INTRODUCTION
Depression is a prevalent mental disorder and affects more than 200 million people worldwide. Most depressive patients are distributed in the regions of Southeast Asia and Western Pacific. Between 2005 and 2015, the prevalence of depressive patients increased by 18.4%, which could represent the growth in the global population. Mortality due to depression reaches 800,000 annually in the age range of 15–29 (WHO, 2020). The current COVID-19 pandemic sees a further increase in the number of cases of depression. One recent study reported that, after adjustment for the pandemic, the estimated prevalence of the major depressive disorder in 2020 was 246 million people from the previously estimated 193 million (COVID-19 Mental Disorders Collaborators, 2021). This makes depression one disorder that needs serious attention and whose therapy is to be prioritized. The economic burden of depressive patients continued to rise over time. A recent study has shown that, in the United States, direct costs incurred by individuals with major depressive disorder rose from USD 111.1 billion in 2010 to USD 114.3 billion in 2018 (Greenberg et al., 2021).
Based on the therapeutic guidelines from the American Psychological Association, the first-line drugs for the treatment of depression include tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, or dopamine agonists, depending on the patient’s clinical condition, drug side effects, and therapy costs (American Psychological Association, 2019). As many as 30% of depressive patients did not respond to drug therapy and 70% of them failed to achieve clinical cure. Compliance is an essential factor contributing to therapy success and better quality of life for depressive patients. Several studies have shown that patient compliance to antidepressant therapy was low; as much as 50% of patients quitted therapy. The reasons for the discontinuation can be divided into two factors: the patient factors, including side effects, drug dependence, and the belief that therapy is unsuccessful; the clinical factors, including low patient education as well as poor follow-up from health practitioners (Sawada et al., 2009; Sansone and Sansone, 2012). These facts have become a justifying factor in embarking on the use of alternative therapies which are safer with easier accessibility. Plant-derived drugs may be the potential candidate in this regard (Kulkarni et al., 2009).
Bitter melon (Momordica charantia) is a widely used medicinal plant due to its various potential health benefits. The studied biological activities include antihyperglycemic, antibacterial, antiviral, antitumor, immunomodulatory, antioxidant, antidiabetic, anthelmintic, antiulcer, antifertility, hepatoprotective, anticancer, and anti-inflammatory activities (Jia et al., 2017). The antioxidant and anti-inflammatory activities open further potential for the activities on the nervous system. To this end and considering the need for alternative antidepressants, we explored the potential use of bitter melon in an animal model of depression.
MATERIALS AND METHODS
Animals
Male Deutschland Denken Yoken (ddY) mice (purchased from PT Biofarma Bandung Indonesia), aged 6–8 weeks, weighing 25–35 g at the beginning of the experiment, were used. They were kept at a constant ambient temperature of 25 ± 3ºC under a 12 hours light/dark cycle with free access to food and water except during behavioral observations. All animal treatments were approved by the Ethical Committee for the Use and Care of Laboratory Animals, Institut Teknologi Bandung (ethical approval reference: 09/KEPHP-ITB/03-2021).
Drugs
Plant determination was carried out in the Herbarium Bandungense at the School of Life Sciences and Technology, Institut Teknologi Bandung (determined as M. charantia L.). Bitter melon fruits harvested on the 28th day were washed, chopped with a food chopper, added to water in a ratio of 1:10, and then filtered. The filtrate was freeze-dried and kept until use. The yield of this process was 4.3%. Fluoxetine was suspended in 1% sodium carboxymethyl cellulose. All drugs were given orally using oral gavage.
Antidepressant activity test
The FST (Porsolt et al., 1977) was utilized to model depression-like behavior. The mouse was forced to swim in a water-filled glass cylinder (30 cm high, 20 cm wide, allowing 15 cm deep water). The immobility time was recorded for each mouse during the last 4 minutes of a total 6-minute observation period. Test substances were administered orally, once daily for 14 days. The bitter melon juice was given at the dose of 50 or 200 mg/kg, while the reference drug fluoxetine was administered at 20 mg/kg. Observations of immobility time, as the index of depression, were carried out on days 7 and 14.
Measurement of cortisol
Following FST on day 14, the mice were sacrificed by cervical dislocation. Samples from the hippocampal and frontal cortical area of the brain were collected, washed with phosphate buffer saline pH 7.4, weighed, and homogenized. The homogenate was then centrifuged at 15,000 rpm for 15 minutes. The supernatant was collected and stored until use at −20ºC. Measurement of cortisol was carried out using ELISA (Bioassay Technology Laboratory) in accordance with the manufacturer’s protocol.
Statistical analyses
In the statistical analyses, one-way analysis of variance (ANOVA) was used to compare means, and LSD tests were used for post-hoc pairwise comparisons. A difference was considered significant at p < 0.05.
RESULTS AND DISCUSSION
To the authors’ knowledge, the current work is the first to study the effect of bitter melon on an animal model of depression. Results on the profile of immobility time in FST following BM treatment are shown in Figure 1. The data showed that BM dose-dependently decreased immobility time on both observation days, significantly different compared to vehicle. On day 7, the immobility time observed after the doses of 50 and 200 mg/kg was 95.67 ± 31.94 sec and 84.00 ± 11.40 sec, respectively, while the time after vehicle was 145.00 ± 14.17 sec (p-value for the difference between BM and vehicle = <0.01 and <0.001, respectively; ANOVA; and post hoc LSD). On day 14, treatment of the doses of 50 and 200 mg/kg resulted in respective immobility times of 79.33 ± 30.66 sec and 39.00 ± 17.45 seconds, with the time after the vehicle of 116.00 ± 13.95 sec (p-value for the difference between BM and vehicle = 0.08 and <0.01, respectively.; ANOVA; and post hoc LSD). The model of depression used in this study was FST with immobility time as the index. FST is one of the most commonly used methods to assess depressive-like behavior in rodents. Immobility during testing is regarded as a measure of behavioral despair, and the method has been repeatedly shown to be sensitive to a wide range of antidepressants (Yankelevitch-Yahav et al., 2015), as shown in the present result.
Data on changes in cortisol concentration accompanying behavioral expression is shown in Figure 2. BM treatment was shown to significantly decrease the level of cortisol in the hippocampal and frontal cortical areas of the brain. At the dose of 50 mg/kg, BM produced the lowest level of cortisol (39.75 ± 4.59 ng/ml, p < 0.05 versus vehicle, ANOVA, and post hoc LSD), but was not significantly different compared to that after the dose of 200 mg/kg, which produced the level of 41.96 ± 4.63 ng/ml (p < 0.05 versus vehicle, ANOVA, and post hoc LSD) or 20 mg/kg fluoxetine. Hypersecretion of cortisol has been regarded as a risk factor for depression (Cosgriff et al., 1990). In addition, it was demonstrated that cortisol hypersecretion found in depressed patients was associated with stress experienced by the subjects (Nemeroff et al., 1996). The hippocampus and frontal cortex, structurally, have been shown to associate with depression in human subjects (Belleau et al., 2019). Furthermore, it was revealed that the morbid hippocampal and frontal cortical condition in depressive patients was associated with elevated levels of baseline cortisol as well as cortisol/dehydroepiandrosterone ratio (Jin et al., 2016; Travis et al., 2016).
To further confirm the antidepressant activity, an assessment of changes in neurotransmitter-related depressive symptoms should be performed. In fact, most pharmacological therapies for the treatment of depressive symptoms are directed toward the normalization of biogenic amine levels (Dulawa et al., 2004; Safhi et al., 2019). However, cortisol has been indicated to be the predictor of psychological therapy response in depressive disorder (Fischer et al., 2017). Furthermore, López et al. (1997) have put forward a hypothesis that the association between changes in 5-HT receptors with suicidal ideation and act might be attributed to or exacerbated by overactivity of the hypothalamus–pituitary–adrenal axis, leading to an elevated level of cortisol. In addition, chronic stress was shown to reduce 5-HT1A receptor mRNA levels, as well as 5-HT1A binding densities in the hippocampus (López et al., 1998). Taking this data into consideration, therefore, one might suggest that cortisol modulates 5-HT receptors, which in turn controls affective states as expressed in the change in mood of depressive patients, further articulating the essential of cortisol in the pathology of depression with hippocampus as a key substrate.
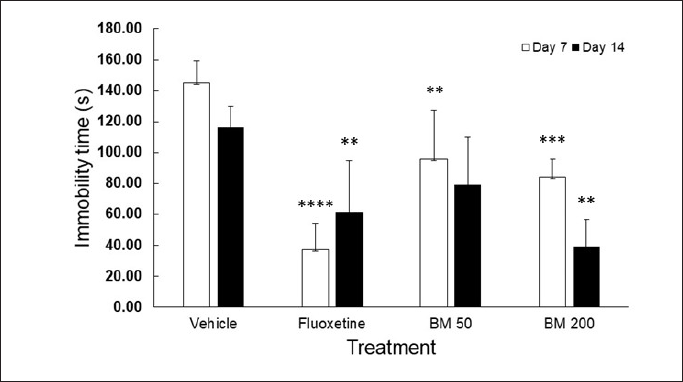 | Figure 1. Effects of bitter melon juice concentrate (BM) on immobility time in FST. The vehicle, fluoxetine (30 mg/kg), and BM (50 or 200 mg/kg) were administered orally for 14 days. Immobility time was recorded on days 7 and 14. Data represents average + SD of threee to four mice. **, ***, ****p < 0.01, 0.001, and 0.0001 versus vehicle, one-way ANOVA, and post hoc LSD. [Click here to view] |
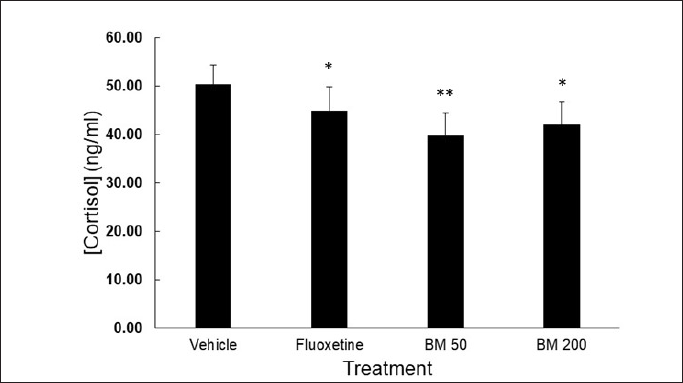 | Figure 2. Effects of bitter melon juice concentrate (BM) on cortisol levels in the hippocampal and frontal cortical areas of the brain. The vehicle, fluoxetine (30 mg/kg), and BM (50 or 200 mg/kg) were administered orally for 14 days. Extraction of the brain areas for cortisol level measurements was carried out soon after the last behavioral assessment. Data represents average + SD of four mice. *, **p < 0.05 and 0.01 versus vehicle, one-way ANOVA, and post hoc LSD. [Click here to view] |
Amid the current COVID-19 pandemic, when household income declines, challenging the provision of healthcare services and their accessibility (Núñez et al., 2021), natural product-derived alternatives become an avenue that people turn to. Thus, depressive patients could take the benefit from consuming bitter melon juice to help improve their symptoms. It has been demonstrated that infections were followed by a battery of body responses, and depression was one of them (Hart, 1988). Indeed, an increased level of cortisol has been observed in COVID-19 patients (Yavropoulou et al., 2022).
The antidepressant effect has been shown in a number of plants of the family Cucurbitaceae, to which bitter melon also belongs. Randawa et al. (2015) demonstrated that methanol extract of Coccinia indica at 400 mg/kg produced behavioral improvement in an animal model using FST. Meanwhile, methanol extract of Cucurbita pepo at 100 mg/kg lowered immobility time in an FST experiment, and the effect was comparable to imipramine at 30 mg/kg (Umadevi et al., 2011). In addition, methanol extract of Benincasa hispida at 100 mg/kg was shown to significantly decrease the immobility time, with MAO-A inhibition as a possible mechanism (Dhingra and Joshi, 2012). Hydroalcoholic extract of Benincasa hispida was studied for its effect on head twitch, a behavioral model associated with the serotoninergic activity. At the dose of 30 mg/kg, the extract was found to dampen the increase in head twitch (Shakya et al., 2019). Another study on a plant from the family Cucurbitaceae was carried out by Rahman et al. (2013), showing that the n-hexane extract Citrullus lanatus at 250 and 500 mg/kg given for 7 days lowered immobility times as tested by FST and tail suspension test (TST), which was comparable to a dose of 10 mg/kg of imipramine. The methanol extract of Lagenaria siceraria at 100 and 200 mg/kg was demonstrated to decrease immobility time which was stronger than the reference drug imipramine at 12.5 mg/kg (Prajapati et al., 2011). Aqueous extract of Cucurbita maxima at 100 mg/kg was demonstrated to blunt immobility time in FST and TST, the activity which was comparable to imipramine at 30 mg/kg (George and Nazmi, 2012). Lastly, Cucurbita moschata water extract was also reported to produce an improvement in the behavioral index in the FST model, which was in line with the increase in blood serotonin, a neurotransmitter essentially affected by depression (Kim et al., 2016).
Antidepressant activity in a plant from the family Cucurbitaceae has also been studied at the level of isolate. The most detailed work in this respect was that on gypenosides, a saponin isolated from the plant Gynostemma pentaphyllum. Gypenosides administered orally for 4 weeks at 50 and 100 mg/kg significantly dampened immobility time in FST testing, and the activities were comparable to that of fluoxetine (Mu et al., 2016). With regard to bitter melon, phytochemical analyses have unveiled alkaloids, flavonoids, glycosides, saponins, steroids, and tannins as the bioactive components (Mada et al., 2013; Oragwa et al., 2013). Based on the chemotaxonomic approach, further studies on the saponin component might be an interesting research focus.
CONCLUSION
Results of the present study demonstrated a decrease in immobility time in a mouse model of depression accompanied by decreased cortisol level after BM treatment. This implies that bitter melon juice concentrate has antidepressant potential, which could be utilized in patients with depressive symptoms. As a “do it yourself” alternative, its use is of particular benefit during the condition in which access to therapy is limited.
ACKNOWLEDGMENT
The authors thank Mr. Sandi Tyas Pangestu for the technical aspects of this work.
FUNDING
This work was funded by the Research and Community Service Program Scheme, Grant no. 3612/IT1.C10/TA/2020.
AUTHORS’ CONTRIBUTIONS
All authors contributed substantially to the design and implementation of this work, including data collection and analysis and preparation of the manuscript.
CONFLICT OF INTERESTS
The authors report no conflict of interests in this work.
ETHICAL APPROVAL
The Ethical Committee for the Use and Care of Laboratory Animals, Institut Teknologi Bandung (ethical approval)
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
American Psychological Association. Clinical practice guideline for the treatment of depression across three age cohorts, 2019. Available via https://www.apa.org/depression-guideline/guideline.pdf (Accessed 25 February 2022).
Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry, 2019; 85(6):443–53. CrossRef
Cosgriff J, Abbott R, Oakley-Browne M, Joyce PR. Cortisol hypersecretion predicts early depressive relapse after recovery with electroconvulsive therapy. Biol Psychiatry, 1990; 28:1007–10. CrossRef
COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet, 2021; 398(10312):1700–12.
Dhingra D, Joshi P. Antidepressant-like activity of Benincasa hispida fruits in mice: Possible involvement of monoaminergic and GABAergic systems. J Pharmacol Pharmacother, 2012; 3(1):60. CrossRef
Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology, 2004; 29(7):1321–30. CrossRef
Fischer S, Strawbridge R, Vives AH, Cleare AJ. Cortisol as a predictor of psychological therapy response in depressive disorders: systematic review and meta-analysis. Br J Psychiatry, 2017; 210(2):105–9. CrossRef
George S, Nazni P. Antidepressive activity of processed pumpkin (Cucurbita maxima) seeds on rats. Int J Pharm Med Bio Sci, 2012; 1(2):225–31.
Greenberg PE, Fournier AA, Sisitsky T, Simes M, Berman R, Koenigsberg SH, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics, 2021; 39(6):653–65. CrossRef
Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev, 1988; 12(2):123–37. CrossRef
Jia S, Shen M, Zhang F, Xie J. Recent advances in momordica charantia: Functional components and biological activities. Int J Mol Sci, 2017; 18(12):2555. CrossRef
Jin RO, Mason S, Mellon SH, Epel ES, Reus VI, Mahan L, Rosser RL, Hough CM, Burke HM, Mueller SG, Wolkowitz OM. Cortisol/DHEA ratio and hippocampal volume: A pilot study in major depression and healthy controls. Psychoneuroendocrinology, 2016; 72:139–46. CrossRef
Kim N, Kim H, Kim M, Kim H, Jeong H. Improvement of depressive behavior by Sweetme Sweet PumpkinTM and its active compound, β-carotene, Life Sci, 2016; 147:39–45. CrossRef
Kulkarni SK, Dhir A, Akula KK. Potentials of curcumin as an antidepressant. Sci World J, 2009; 9:1233–41. CrossRef
López JF, Vázquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Ann N Y Acad Sci, 1997; 836:106–34. CrossRef
López JF, Chalmers DT, Little KY, Watson SJ. A.E. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry, 1998; 43(8):547–73. CrossRef
Mada SB, Garba A, Mohammed HA, Muhammad A, Olagunju A. Antimicrobial activity and phytochemical screening of aqueous and ethanol extracts of Momordica charantia L . leaves. J Med Plants Res, 2013; 7(10):579–86
Mu RH, Fang XY, Wang SS, Li CF, Chen SM, Chen XM, Liu Q, Li YC, Yi LT. Antidepressant-like effects of standardized gypenosides: involvement of brain-derived neurotrophic factor signaling in hippocampus. Psychopharmacology, 2016; 233(17):3211–21. CrossRef
Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry, 1996;1(4):336–42.
Núñez A, Sreeganga SD, Ramaprasad A. Access to Healthcare during COVID-19. Int J Environ Res Public Health, 2021; 18(6):2980. CrossRef
Oragwa LN, Efiom OO, Okwute SK. Phytochemicals , antimicrobial and free radical scavenging activities of Momordica charantia Linn (Palisota Reichb) seeds. Afr. J. Pure Appl. Chem. Full, 2013; 7(12):405–9.
Porsolt RD., Bertin A., Jalfre M. Behavioural despair in mice: A primary screening test for antidepressants, Arch Int Pharmacodyn, 1977; 229:327–36.
Prajapati R, Umbarkar R, Parmar S, Sheth N. Antidepressant like activity of Lagenaria siceraria (Molina) Standley fruits by evaluation of the forced swim behavior in rats. Int J Nutr Pharmacol Neurol Dis, 2011; 1:152–6. CrossRef
Randhawa K, Kumar D, Jamwal A, Kumar S. Screening of antidepressant activity and estimation of quercetin from Coccinia indica using TLC densitometry. Pharm Biol, 2015; 53(12):1867–74. CrossRef
Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci, 2012; 9(5–6):41–6.
Sawada N, Uchida H, Suzuki T, Watanabe K, Kikuchi T, Handa T, Kashima H. Persistence and compliance to antidepressant treatment in patients with depression: a chart review. BMC Psychiatry, 2009; 9:38. CrossRef
Safhi MM, Qumayri HM, Masmali AUM, Siddiqui R, Alam MF, Khan G, Anwer T. Thymoquinone and fluoxetine alleviate depression via attenuating oxidative damage and inflammatory markers in type-2 diabetic rats. Arch Physiol Biochem, 2019; 125(2):150–5. CrossRef
Shakya A, Bhat HR, Ghosh SK. Assessment of Neurobehavioral properties of hydroalcoholic extract of Benincasa hispida (Thunb.) Cogn. fruit pulp in mice. J Biol Act Prod Nat, 2019; 9(4):299–310. CrossRef
Travis SG, Coupland NJ, Hegadoren K, Silverstone PH, Huang Y, Carter R, Fujiwara E, Seres P, Malykhin NV. Effects of cortisol on hippocampal subfields volumes and memory performance in healthy control subjects and patients with major depressive disorder. J Affect Disord, 2016; 201:34–41. CrossRef
Umadevi P, Murugan S, Suganthi J, Subakanmani, S. Evaluation of antidepressant like activity of Cucurbita pepo seed extracts in rats. Int J Curr Pharm Res, 2011; 3(1):108–13.
WHO. 2020. International statistical classification of diseases and related health problems (11th Revision). Available at: https://icd.who.int/en (Accessed 17 February 2021).
Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp, 2015; (97):52587. CrossRef
Yavropoulou MP, Filippa MG, Mantzou A, Ntziora F, Mylona M, Tektonidou MG, Vlachogiannis NI, Paraskevis D, Kaltsas GA, Chrousos GP, Sfikakis PP. Alterations in cortisol and interleukin-6 secretion in patients with COVID-19 suggestive of neuroendocrine-immune adaptations. Endocrine, 2022; 75(2):317–27. CrossRef