INTRODUCTION
Cancer is a non-communicable disease as a result of abnormal cell proliferation in tissues, which are characterized as either benign or malignant. Cervical cancer is a common gynecologicaly cancer that most often causes death in women (Small et al., 2017). Cervical cancer is a malignant tumor originating from the endocervical and ectocervical epithelial junction caused by persistent infection from human papilloma virus (HPV) types 16 and 18 (Eifel, 2018). Both HPV types 16 and 18 cause 75% of invasive cervical cancer (Akbar et al., 2019). In 2018, the World Health Organization reported cervical cancer in 32,496 woman (WHO, 2018).
HPV produces two proteins that play a vital role in cervical cancer pathogenesis:, E6 protein, and E7 protein (Yang, 2013). Both E6 protein and E7 protein can inactivate apoptotic proteins in cervical epithelial cells. The E6 protein is the main protein that causes oncogenesis by binding to the p53 tumor suppressor protein, whereas the E7 protein binds and inactivates the retinoblastoma (Rb) protein in infected cells and activates the cell cycle program (Graham, 2017; Small et al., 2017). If cell apoptotic proteins are inactivated and degraded, then cell proliferation will be abnormal and the final result is cervical cancer.
The main reason for high cervical cancer prevalence and mortality is metastasis to other sites in the body. Cervical cell cancer metastasis involves a process called the epithelial-mesenchymal transition (EMT), especially EMT type 3 (Jiang et al., 2016; Lee and Shen, 2012). Snail-1 is a zinc-finger transcription factor that is regulated by the PI3K/Akt signaling pathway and increases EMT by downregulating E-cadherin and upregulating vimentin (Myong, 2012; Xu et al., 2015). EMT changes epithelial cells to mesenchymal cells, causing loss of cell adhesion and polarity (Ha et al., 2013). These changes in characteristics increase migration capacity, invasion, apoptosis resistance, and extra-cellular matrix production (Bermudez et al., 2015).
Patients with metastatic cervical cancer need more advanced treatments. In Indonesia, cervical cancer treatment includes operative treatment, chemotherapy, and radiation therapy (Permatasari et al., 2019). Recently, chemotherapy is the most common for treating metastatic cervical cancer, but using chemotherapy sometimes results in the worst side effects because the health and viability of the cells in the surrounding healthy tissues around the cancer also decrease and have a tendency to undergo necrosis. In addition, the chemotherapy treatment price is too expensive (Van Minh et al., 2017). Therefore, more effective and cheaper treatment solutions are needed. One such avenue is using natural extracts that are easily found in Indonesia, as some have proven to contain anti-cancer effects.
Indonesia is one of the biggest clove (Syzygium aromaticum) exporters in the world. Clove has an active substance called eugenol (4-allyl-2-methoxyphenol). Eugenol concentrations inside clove oil are approximately between 70%– and 96% (Bezerra et al., 2017). Eugenol contains many functional groups, such as allyl (-CH2-CH=CH2), phenol (OH), and methoxy (−OCH3) (Towaha, 2012). Recently, experiments have revealed that the active clove oil substance, eugenol, has anti-tumor properties (Jaganathan, 2012). Our previous study revealed that eugenol’s anti-tumor mechanisms induce apoptosis in cancer cells by enhancing p53 protein concentrations (Permatasari et al., 2019). However, there is no research on eugenol as an anti-metastasis agent. Therefore, this study aimed to investigate eugenol’s inhibitory effects on cervical cancer metastasis.
MATERIALS AND METHODS
Eugenol isolation from clove oil by fractional distillation
As much as 150 ml of clove oil (Malang, Indonesia) was added into a round bottom flask and underwent a series of fractional distillations connected by vacuum. The pressure was controlled at 100 mmHg and distillation was carried out. The distillate was obtained at a temperature of 80°C–82°C and collected in an Erlenmeyer flask. The isolated eugenol was measured by its specific gravity and analyzed by infrared spectrophotometry and high performance liquid chromatography, as previously described (Fitri and Kawira, 2006).
HeLa cervical cancer cell culture
Cervical cancer HeLa cells were purchased from the Biomedical Laboratory, Medicine Faculty, Brawijaya University (Malang, Indonesia). Cells were incubated with 5% CO2 at 37°C. HeLa cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 media (Roswell Park Memorial Institute, New York, NY) supplemented with 10% fetal bovine serum (FBS), and antibiotics (100 UI/ml penicillin, 100 μl/ml streptomycin), and maintained at a pH of 7.2–7.4. HeLa cells were routinely grown and harvested with trypsin–ethylenediaminetetraacetic acid solution (You et al., 2010).
Cell migration using the wound healing assay
Cells were grown until they become monolayers in 24-well plates. A p10 micropipette tip was used to manually scratch a single line in each well. The dislodged cells were washed away using RPMI medium. After the scratches were formed, cells were grown in starvation medium (FBS 0.1%) with varying doses of eugenol (0, 50, 100, and 200 μM) as previously described (Permatasari et al., 2019). The wound-closing process was observed every 3 hours until the scratch in the control had fully closed. Cell pictures of each condition were taken at the same time. The scratch width was calculated using the ImmunoRatio ImageJ application.
E-cadherin, vimentin, and snail-1 expression using immunofluorescence
HeLa cell culture was carried out in 24-well plates with a 13 mm round-shaped glass cover at the bottom of each well (Matsunami, Japan). The primary antibodies used were anti-mouse E-cadherin (Santa Cruz, CA), anti-rabbit vimentin (Cell Signaling Technology, Danvers, MA), and anti-mouse FITC Snail-1 (Santa Cruz, CA). The secondary antibodies used were: Alexa Fluor-546 goat anti-mouse IgG (Invitrogen, Carlsbad, CA), Alexa Fluor-488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA), and Alexa Fluor-488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA). The nucleus was stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO).
Data collection and data analysis
The results are expressed as the mean ± SD of three independent experiments. The data were analyzed using GraphPad Prism 8. Measurements were statistically analyzed using the one-way analysis of variance (ANOVA), Tukey’s multiple comparison test, and Pearson’s correlation. A p-value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
To understand if eugenol has anti-metastatic effects on cervical cancer, we investigated ifwhether eugenol affects cell migration. HeLa cells in scratched wells were incubated with eugenol at various concentrations (0, 50, 100, and 200 μM) in starvation medium (FBS 0.1%). Scratch distances were observed under a light microscope after incubating for 72 hours. The HeLa cell morphology after eugenol exposure at varying concentrations for 72 hours is shown in Figure 1A. Figure 1B shows the scratch widths of control and eugenol-treated cells, before and after incubation. Based on Figure 1B, two populations from each treatment groups with particular eugenol concentrations (0, 50, 100, and 200 μM) formed a gap with lengths of 868.8, 890.2, 921.1, and 887.5 μm, respectively. Distance measurement was carried out after 72 hours of incubation, resulting in distance adjustment between the opposite populations in the same chamber. The control group showed a 405.1 μm space after the incubation. On the other hand, groups immersed with eugenol showed a wider gap of the two cell populations, with the distance increment directly proportional to the administered eugenol concentrations: 512.1 μm, 619.7 μm, and 750.4 μm for 0 μM, 50 μM, 100 μM, and 200 μM of eugenol, respectively. Cell migration percentage can be examined from the data above by calculating the width difference between before and after incubation. The percentage of cell migration on the HeLa cell culture with eugenol 0 μM is 53.4%. Furthermore, the HeLa cell immersed with eugenol concentrations (50 μM, 100 μM, and 200 μM) revealed a decrease of migration percentage by 42.5%, 23.7%, and 15.4%, respectively.
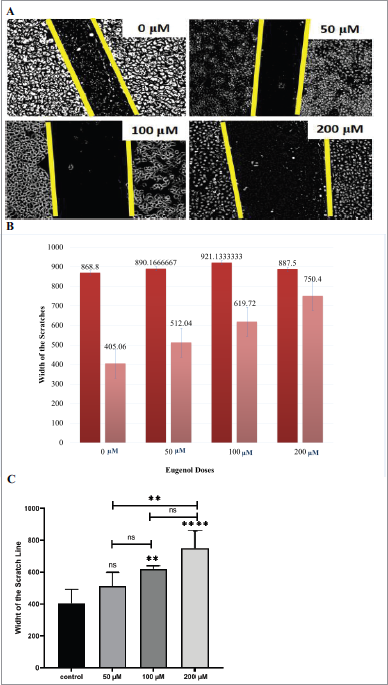 | Figure 1. The eugenol effect on HeLa cells migration. Theeffect (A) HeLa cell morphology after eugenol exposure at varying concentrations for 72 hours. (B) The scratch widths before and after, for control and eugenol-treated cells. (C) Scratch width from the wound healing assay calculated using the ImmunoRatio Image J application. The figure shows The mean ± SD is shown for three individual trials. One-way ANOVA and Tukey’s multiple comparison test: ** p < 0.01;; and **** p < 0.0001; ns =: not significant. [Click here to view] |
The wound healing scratch width ratio was calculated using the ImageJ ImmunoRatio application using the mean from three individual experiments (Fig. 1C). Overall, we observed that HeLa cell migration was inhibited when treated with eugenol. At the highest dose (200 μM), the migration rate was decreased by 3.38 ± 1.22-fold over the control (p < 0.0001). These results indicate that HeLa cell migration is significantly decreased in a dose-dependent manner, as evident by Pearson’s correlation coefficient (r = 0.9679).
Cervical cancer metastasis involves a process called the EMT. Decreased E-cadherin expression is associated with the EMT process. Therefore, we investigated the E-cadherin expression in HeLa cells incubated with various doses of eugenol (0, 50, 100, and 200 μM) for 24 hours. We found that there was an increase in E-cadherin levels, especially at 100 μM and 200 μM (Fig. 2A).
Recent studies revealed that carcinoma cells that finished their EMT process will obtain a mesenchymal phenotype and expressed mesenchymal markers, such as vimentin. We evaluated the vimentin expression in HeLa cells that were grown in the presence of eugenol using an immunofluorescence method. There was a significant decrease in vimentin expression, especially at 100 μM and 200 μM (Fig. 2B).
Snail-1 is a zinc-finger family transcription factor that plays an important role in the EMT process and cervical cancer cell invasion. The Snail-1 protein represses the gene that encodes for E-cadherin and stimulates the gene that encodes for vimentin. To determine the eugenol effect on Snail-1-mediated EMT, we examined Snail-1 expression in HeLa cells incubated with varying concentrations of eugenol (0, 50, 100, and 200 μM). The results showed a decrease in Snail-1 protein expression in a dose-dependent manner (Fig. 2C). At 200 μM, Snail-1 protein was completely absent.
In this research, we investigated if eugenol, an extract from clove oil (S. aromaticum), had anti-metastatic effects on cervical cancer. Eugenol has anti-cancer activity for some cancer types and it wasshowed pro-apoptotic effects in cervical cancer (Towaha, 2012). Permatasari et al. (2019), demonstrated that eugenol induces apoptosis in HeLa cells. Eugenol’s cytotoxic effect in HeLa cells was in the 50–200 μM range. This apoptotic occurrence is mediated by Caspase-3 and p53 protein expression (Permatasari et al., 2019).
Baharara et al. (2015) found that eugenol can suppress breast cancer cell migration mainly through decreased MMP-9 and Paxilin gene expression.,. Unfortunately, there is still no research on eugenol in inhibiting cervical cancer migration. The results of this study reveal the anti-carcinogenic effects of eugenol, especially in HeLa cells. We show one of the anti-carcinogenic eugenol effects on cervical cancer was inhibiting cell migration. The migration inhibition iswas achieved as eugenol concentrations increased, as shown by the Pearson’s correlation value, (r = 0.9679). HeLa cells treated with 200 μM eugenol exhibited the most effective cell migration inhibition. This was evident by the difference in scratch width pre- and post-scratch between the control and eugenol treated cells. The control condition closed up the wound more than the eugenol -treated cells.
Cervical cancer metastasis involves EMT (Jiang et al., 2016). EMT is stimulated by epithelial cell gene expression. Some of these genes can be traced by measuring the amount of protein product present in the cell. The result in EMT gene expression changes the overall characteristic of epithelial cells to develop mesenchymal cell characteristics. To that end, cell polarity and epithelial cell attachment to the basal membrane is lost, and metastasis occurs allowing the cell to detach and enter into the blood circulation (Kalluri and Weinberg, 2009).
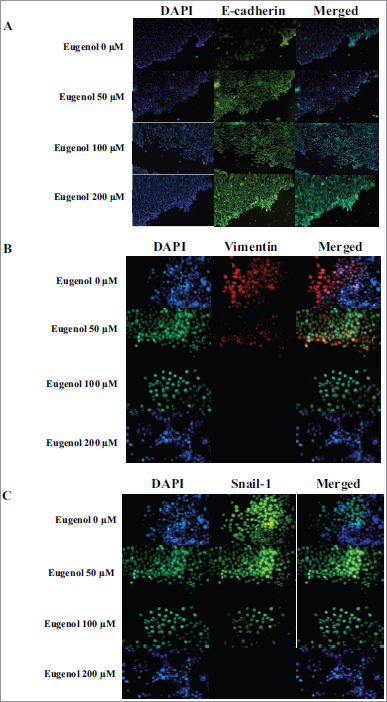 | Figure 2. Eugenol effect on E-cadherin, vimentin, and Snail-1 protein expression. (A) Increased E-cadherin protein expression was observed after eugenol treatment. HeLa cells were stained for E-cadherin (green). (B) Decreased vimentin protein expression was observed after eugenol treatment. HeLa cells were stained for vimentin (red). (C) Decreased Snail-1 protein expression after eugenol treatment. HeLa cells were stained for Snail-1 (green). DAPI (blue) indicates nuclei for all panels. [Click here to view] |
Recent studies have identified several protein markers for cervical cancer progression. Myong revealed that the E-cadherin, vimentin, and Snail proteins can be used as EMT markers. The conclusion was supported by the following results. Invasive cells showed reduced E-cadherin expression and elevated vimentin expression compared to the control group. And Snail-1 protein upregulation in the invasive carcinoma group correlated with the loss of E-cadherin expression and gain of vimentin expression (Myong, 2012).
E-cadherin functions as a cell adhesion molecule and is a marker for EMT. The loss of E-cadherin expression is associated with increased EMT and frequently occurs during tumor metastasis (Petrova et al., 2016). The EMT process usually does not occur if E-cadherin expression is increased in cancer cells. This research shows E-cadherin expression increased in HeLa cells treated with eugenol, especially at 200 μM. This is consistent with the loss of the EMT process inhibited by administering eugenol to HeLa cells.
Carcinoma cells, after finishing the EMT process, obtained a mesenchymal phenotype and expressed mesenchymal markers such as vimentin (Lee and Shen, 2012). We show that vimentin expression decreased in HeLa cells treated with eugenol. The vimentin expression was significantly reduced at concentrations of 100 and 200 μM (Figure. 2B). These results indicate eugenol is able to inhibit EMT in cervical cancer.
Snail-1, which is a powerful EMT regulator, suppresses E-cadherin expression by binding to its promoter. In addition, Snail-1 controls matrix metalloproteinase proteolytic activity associated with EMT and stromal invasion, which contributes to the upregulation of mesenchymal markers like vimentin and fibronectin (Jin, 2010; Myong, 2012; Qureshi et al., 2015). We observed Snail-1 protein expression in HeLa cells that were treated with eugenol. There was a significant decrease in Snail-1 protein expression in a dose-dependent manner. At 200 μM concentration, Snail-1 protein expression was not observed. These results indicate that eugenol is able to inhibit EMT by inhibiting Snail-1 protein expression, causing a subsequent increase in E-cadherin protein expression and a decrease in vimentin protein expression. Interestingly, vimentin protein expression disappeared at 100 μM, whereas Snail-1 was still expressed. This observation suggests that the eugenol effect was more dominant on vimentin directly than through Snail-1 regulation.
CONCLUSION
In summary, for the first time we established that eugenol can inhibit HeLa cervical cancer cell migration. The eugenol mechanism in inhibiting migration was mediated by a decrease in vimentin and Snail-1 expression and by an increase in E-cadherin expression. However, the loss of vimentin expression preceded Snail-1 in inhibiting migration. Further research is needed to fully ascertain the eugenol inhibiting mechanism for HeLa cell migration.
ACKNOWLEDGMENTS
This experiment was supported by Faculty of Medicine Brawijaya University, Malang, Indonesia. The authors would like to thank the Biochemistry Department for providing eugenol and several antibodies. We also appreciate all Biomedical Laboratory workers and helps for analyses, discussions, and suggestions.
CONFLICT OF INTEREST
The autors declare no conflict of interest.
FUNDING
The entire experiment received a fund from Hibah Guru Besar Lembaga Penelitian dan Pengabdian Masyarakat Universitas Brawijaya.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akbar W, Rauf S, Riu DS, St Maisuri TC. Concordance of human papillomavirus type 16 and 18 in cervical and oral specimen of cervical cancer patients. Indones J Obstet Gynecol, 2019; 7(1):69–73. CrossRef
Baharara J, Ramezani T, Mousavi M, Kouhestanian K. Eugenol suppressed metastasis of breast carcinoma cells and migration by regulation of MMP-9 & paxilin gene expression. Sch J Agric Vet Sci, 2015; 2(2B):125–30.
Bermudez A, Bhatla N, Leung E. Cancer of the cervix uteri. Int J Gynecol Obstet, 2015; 131:S88–95. CrossRef
Bezerra DP, Militão GC, De Morais MC, De Sousa DP. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients, 2017; 9(12):1367. CrossRef
Eifel, Patricia J., Ann H. Klopp, Jonathan S. Berek, and Panagiotis A. Konstantinopoulos. "Cancer of the cervix, vagina, and vulva." DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. Wolters Kluwer Health Pharma Solutions (Europe) Ltd, 2018. 1172-1210.
Fitri N, Kawira JA. Perbandingan variabel pada isolasi dan pemurnian eugenol dari minyak daun cengkeh. Media Penelitian dan Pengembangan Kesehatan, 2006; 16(2):156449.
Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci, 2017; 131(17):2201–21. CrossRef
Ha GH, Park JS, Breuer EK. TACC3 promotes epithelial–mesenchymal transition (EMT) through the activation of PI3K/Akt and ERK signaling pathways. Cancer Lett, 2013; 332(1):63–73. CrossRef
Jaganathan SK, Supriyanto E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules, 2012; 17(6):6290–304. CrossRef
Jiang Z, Song Q, Zeng R, Li J, Li J, Lin X, Chen X, Zhang J, Zheng Y. MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Onco Targets Ther, 2016; 7(29):45622. CrossRef
Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia L, Wu Y, Zhou BP, Feng Y. Snail is critical for tumor growth and metastasis of ovarian carcinoma. Int J Cancer, 2010; 126(9):2102–11. CrossRef
Kalluri R, Weinberg RA. Erratum: the basics of epithelial-mesenchymal transition. J Clin Investig, 2009; 119(6):1420–8. CrossRef
Lee MY, Shen MR. Epithelial-mesenchymal transition in cervical carcinoma. Am J Transl Res. 2012; 4(1):1.
Myong NH. Loss of E-cadherin and acquisition of vimentin in epithelial-mesenchymal transition are noble indicators of uterine cervix cancer progression. Korean J Pathol. 2012; 46(4):341. CrossRef
Permatasari HK, Kusuma ID, Mayangsari E. Minyak Cengkeh (Syzygium aromaticum) menginduksi apoptosis pada sel kanker servik HeLa melalui peningkatan kadar protein p53. J Kedokt Brawijaya. 2019; 30(3):185–90. CrossRef
Petrova YI, Schecterson L, Gumbiner BM. Roles for E-cadherin cell surface regulation in cancer. Mol Biol Cell, 2016; 27(21):3233–44. CrossRef
Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: its role in tumour progression and response to therapy. Cancer Lett. 2015; 356(2):321–31. CrossRef
Small Jr W, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR, Viswanathan AN, Gaffney DK. Cervical cancer: a global health crisis. Cancer. 2017; 123(13):2404–12. CrossRef
Towaha J. Manfaat eugenol cengkeh dalam berbagai industri di Indonesia. Perspektif. 2012; 11(2):79–90.
Van Minh H, My NT, Jit M. Cervical cancer treatment costs and cost-effectiveness analysis of human papillomavirus vaccination in Vietnam: a PRIME modeling study. BMC Health Serv Res, 2017; 17(1):353. CrossRef
World Health Organization. Sexual and reproductive health cervical cancer (online). WHO, Geneva, Switzerland. 2018. Available from: https://www.who.int/reproductivehealth/topics/cancers/en/ (Accessed 20 February 2020).
Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Mig, 2015; 9(4):317–24. CrossRef
Yang HJ. Aberrant DNA methylation in cervical carcinogenesis. Chin J Cancer, 2013; 32(1):42. CrossRef
You BR, Moon HJ, Han YH, Park WH. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem Toxicol, 2010; 48(5):1334–40. CrossRef