/LAMA dual therapies because of the inclusion of an extra third drug in the triple therapy. This was balanced by lower non-drug costs associated with triple therapy owing to a lesser number of exacerbations and hospital days compared to LABA/LAMA dual therapy. When triple therapy is compared to ICS/LABA dual therapy, non-drug costs were higher for triple therapy not only because the exacerbation benefit is shared by both ICS/LABA dual therapy and triple therapy but also because triple therapy resulted in higher forced expiratory volume in 1 second (FEV1) benefit, prolonged survival, and the resultant increase in the utilization of healthcare resources (Ismaila et al., 2019).
In the included studies, cost-effectiveness was sensitive to treatment effects, utility scores, healthcare costs, and SGRQ scores. Various reasons may be attributed to the heterogeneous nature of these parameters. The frequency and severity of exacerbations and resultant hospitalizations are greatly affected by patient characteristics and disease conditions between countries (McGarvey et al., 2015). All the included studies were manufacturer funded and applied economic modeling approach to existing clinical trial data to generate results which may be a source of uncertainty in the sensitivity analysis. The cost of treating exacerbations has wide variations in different countries depending upon the gross domestic product and per capita income of the country. Nevertheless, as the ICER of the present study falls under the Willingness to pay of US$50,000/QALY, which is generally described as cost-effectiveness threshold in the US (Dubois, 2016), results of this study justify the excess costs of triple therapy as they are balanced by the added utility benefits with triple therapy in moderate to severe symptomatic COPD patients.
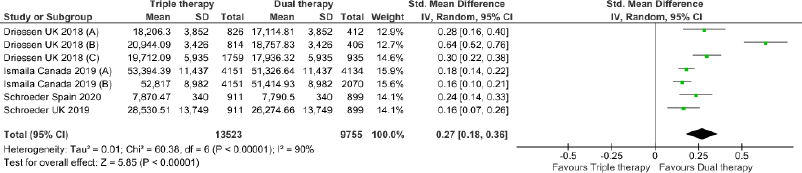 | Figure 2. Comparison of cost between triple therapy and dual therapy in COPD patients.
[Click here to view] |
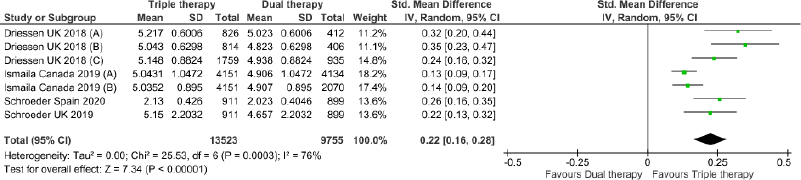 | Figure 3. Comparison of QALY between triple therapy and dual therapy in COPD patients.
[Click here to view] |
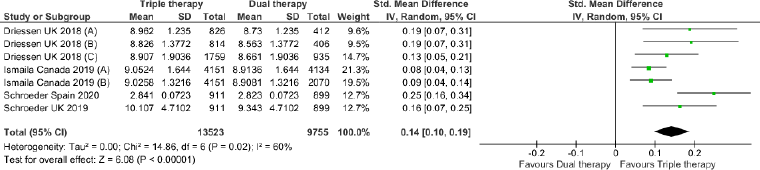 | Figure 4. Comparison of LY between triple therapy and dual therapy in COPD patients.
[Click here to view] |
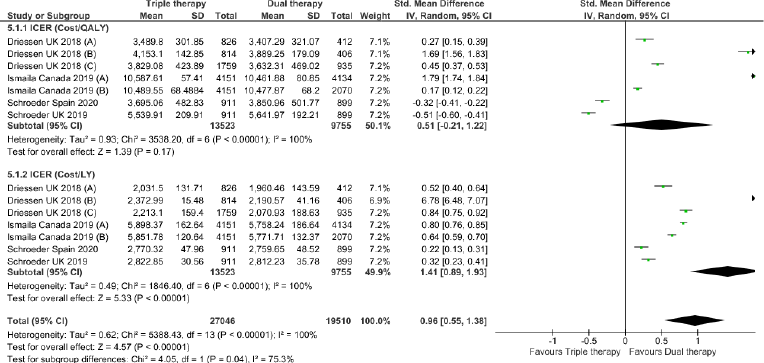 | Figure 5. Incremental cost-effectiveness ratio of triple versus dual therapy in COPD patients.
[Click here to view] |
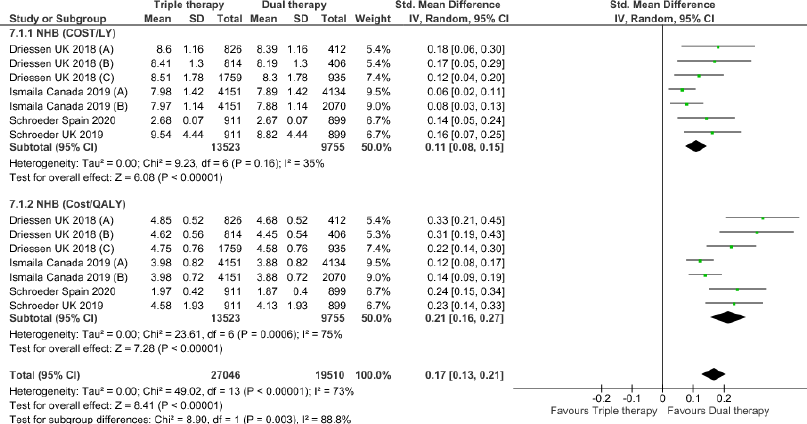 | Figure 7. Incremental Net Health Benefit (INHB) of triple versus dual therapy in COPD patients.
[Click here to view] |
As all the included studies were model analyses of clinical trial data, they did not include real-world effectiveness data. Treatment effect, discontinuation rates, and medication adherence were assumed to remain consistent over the lifetime horizon period. Sensitivity analysis and publication bias were not assessed due to the low number of available studies for meta-analysis. The majority of the values of baseline variables in the included studies were not available directly from clinical trial data and were derived from the risk equations of the Galaxy COPD disease progression model which may make the results of this meta-analysis uncertain. Taking into account the drawbacks of modeling approaches, future research on cost-effectiveness should put more emphasis on actual data from practical life effectiveness studies.
REFERENCES
Blasi F, Cesana G, Conti S, Chiodini V, Aliberti S, Fornari C, Mantovani LG. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One, 2014; 9(6):e101228. CrossRef
Crespo C, Monleon A, Díaz W, Ríos M. Comparative efficiency research (COMER): meta-analysis of cost-effectiveness studies. BMC Med Res Methodol, 2014; 14:139. CrossRef
Driessen M, Shah D, Risebrough N, Baker T, Naya I, Briggs A, Ismaila AS. Cost-effectiveness of umeclidinium as add-on to ICS/LABA therapy in COPD: a UK perspective. Respir Med, 2018; 145:130–7. CrossRef
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ economic evaluation working party. BMJ, 1996; 313:275–83. CrossRef
Dubois RW. Cost-effectiveness thresholds in the USA: are they coming? Are they already here? J Comp Eff Res, 2016; 5(1):9–11. CrossRef
Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis, 2016; 20(1):11–23. CrossRef
Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, Bourne E, Ballal S, Darken P, DeAngelis K, Aurivillius M, Dorinsky P, Reisner C. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a doubleblind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med, 2018; 6:747–58. CrossRef
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. Available via https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORTver1.0wms.pdf (Accessed 24 December 2020).
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.2. 2021. Available via www.training.cochrane.org/handbook (Accessed 10 March 2021)
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health, 2013; 16:231–50. CrossRef
Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis, 2020; 15:439–60. CrossRef
Ismaila AS, Risebrough N, Schroeder M, Shah D, Martin A, Goodall EC, Ndirangu K, Criner G, Dransfield M, Halpin DM, Han MK, Lomas DA. Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: the IMPACT trial. Int J Chron Obstruct Pulmon Dis, 2019; 14:2681–95. CrossRef
Lipson DA, Barnacle H, Birk R, Brealey N, Locantore N, Lomas DA, Ludwig-Sengpiel A, Mohindra R, Tabberer M, Zhu CQ, Pascoe SJ. FULFIL trial: once daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 2017; 196:438–46. CrossRef
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, Kilbride S, Lange P, Lomas DA, Martinez FJ, Singh D, Tabberer M, Wise RA, Pascoe SJ, IMPACT investigators. Once daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med, 2018; 378:1671–80. CrossRef
Lopez-Campos JL, Carrasco Hernández L, Muñoz X, Bustamante V, Barreiro E. Current controversies in the stepping up and stepping down of inhaled therapies for COPD at the patient level: stepping up and down COPD therapies. Respirology, 2018; 23(9):818–27. CrossRef
Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology, 2016; 21(1):14–23. CrossRef
McFarlane PA, Bayoumi AM. Acceptance and rejection: cost-effectiveness and the working nephrologist. Kidney Int, 2004; 66:1735–41. CrossRef
McGarvey L, Lee AJ, Roberts J, Gruffydd-Jones K, McKnight E, Haughney J. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med, 2015; 109(2):228–37. CrossRef
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev, 2015; 4:1. CrossRef
Rutten-van Mölken M, Lee TA. Economic modeling in chronic obstructive pulmonary disease. Proc Am Thorac Soc, 2006; 3(7):630–4. CrossRef
Schroeder M, Benjamin N, Atienza L, Biswas C, Martin A, Whalen JD, Izquierdo Alonso JL, Riesco Miranda JA, Soler-Cataluña JJ, Huerta A, Ismaila AS. Cost-effectiveness analysis of a once-daily single-inhaler triple therapy for patients with chronic obstructive pulmonary disease (COPD) using the FULFIL trial: a Spanish perspective. Int J Chron Obstruct Pulmon Dis, 2020; 15:1621–32. CrossRef
Schroeder M, Shah D, Risebrough N, Martin A, Zhang S, Ndirangu K, Briggs A, Ismaila AS. Cost-effectiveness analysis of a single-inhaler triple therapy for patients with advanced chronic obstructive pulmonary disease (COPD) using the FULFIL trial: a UK perspective. Respir Med X, 2019; 1:100008. CrossRef
Siler TM, Kerwin E, Singletary K, Brooks J, Church A. Efficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomized, double-blind studies. COPD, 2016; 13:1–10. CrossRef
Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studies. Respir Med, 2015; 109:1155–63. CrossRef
Soler-Cataluna JJ, Sauleda J, Valdes L, Marin P, Aguero R, Perez M, Miravitlles M. Prevalence and perception of 24-h symptom patterns in patients with stable chronic obstructive pulmonary disease in Spain. Arch Bronconeumol, 2016; 52(6):308–15. CrossRef
Sousa AR, Riley JH, Church A, Zhu CQ, Punekar YS, Fahy WA. The effect of umeclidinium added to inhaled corticosteroid/long-acting beta2-agonist in patients with symptomatic COPD: a randomised, double-blind, parallel group study. NPJ Prim Care Respir Med, 2016; 26:16031. CrossRef
Stinnett AA. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making, 1998; 18(2):S68–80. CrossRef
Suissa S, Drazen JM. Making sense of triple inhaled therapy for COPD. N Engl J Med, 2018; 378(18):1723–24. CrossRef
Taylor J. 1997. An introduction to error analysis: the study of uncertainties in physical measurements. Sausalito, CA: University Science Books.
Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD, 2010; 7(3):214–28. CrossRef
Willan AR. Incremental net benefit in the analysis of economic data from clinical trials, with application to the CADET-Hp trial. Eur J Gastroenterol Hepatol, 2004; 16:543–9. CrossRef