INTRODUCTION
Anxiety is a normal emotional response of defense against tensional factors that imply a certain degree of danger. It can be a psychiatric disorder, depending on the intensity and its impact on the activity of the person. Indeed, it is inserted normally as either a main symptom or annex with a wide variety of neuropathological illnesses (Fernando et al., 2017). Psychiatric ailment, sleep disorders, and convulsions, among other central nervous system (CNS) disorders characterize part of the utmost threats to public health. Besides, psychiatric disorders and subjective mental health actually are linked to common musculoskeletal diseases (Heikkinen et al., 2019). Depression has substantially increased the since 1990s and is the core cause of the global concern of nontransmissible illness, basically motivated by population growth and aging. Although depression problem is the foremost cause in both genders, the heavyweight of depression is 50% higher for females than males (Murray and Lopez, 1996). Negative consequences in afflicted individuals are represented by unpleasant, painful daily life, an elevated hazard for additional ailments and death by suicide. During this decade is estimated to converted into tenth greatest common reason of decease in adult individuals. (Krause et al., 2019). The epidemic of neurological diseases is anticipated to be even more troublesome and incapacitant, except if global actions are taken radically [World Health Organization (WHO), 2004].
Traditional medicine globally contributes to increasing the access of people to healthcare around the world. Nowadays, globally, health authorities have identified the need to develop a reliable and integrative procedure to healthcare that consent governments, healthcare physicians, and, most significantly, those users of healthcare services, to access a safe, respectful, cost-efficient, and effective way of traditional medicine with recognized quality, safety, and efficacy [World Health Organization (WHO), 2013].
Phoradendron bathyoryctum Eichler (Santalaceae) is a parasitic plant of many forest species such as Guajayvì (Cordia americana, Boraginaceae) and lapacho (Tabebuia impetiginosa). In Paraguayan popular medicine, this plant is empirically used alleging tonic activity on cardiovascular and the CNS (Gonzales Torrez, 1992). Pharmacological studies on P. bathyoryctum are scarce, and, in the consulted databases, not much information is found on CNS properties. However, other species of Phoradendron have been previously studied, showing gastroprotective activity (González, 2000), anti-HIV activity (Kashiwada, 1998), among others. The presence of tannins, saponins, flavonoids, and coumarins was determined as chemical components of the Phoradendron genus (González, 2000). In Argentinian popular medicine, Phoradendron liga Eichler (Gillies ex Hook. and Arn.) is used and is claimed to have hypotensive properties for treatment of feminine conditions (placenta delivery, menstruation, and wounds) (Suárez, 2019). In addition, the most recognized pharmacological usages are for its cardiovascular putative impact (Benigni et al., 1964; Wagner et al., 1986). Ethnopharmacological information had verified that P. pruinosum and P. liga are used by inhabitants of Argentina (northeast area) for treatment of cardiac affections and Phoradendron hieronymi for respiratory bronchospasm (Martínez Crovetto, 1981; Toursarkissian, 1980; Wagner et al., 1986). In addition, in vitro studies carried out by Jiménez-Estrada et al. (2013) with Phoradendron californicum showed slight antioxidant and antiproliferative activity.
As stated previously, pharmacotoxicological information is scarce and the popular use of P. bathyoryctum is intensive, which together conditioned an imperious requirement for pharmacological validation to gather evidence of its influence on gross or specific animal behavior. Consequently, the purpose of the study was to characterize the acute toxicity, influence on gross behavior, effect on barbiturates-induced hypnosis, and the influence on behavioral performance of mice subjected to elevated plus-maze (EPM) and FST after oral treatment with P. bathyoryctum.
MATERIALS AND METHODS
Plant material and extract preparation
Whole plant materials of P. bathyoryctum Eichler (CEPb) were collected from Luque, Paraguay, and authenticated, and a certificate sample was kept under code number CC&DI 1352 at the herbarium of Botany Department (Faculty of Chemical Sciences, National University of Asuncion). Fresh plant samples were dried in a shade environment protected from sunlight and then cut and ground until a fine powder was obtained, which was finally subjected to hydroethanolic extraction with a mixture of ethanol 70 and water 30 by the reflux method for 1 hour. The purpose of using a mixture of ethanol/water is to get a maximal extraction of compounds from the sample. The procedure was replicated three times, the resultant extracts were joined, and the solvent was removed under reduced pressure. Finally, the crude hydroethanolic extract of P. bathyoryctum (CEPb) was subjected to lyophilization to avoid the presence of ethanol in the extract and evading its influence on mice behaviors. Therefore, an ethanol-free extract powder was used for all pharmacological tests.
Animals
All pharmacological assays were assessed in Swiss albino mice (20–35 g) of both sexes gained from the Animal Center of Faculty of Medicine of the National University of Itapúa. The experimental room was maintained in a controlled illumination cycle of 12 hours light and 12 hours darkness, a temperature of 22°C–25°C, and 50%–60% of humidity. The animals were fed with commercial diets with an unrestricted opportunity to drinking water and all animals were fasted overnight, as standard procedure, before each experiment. All experimental techniques were implemented in compliance with international standards of animal protection. Prior to the experiments, the protocol was submitted, revised, and accepted by the institutional Ethical Committee in Scientific Research of the Faculty of Medicine on April 15, 2015 (CEI-002-15).
Drugs used in this study
Sodium chloride was purchased from Sigma Chemical Company (St. Louis, MO), Diazepam from Roche Pharmaceutical Co., Ltd. (Argentine), sodium pentobarbital from Abbott (Japan), and Clomipramine from Novartis Argentina S.A. (Argentine), which were obtained locally.
Acute toxicity assay
The fixed-dose procedure (FDP), presently included in the OECD Guide 425, was employed for acute toxicity evaluation (OECD, 2001, 2008; Stallard and Whitehead, 2004) using oral (p.o.) and intraperitoneal (i.p.) route. Male mice (20–35 g, b.w.) were treated with of CEPb in a stepwise procedure, using the FDP (500, 1,000, 2,000, and 3,000 mg/kg p.o., and 300, 600, 900, and 1,200 mg/kg i.p.) in groups of five mice searching lethality lethal dose (LD50). After treatments, animals were scrutinized for lethal response through the initial 24 hours and the following 14 days. Afterward, rodents were sacrificed and the thoracic and abdominal organs were dissected and carefully examined visually and also all matching organs, compared with those of the control group.
Gross behavior assay
Description of the general behavior of animals treated with CEPb was studied by individually locating mice within a transparent acrylic box in two different series. Groups of five female mice (20–35 g) received orally saline solution (0.1 ml/10 g of body weight), 500, 1,000, 2,000, and 3,000 mg/kg of CEPb and intraperitoneally saline solution, 300, 600, 900, and 1,200 mg/kg of CEPb, respectively. Observations were made during the first 3 hours and at 24, 48, 72 hours, and up to 14 days after the administration of CEPb. Central and peripheral behaviors were recorded (Irwin, 1968; Roux et al., 2005) such as spontaneous mobility, respiratory frequency, piloerection and exophthalmia, stereotyped movements, self-cleaning and caudal chewing behaviors, clonic seizures, tonic, fine, and strong tremor, sialorrhea, fasciculation, mydriasis, tail erection, tremor in the tail, pupillary dilation, sedation, catatonia, palpebral ptosis, analgesia, anesthesia, loss of the motor coordination reflex, ataxia, dyspnea, passivity, environmental alignment, dorsal tone decrease, and loss of blood pressure in an individual session.
Pentobarbital-induced hypnosis assay
Adult albino female mice (20–35 g) were arbitrarily assigned into different groups (6 animals per cage) and administered orally with doses of 1, 10, and 100 mg/kg of CEPb and saline (0.1 ml/10 g, b.w.). Besides, in a different experimental series, intraperitoneal doses of 1, 10, and 100 mg/kg of CEPb and saline (0.1 ml/10 g, b.w.) were dosed to different groups of mice (n = 6). A positive control group intraperitoneally received (0.5 mg/kg), a CNS depressant agent. Doses selected for pharmacological assays were 10 times lower than the maximal dose used in toxicological studies. One hour after dosing, sodium pentobarbital 35 mg/kg (i.p.) was injected into each animal. Accordingly, the induction time (loss of posture) and the sleep time in minutes (from loss of posture to awakening) were recorded for each mouse (Hellión-Ibarrola et al., 1999; Rodrigues and Carlini, 2005).
Elevated plus-maze (EPM) assay
Pellow et al. (1985, 1986) proposed the use of the EPM model to explore anxiety in rats. Correspondingly, it was validated by Lister (1987, 1990) and upgraded by File et al. (2004) for mice, consisting of two opposite open arms (30 × 5 × 25 cm) and two closed arms (30 × 5 × 25 cm), also opposed, in the form of a Greek cross. A central stage (5 × 5 cm) connects both the open and closed arms of the EPM. Transparent Plexiglas was used to make the platform, the lateral walls of the closed arms, while the base was made from black Plexiglas and finally the device was elevated to 45 cm above floor level with wooden support. The device was located in a room illuminated with a red light (15 W) and, after each test, it was cleaned with tissue paper immersed in a 10% ethanol solution.
Groups of 10 male mice (18–30 g) were dosed with (a) CEPb (1, 10, and 100 mg/kg, p.o.), (b) vehicle (2.5% ethanol), and (c) Diazepam 0.5 mg/kg, i.p, in individual sequence, respectively. One hour after the treatments, the animals were placed individually on central platform facing an enclosed arm and their behavior was registered for 5 minutes. The group was treated with a nonsedative dose of Diazepam (positive anxiolytic control), located into EPM 20 minutes after i.p. treatment. The behavioral measures recorded in the EPM were the frequency of entrances and the time employed in the arms (open and closed) and in the central platform. The total frequency of entrances was obtained from the simple sum of the input frequencies in the open arms and in the closed arms. For statistical analysis of the data and drawing of the graphs, the percentage of entries in the open arm was calculated by dividing the frequency of entry in the open arms by the total frequency of entries, and this index was multiplied by 100. In an equivalent way, the percentage of time that the animals remain in the open arms in relation to the sum of the time of permanence in the open and closed arms was calculated, and the quotient obtained was multiplied by 100.
Forced swimming test (FST or behavioral despair)
The experimental procedure initially described and improved by Porsolt et al. (2000) was used to measure possible antidepressant properties. Concisely, the animals treated 1 hours earlier were forced to swim individually for 6 minutes, in cylinders (Plexiglas of 25 cm high and 15 cm in diameter) filled until 15 cm of tap water (25°C). The total motionlessness duration (in seconds) was determined throughout the last 4 minutes of a single 6-minutes test session. Each mouse was judge to be motionless when it desists fighting and maintaining floating quietly in the water, making only the tiniest and essential motor activities to keep its head aside of water. Male albino mice (18–30 g) were arbitrarily allocated in five groups (10 animals/each) and placed in the laboratory environment, having unrestricted access to diet and drinking water. One hour before force swimming assay, groups of male mice were dosed orally with acute single doses of CEPb (1, 10, and 100 mg/kg) and vehicle (0.1 ml/10 g, b.w.), respectively. One group was treated intraperitoneally with Clomipramine (32 mg/kg) as antidepressant control. Afterward, animals treated 1 hours earlier were forced to swim individually in the same conditions and in corresponding timing. After each individual swimming session, the water was discharged and the cylinder was thoroughly cleaned up with tap water with a final cleaning with tissue paper embedded in 10% ethanol solution before freshwater was filled in the cylinder. Animals removed from the water were allowed to dry before returning to their respective cages.
Statistical analysis
The results were plotted as means ± standard deviation (SD), and statistical analysis of the data was made by analysis of variance, followed by Dunnett’s multiple comparison test using GraphPad Prism 7.0 software (GraphPad Software, Inc., San Diego, CA). Differences between experimental groups were esteemed to be statistically significant when probability level p was less than 0.05.
RESULTS AND DISCUSSION
Acute toxicity and effect on general behavior in mice
Doses of up to 1,200 mg/kg (i.p.) and up to 3,000 mg/kg (p.o.) of CEPb did not provoke lethality in mice throughout 24 hours or in the 14 successive observation days. As a consequence, the LD50 of CEPb is considered superior to the doses previously mentioned. All animals were sacrificed and the thoracic and abdominal organs were examined by direct visual inspection and none of them denoted changes in color, size, and morphological conditions when compared to the control group. Doses higher than those mentioned above were not used because it is considered excessive and nonrational. Furthermore, a weak effect on the general behavior of mice submitted to the influence of CEPb was observed. Certainly, oral doses of 500 mg/kg provoked an increase in motility, exploratory, grooming behaviors, and respiratory rate, whereas 1,000, 2,000, and 3,000 mg/kg p.o. of CEPb provoked a decrease in motility and respiratory rate; also, increased piloerection, passivity, palpebral ptosis, tail erection, and grooming behaviors were observed. In addition, intraperitoneal doses of 300, 600, 900, and 1,200 mg/kg of CEPb decreased motility, exploratory behavior, and respiratory rate; besides, augmentations of piloerection, passivity, palpebral ptosis, tail erection, grooming, and abdominal contortions were denoted and are summarized in Table 1.
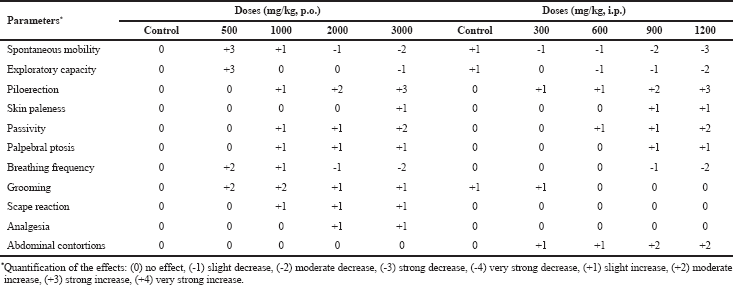 | Table 1. Influence of administration (p.o. and i.p.) of the hydroalcoholic extract of P. bathyoryctum (CEPb) on mice general behavior (n = 5). [Click here to view] |
There is no effect of the extract on mice behavioral parameters when compared to the vehicle group.
The outcomes of the present work show that the CEPb exhibits low toxicity, reflected by the nonexistence of lethality with doses tested by both oral and intraperitoneal routes. Besides, a weak effect on the general behavior of mice, submitted to a huge range of doses of CEPb, reflects a null modification of functions governed by the CNS accounting for a safe natural product.
Effect of CEPb on barbiturate-induced hypnosis in mice
Groups of animals treated orally (1, 10, and 100 mg/kg) and intraperitoneally (1, 10, and 100 mg/kg) with CEPb did not modify neither induction time (data are not showed) nor sleeping time of mice submitted to sodium pentobarbital injection (35 mg/kg, i.p.) in comparison with the group treated by vehicle (Figs. 1 and 2). As supposed, the positive depressant control (Diazepam 0.5 mg/kg i.p.) augmented the sleeping time (p.o.: 98.7 ± 6.1 minutes, p < 0.001, and i.p.: 99.2 ± 7.0 minutes, p < 0.001), in contrast with vehicle submitted group (p.o.: 46.4 ± 4.2 minutes and i.p.: 66.9 ± 6.3 minutes). This method is recognized as a useful technique to perceive drugs with depressant or stimulating action on brain functions (Rodrigues and Carlini, 2005). CEPb did not produce significant effects using two routes of administration in mice.
Effect of CEPb on mice behavioral performance in the EPM assay
Oral administration of CEPb in doses of 10 (50.3 ± 17, p < 0.01) and 100 mg/kg (65.7 ± 23.6, p < 0.001) denoted an increment (Fig. 3) in % of the open arm permanence in the EPM in a dose-dependent response in comparison to the vehicle-treated group (12 ± 8.5). Similarly, 0.5 mg/kg Diazepam (98.6 ± 7.0, p < 0.0001) provoked a substantial increase in behavioral performance in % of permanence in EPM. Besides, the % of entries in the open arm of EPM (Fig. 4) was incremented significantly with doses of 10 (61.5 ± 6.3, p < 0.001) and 100 mg/kg (54.5 ± 9.1, p < 0.01) in comparison to vehicle-treated group (32.2 ± 11.9). Likewise, the % of entries in the open arm of EPM was increased by 0.5 mg/kg Diazepam (91.25 ± 5.9, p < 0.0001) supporting the method used. Moreover, the performance in the enclosed arm denoted a decrease in the % of entries with a dose of 100 mg/kg of CEPb- (42.5 ± 6.0, p < 0.05) and Diazepam-treated groups (8.8 ± 6.0, p < 0.0001) as shown in Figure 5 and compared with the control group (67.8 ± 11.9). Figure 6 shows the significant decrease in the % permanence in the enclosed arm, in mice treated with oral doses of 10 (50 ± 13.9, p < 0.001) and 100 (35.8 ± 19.5, p < 0.00001) mg/kg of CEPb, compared to the performance of the group treated with the vehicle (88 ± 8.5). Instead, the administration of 1, 10, and 100 mg/kg p.o. of the CEPb in mice did not cause significant changes in the ethological parameters observed in the trial. Additionally, Diazepam (0.5 mg/kg i.p.) induced (2.0 ± 3.0) a clear anxiolytic-like effect as spectated from a positive control in comparison to the vehicle group. The lower dose was unable to denote a significant anxiolytic-like effect in mice submitted to EPM.
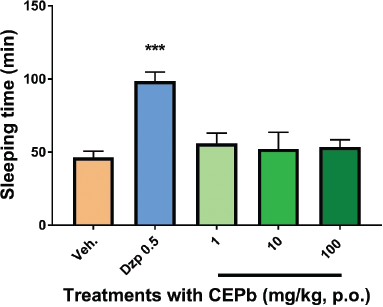 | Figure 1. Influence of oral administration of a single dose of vehicle (0.1 ml/10 g, b.w.), Diazepam (0.5 mg/Kg i.p.), and the respective dose of P. bathyoryctum (CEPb 1, 10, and 100 mg/kg p.o.) in the barbiturate-induced sleep in mice (n = 6). Each bar describes the means ± SD of the sleeping time. No significant effect was observed with all doses of CEPb used, whereas Diazepam (positive central depressant drug) increased the sleeping time (***p < 0.0001). [Click here to view] |
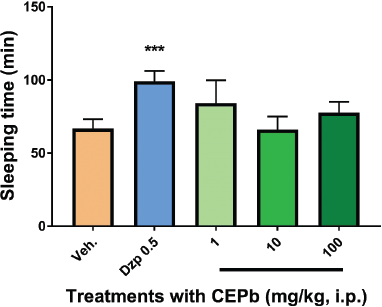 | Figure 2. Influence of a single intraperitoneal dose of vehicle (0.1 ml/10g, b.w.), Diazepam (0.5 mg/Kg, i.p.), and the respective dose of P. bathyoryctum (CEPb 1, 10, and 100 mg/kg, i.p.) in the barbiturate-induced sleep in mice (n = 6). Each bar describes the means ± SD of sleeping time. No significant effect was observed with all doses of CEPb used, whereas Diazepam (positive central depressant drug) increased the sleeping time (***p < 0.0001). [Click here to view] |
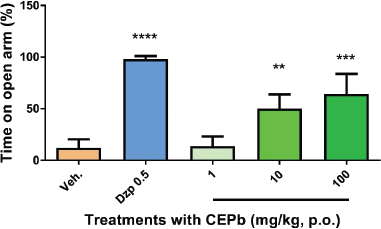 | Figure 3. Behavioral influence of single oral dose of vehicle (0.1 ml/10 g, b.w.), Diazepam (0.5 mg/kg i.p.), and the respective dose of P. bathyoryctum (CEPb 1, 10, and 100 mg/kg p.o.) registered from mice submitted in the EPM test (n = 10). Each bar describes the means ± SD of time consumed in the open arm. A significant increase in the % of time spent on the open arm was observed, with doses of 10 (**p < 0.001) and 100 mg/kg (***p < 0.0001) compatible with an anxiolytic-like effect. Diazepam was used as a positive anxiolytic agent (****p < 0.00001). [Click here to view] |
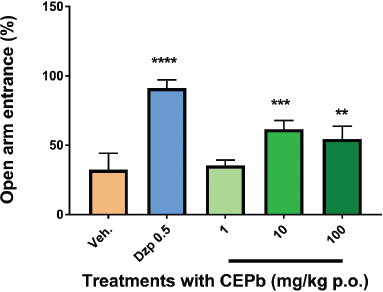 | Figure 4. Behavioral influence of single oral dose of vehicle (0.1 ml/10 g, b.w.), Diazepam (0.5 mg/kg i.p.), and the respective dose of P. bathyoryctum (CEPb 1, 10, and 100 mg/kg p.o.) registered from mice submitted in the EPM test (n = 10). Each bar describes the means ± SD of % of access in the open arm. A significant increase in the % of access in the open arm was observed, with doses of 10 (***p < 0.0001) and 100 mg/kg (**p < 0.001) compatible with an anxiolytic-like effect. Diazepam was used as a positive anxiolytic agent (****p < 0.00001). [Click here to view] |
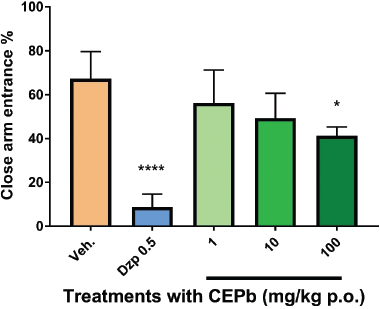 | Figure 5. Behavioral influence of single oral dose of vehicle (0.1 ml/10 g, b.w.), Diazepam (0.5 mg/kg i.p.), and the respective dose of P. bathyoryctum (CEPb 1, 10, and 100 mg/kg p.o.) registered from mice submitted in the EPM test (n = 10). Each bar describes the means ± SD of % close arm entries. A significant decrease in the % of close arm entrance was observed, with doses of 100 mg/kg (*p < 0.05) compatible with an anxiolytic-like effect. Diazepam was used as a positive anxiolytic agent (****p < 0.00001). [Click here to view] |
Benzodiazepines are psychoactive drugs of clinical use, which were initially subjected to psychopharmacological studies in animals, from which their differential benefits (sedatives, anxiolytics, antidepressants, and anticonvulsants) emerged, as well as adverse effects (File et al., 2004). The EPM was invented to deliver a behavioral scale of anxiety/antianxiety in rodents relatively free from the influence of a global motor activity. The technique has been broadly authenticated by Pellow et al. (1985, 1986) by means of behavioral, physiological, and pharmacological procedures. According to File et al. (2004), the EPM test depends on the intrinsic conflict between exploration of a novel area and avoidance of its unwilling features. Certainly, it is able to discriminate between anxiety (anxiogenic effects of drugs, drug withdrawal, and predator odor) and antianxiety (the anxiolytic effects of neurotoxic lesions of serotonergic neurons) behaviors provoked by specific experimental stimulus. The CEPb is effective as anxiolytic, demonstrated by the ability to provoke overwhelming behaviors (parameters in the open arm) in response to stimuli or anxiogenic factors presented in the EPM. Really, oral dosing of CEPb (10 and 100 mg/kg) denoted, for the first time, increment in both the permanence and the % of access in the open arm of EPM, in a dose-dependent response in comparison to control (vehicle) group indicating a clear antianxiety effect. Actually, the best measures of anxiety are consequences from analysis of the formal EPM component, where both the percentage of time consumed and the number of access onto the open arms are major parameters. Indeed, these indexes are increased by anxiolytic and reduced by anxiogenic drug treatments (Cryan and Mombereau, 2004; Cryan et al., 2002; File et al., 2004). Nevertheless, the best measure of locomotor activity is the number of closed arm access. Truly, a diminution in the % of entries and the permanence of animals in the enclosed arm induced by CEPb was denoted. Actually, the majority of the initial screening searching new anxiolytic agents from natural sources are submitted to the EPM test. Therefore, some examples of plants validated recently are Lippia citriodora (Razavi et al., 2017), Kyllinga brevifolia (Hellión-Ibarrola et al., 2012), and Aloysia polystachya (Hellión-Ibarrola et al., 2008) among others.
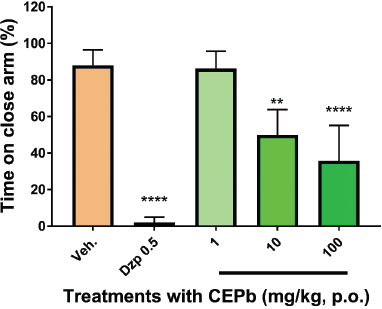 | Figure 6. Behavioral influence of single oral dose of vehicle (0.1 ml/10 g, b.w.), Diazepam (0.5 mg/kg i.p.), and the respective dose of P. bathyoryctum (CEPb 1, 10, and 100 mg/kg p.o.) registered from mice submitted in the EPM test (n = 10). Each bar describes the means ± SD of time spent in a close arm. Significant decrease in the % of time permanence was observed, with doses of 10 (**p < 0.001) and 100 mg/kg (****p < 0.00001) compatible with an anxiolytic-like effect. Diazepam was used as a positive anxiolytic agent (****p < 0.00001). [Click here to view] |
Effect of CEPb on behavioral performance of mice in the FST
Single oral doses of CEPb (110 mg/kg, 5.6 ± 9.6, p < 0.0001, and 10 mg/kg, 19.15 ± 14.8, p < 0.0001) were provided to different groups of male mice 1 hour before the test, and caused a statistically significant reduction in the immobility time in contrast to the control (76.3 ± 25.9) as shown in Figure 7. As spectated, the behavioral profile of Clomipramine (5.5 ± 4.2, p < 0.0001) in FST denoted a compatible antidepressant-like effect, thus validating the method used. It is important to denote that a higher dose has no influence on the immobility time of animals submitted to FST.
Certainly, CEPb is effective as an antidepressant, considering its ability to provoke continuous fighting or escape behavior in the FST in a similar intensity of the positive antidepressant control Clomipramine. Currently, FST is undoubtedly more extensively and normally used for sensing antidepressant activity. It is principally selected by virtue of its comparative consistency across research laboratory and its capacity to perceive activity in an extensive range of clinically effective antidepressants. Actually, this assessment is broadly operated to evaluate behavior-related phenotypes in mice genetically transformed (depressive/antidepressive model). The assay is constructed on observed rodent’s swimming behavior. Initially, a furious escape-oriented movement is induced and quickly the animal develops an immobile posture when is placed into an unavoidable swimming cylinder. Indeed, if the assay is preceded by antidepressant drug treatments, the animals will powerfully persist alluring in escape-driven behaviors for a longer time in contrast with animals treated with the vehicle. In mice, one acute pretreatment with antidepressant agents is satisfactory to produce a firm motionlessness behavior, while commonly two trials are essential in the rat for identical behavior and the mechanism is unknown (Cryan and Mombereau, 2004; Cryan et al., 2002). Afterward, from searching the literature, a huge amount of medicinal plant with putative antidepressant activity, validated using mice FST, can be found. Some of them are Uncaria rhynchophylla (Geng et al., 2019), Origanum majorana essential oil (Abbasi-Maleki et al., 2019), Xiaochaihutang, a traditional Chinese medicine prescription (Zhang et al., 2019), Anthemis wiedemanniana Fisch. and Mey (GüraÄŸaç Dereli et al., 2018), Poria cocos (Schw.) Wolf (Zhang et al., 2018), K. brevifolia (Hellión-Ibarrola et al., 2016), and A. polystachya (Hellión-Ibarrola et al., 2008) among many others.
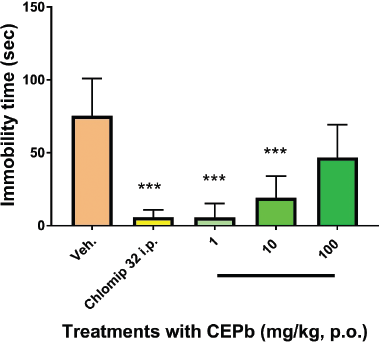 | Figure 7. Behavioral influence of single oral dose treatment with vehicle (0.1 ml/10 g, b.w.), Clomipramine (32 mg/kg i.p.), and the respective dose of P. bathyoryctum (CEPb 1, 10, and 100 mg/kg, p.o.) registered in mice submitted to FST (n = 10). Each bar describes the means ± SD of the immobility time of different groups. A significant decrease in the immobility time was observed with doses of 1 and 10 mg/kg (***p < 0.0001) compatible with antidepressant-like effect. Clomipramine was used as a positive antidepressant agent (***p < 0.0001). [Click here to view] |
The chemical composition of P. bathyoryctum is unknown. However, other species of the genus have increasing chemical information which offer a differential spectrum of biological activities. Consequently, two acidic compounds (morolic and moronic) isolated from Phoradendron reichenbachiana have demonstrated antidiabetic and antihyperglycemic effects in chemically induced diabetes (noninsulin-dependent) model in rats (Ramírez-Espinosa et al., 2013). Phoradendron serotinum showed moderate toxic activity in mice and also exhibited an antitumor effect (TC-1 tumor model in mice). Besides, in vivo immunomodulation, apparently related to the production of cytokines, was detected (Alonso-Castro et al., 2011, 2012). Rios et al. (2012) demonstrated the NO-dependent vasodilator effect of five structurally related triterpene molecules (ursolic acid, moronic acid, morolic acid, betulinic acid, and 3, 4-seco-olean-18-ene-3,28-dioic acid) obtained from P. reichenbachiana using isolated rat aorta. López-Martínez et al. (2013) have described the occurrence of linoleate of β-sitosterol and stigmasterol, β-sitosterol, stigmasterol, triacontanol, squalene, α- and β-amyrin, lupeol, lupenone, aldehydes (betulinic, betulin, and oleanolic), acids (betulinic, betulonic, moronic, morolic, oleanolic), and the flavonoids acacetin and acacetin 7-methyl ether as components of P. brachystachya. Acacetin Acacetin 7-methyl ether has human monoamine oxidase B (MAO-B) inhibitory activity and is potentially useful in Parkinson’s disease among others neurodegenerative conditions (Chaurasiya et al., 2019). Despite broad pharmacological information on Phoradendron genus, as stated clearly, we have to mention some limitations of this study. First, the anxiolytic and antidepressant-like activity were performed using crude extract; however, the chemical composition of P. bathyoryctum is unknown and we cannot assign a putative activity to any molecule(s) as responsible for the observed results. Furthermore, without a solvent fractionation, separation/isolation of at least a major component of P. bathyoryctum, we cannot assure any conclusion about the behavioral activity. Subsequently, a final direction of the studies will depend on complementary chemical and pharmacological studies and the concordance or not of results could strengthen or negate this preliminary finding.
Furthermore, at this stage, for the first time, we denoted valuable knowledge about this natural product. Undoubtedly, the isolation of the active compound(s) or main component and the determination of the molecular mechanism(s) involved in anxiolytic or antidepressant-like action are the next goals. In addition, depending on the final outcome, this validated natural resource with an acceptable safety/efficacy profile would be a candidate to be converted to a phytomedicine.
CONCLUSION
Based on results, the hydroethanolic extract of P. bathyoryctum is safe and exempted from acute toxicity and exhibited an orally effective antianxiety and antidepressant in preclinical condition using male mice. This finding encourages us to design new chemical and biological experiments to characterize molecule(s) and the molecular mechanism responsible for these pharmacological activities.
ACKNOWLEDGMENTS
This research was executed under the support and donation of facilities from the Faculty of Medicine (National University of Itapua). Also, the authors thank the members of the Botany Department from the Faculty of Chemical Sciences (National University of Asunción) for the identification and authentication of plant material.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval is provided by the institutional Ethical Committee in Scientific Research of the Faculty of Medicine on April 15, 2015 (CEI-002-15) .
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbasi-Maleki S, Kadkhoda Z, Taghizad-Farid R. The antidepressant-like effects of Origanum majorana essential oil on mice through monoaminergic modulation using the forced swimming test. J Tradit Complement Med, 2019; 10(4):1–9. CrossRef
Alonso-Castro AJ, Juárez-Vázquez MDC, Domínguez F, González-Sánchez I, Estrada-Castillón E, López-Toledo G, Chávez M, Cerbón MA, García-Carranca A. The antitumoral effect of the American mistletoe Phoradendron serotinum (Raf.) M.C. Johnst. (Viscaceae) is associated with the release of immunity-related cytokines. J Ethnopharmacol, 2012; 142:857–64. CrossRef
Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A. Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol, 2011; 133: 945–72. CrossRef
Benigni R, Capra C, Cattorini PE. Piante Medicinali, Chimica, Farmacologia e Terapia. Vol II, Inverni della Beffa, Milán, Italy, pp 1760–82, 1964.
Chaurasiya ND, Zhao J, Pandey P, Doerksen RJ, Muhammad I, Tekwani BL. Selective inhibition of human monoamine oxidase B by acacetin 7-methyl ether isolated from turnera diffusa (Damiana). Molecules, 2019; 24:1–15. CrossRef
Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry, 2004; 9:326–57. CrossRef
Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol, 2002; 436:197–205. CrossRef
Fernando M, Peres P, Mercante JPP, Tobo PR, Kamei H, Bigal ME. Anxiety and depression symptoms and migraine : a symptom-based approach research. J Headache Pain, 2017, 18:1–8. CrossRef
File SE, Lippa AS, Bernard B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci, 2004; 8:8.3.1–22. CrossRef
Geng CA, Yang TH, Huang XY, Ma YB, Zhang XM, Chen JJ. Antidepressant potential of Uncaria rhynchophylla and its active flavanol, catechin, targeting melatonin receptors. J Ethnopharmacol, 2019; 232:39–46.
Gonzales Torrez DM. Catálogo de Plantas Medicinales (y Alimenticias y Utiles) Usadas en el Paraguay. Asunción, Paraguay: El País, pp. 312 and 452, 1992.
González E, Iglesias I, Carretero E, Villar A, Gastric cytoprotection of bolivian medicinal plants. J Ethnopharmacol, 2000; 70(3):329–33. CrossRef
GüraÄŸaç Dereli FT, Ilhan M, Küpeli Akkol E. Discovery of new antidepressant agents: In vivo study on Anthemis wiedemanniana Fisch. & Mey. J Ethnopharmacol, 2018; 226:11–6. CrossRef
Heikkinen J, Honkanen R, Williams L, Leung J, Rauma P, Quirk S, Koivumaa-Honkanen H. Depressive disorders, anxiety disorders and subjective mental health in common musculoskeletal diseases: a review. Maturitas, 2019; 127:18–25. CrossRef
Hellión-Ibarrola MC, Ibarrola DA, Montalbetti Y, Kennedy ML, Heinichen O, Campuzano M, Ferro EA, Alvarenga N, Tortoriello J, De Lima TCM, Mora S. The antidepressant-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. Phytomedicine, 2008; 15:478–83. CrossRef
Hellión-Ibarrola MC, Ibarrola DA, Montalbetti Y, Villalba D, Heinichen O, Ferro EA. Acute toxicity and general pharmacological effect on central nervous system of the crude rhizome extract of Kyllinga brevifolia Rottb. J Ethnopharmacol, 1999; 66:271–6. CrossRef
Hellión-Ibarrola MC, Montalbetti Y, Heinichen OY, Kennedy ML, Campuzano MA, Alvarenga N, Ibarrola DA. Antidepressant-like effect of Kyllinga brevifolia rhizomes in male mice and chemical characterization of the components of the active ethyl acetate fraction. J Ethnopharmacol, 2016; 194:1005–11. CrossRef
Hellión-Ibarrola MC, Montalbetti Y, Heinichen OY, Kennedy ML, Campuzano MA, Ibarrola DA. Anxiolytic-like and sedative effects of Kyllinga brevifolia in mice. Braz J Pharmacogn, 2012; 22(6):1323–9. CrossRef
Irwin, S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia, 1968; 13(3):222–57. CrossRef
Jiménez-Estrada M, Velázquez-Contreras C, Garibay-Escobar A, Sierras-Canchola D, Lapizco-Vázquez R, Ortiz-Sandoval C, Burgos-Hernández A, Robles-Zepeda RE. In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from northwest of Mexico. BMC Complement Altern Med, 2013; 13:12. CrossRef
Kashiwada Y, Wang HK, Nagao T, Kitanaba S, Anti AIDS agents.30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod, 1998; 61(9):1090–5. CrossRef
Krause M, Gutsmiedl K, Bighelli I, Schneider-Thoma J, Chaimani A, Leucht S. Efficacy and tolerability of pharmacological and non-pharmacological interventions in older patients with major depressive disorder: a systematic review, pairwise and network meta-analysis. Eur Neuropsychopharmacol, 2019; 29:1003–22. CrossRef
Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther, 1990; 46:321–40. CrossRef
Lister RG. The use of the pluz-maze to measure anxiety in the mouse. Psychopharmacology, 1987; 92:180–5. CrossRef
López-Martínez S, Navarrete-Vázquez G, Estrada-Soto S, León-Rivera I, Rios MY. Chemical constituents of the hemiparasitic plant Phoradendron brachystachyum DC Nutt (Viscaceae). Nat Prod Res, 2013; 27(2):130–6. CrossRef
Martínez Crovetto R. Las plantas utilizadas en Medicina Popular en el Noroeste de Corrientes (República Argentina). Miscelánea N° 69. S.M. de Tucumán, Argentina: Fundación Miguel Lillo, pp 1–139, 1981.
Murray CJ, Lopez AD. Global burden of disease and injury series the global burden of disease. OMS, Geneva, Switzerland pp 1–46, 1996.
OECD. Acute oral toxicity – Fixed Dose Procedure (chptr). In: Organisation for Economic Co-operation and Development. OECD guidelines for the testing of chemicals. OECD, Paris, France, pp 1–14, 2001.
OECD. Test No. 425: acute oral toxicity: Up-and-Down procedure. Test 1–21. OECD, Paris, France, 2008.
Pellow S. Anxiolitic and anxiogenic drug effects in a novel test of anxiety: are exploratory models of anxiety in rodents valid? Methods Find Exp Clin Pharmacol, 1986; 8(9):557–65.
Pellow S, Chopin P, File SE, Briley M. Validation of open, closed arm entries in a elevated pluz-maze as a measure of anxiety in rat. J Neurosci Methods, 1985; 14:149–67. CrossRef
Porsolt RD, Brossard G, Roux S. Models of affective illness: forced swimming and tail suspension test in rodents. Curr Protoc Pharmacol, 2000; 1:5.8.1–9. CrossRef
Ramírez-Espinosa JJ, García-Jiménez S, Rios MY, Medina-Franco JL, López-Vallejo F, Webster SP, Binnie M, Ibarra-Barajas M, Ortiz-Andrade R, Estrada-Soto S. Antihyperglycemic and sub-chronic antidiabetic actions of morolic and moronic acids, in vitro and in silico inhibition of 11β-HSD 1. Phytomedicine, 2013; 20(7):571–6. CrossRef
Razavi BM, Zargarani N, Hosseinzadeh H. Anti-anxiety and hypnotic effects of ethanolic and aqueous extracts of Lippia citriodora leaves and verbascoside in mice. Avicenna J Phytomed, 2017; 7:353–65.
Rios MY, López-Martínez S, López-Vallejo F, Medina-Franco JL, Villalobos-Molina R, Ibarra-Barajas M, Navarrete-Vazquez G, Hidalgo-Figueroa S, Hernández-Abreu O, Estrada-Soto S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: ex vivo and in silico studies. Fitoterapia, 2012; 83(6):1023–9. CrossRef
Rodrigues E, Carlini EA. Ritual use of plants with possible action on the central nervous system by the Krahô Indians, Brazil. Phytother Res, 2005; 19:129–35. CrossRef
Roux S, Sablé E, Porsolt RD. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr Protoc Pharmacol, 2005; 10:10.10.1–23. CrossRef
Stallard N, Whitehead A. A statistical evaluation of the fixed dose procedure. Altern Lab Anim, 2004; 32(Suppl 2):13–21. CrossRef
Suárez ME. Medicines in the forest: ethnobotany of wild medicinal plants in the pharmacopeia of the Wichí people of Salta province (Argentina). J Ethnopharmacol, 2019; 231:525–44. CrossRef
Toursarkissian M. Plantas medicinales de la Argentina. Sus nombres botánicos, vulgares, usos y distribución geográfica. Hemisferio Sur, Buenos Aires, Argentina, p 79, 1980.
Wagner ML, Vaccaro MC, Gurni AA, Coussio JD, y Rondina RVD. Estudio de la variabilidad en compuestos aminados de diferentes ejemplares del género Phoradendron que crecen en las zonas centro-oeste y misioneras argentinas. Acta Farm Bonaer, 1986; 5(3):139–48.
World Health Organization (WHO). The global burden of disease. Updat. World Health Organization, Geneva, Switzerland, p 146, 2004.
World Health Organization (WHO). WHO traditional medicine strategy 2014-2023. World Health Organization, Geneva, Switzerland, pp 1–76, 2013.
Zhang K, Wang Z, Pan X, Yang J, Wu C. Antidepressant-like effects of Xiaochaihutang in perimenopausal mice. J Ethnopharmacol, 2019; 248:112318. CrossRef
Zhang W, Chen L, Li P, Zhao J, Duan J. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int J Biol Macromol, 2018; 120:1696–704. CrossRef